Macroscopic Friction Studies of Alkylglucopyranosides as Additives for Water-Based Lubricants
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
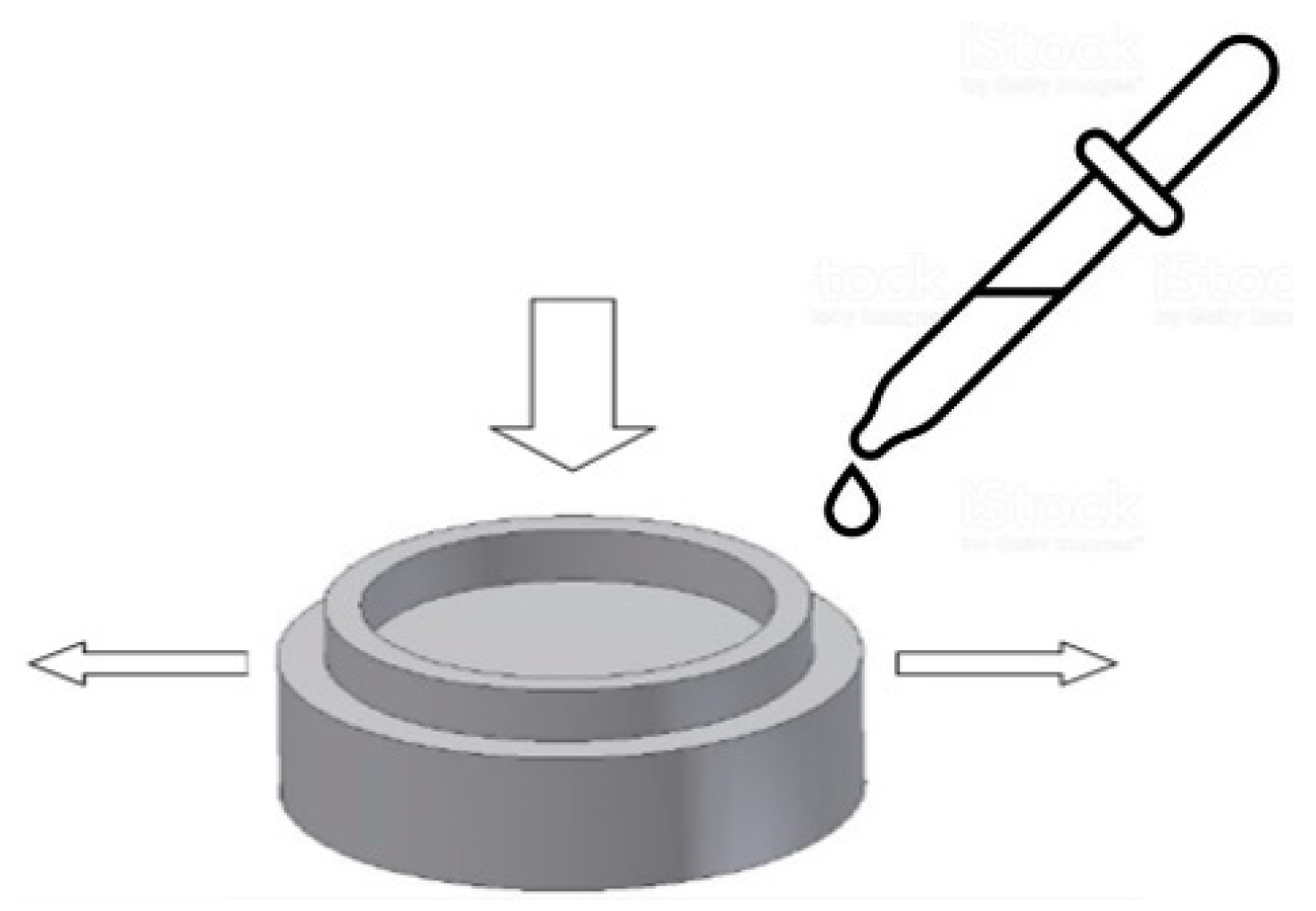
2.2. Methods
3. Results
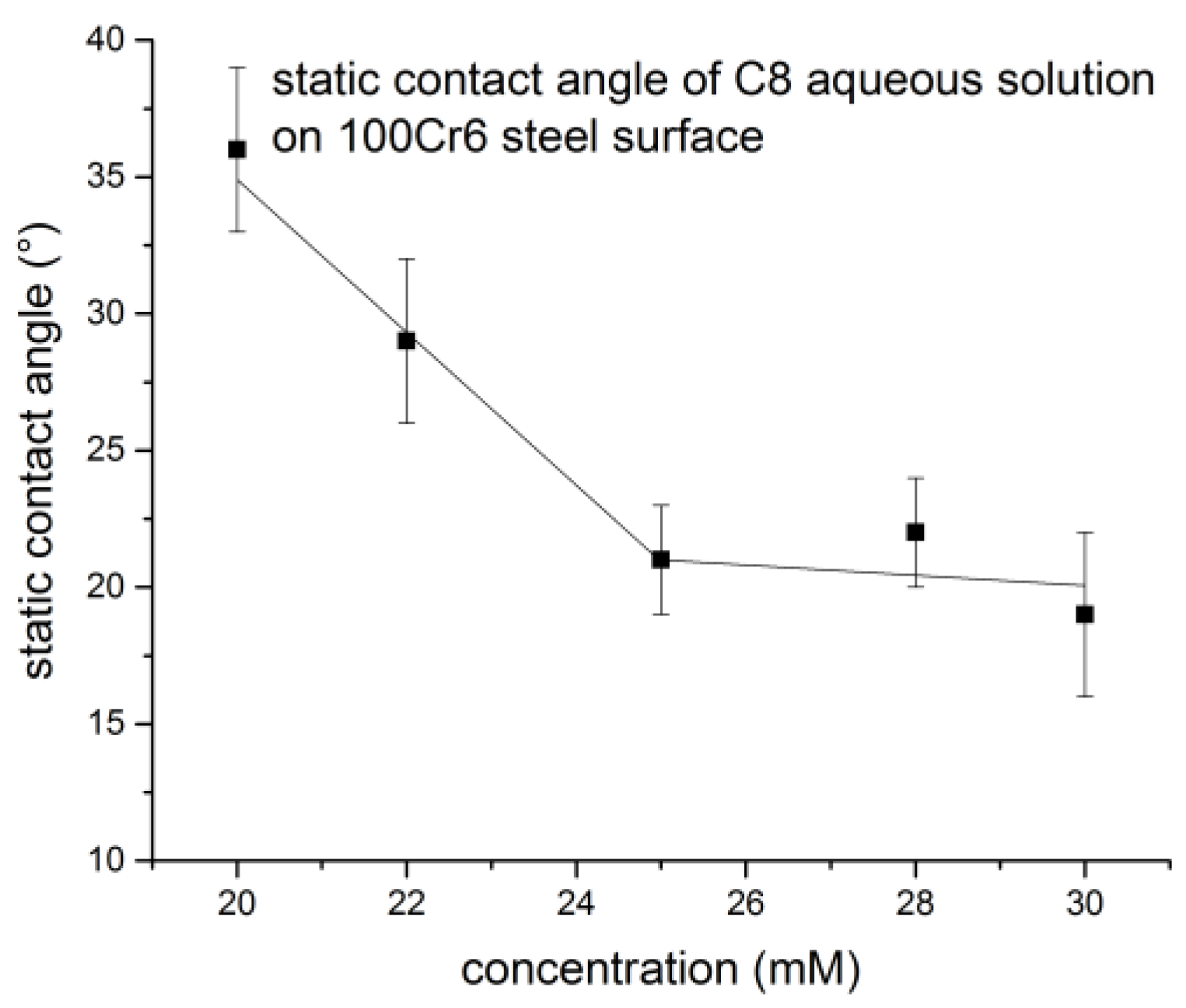
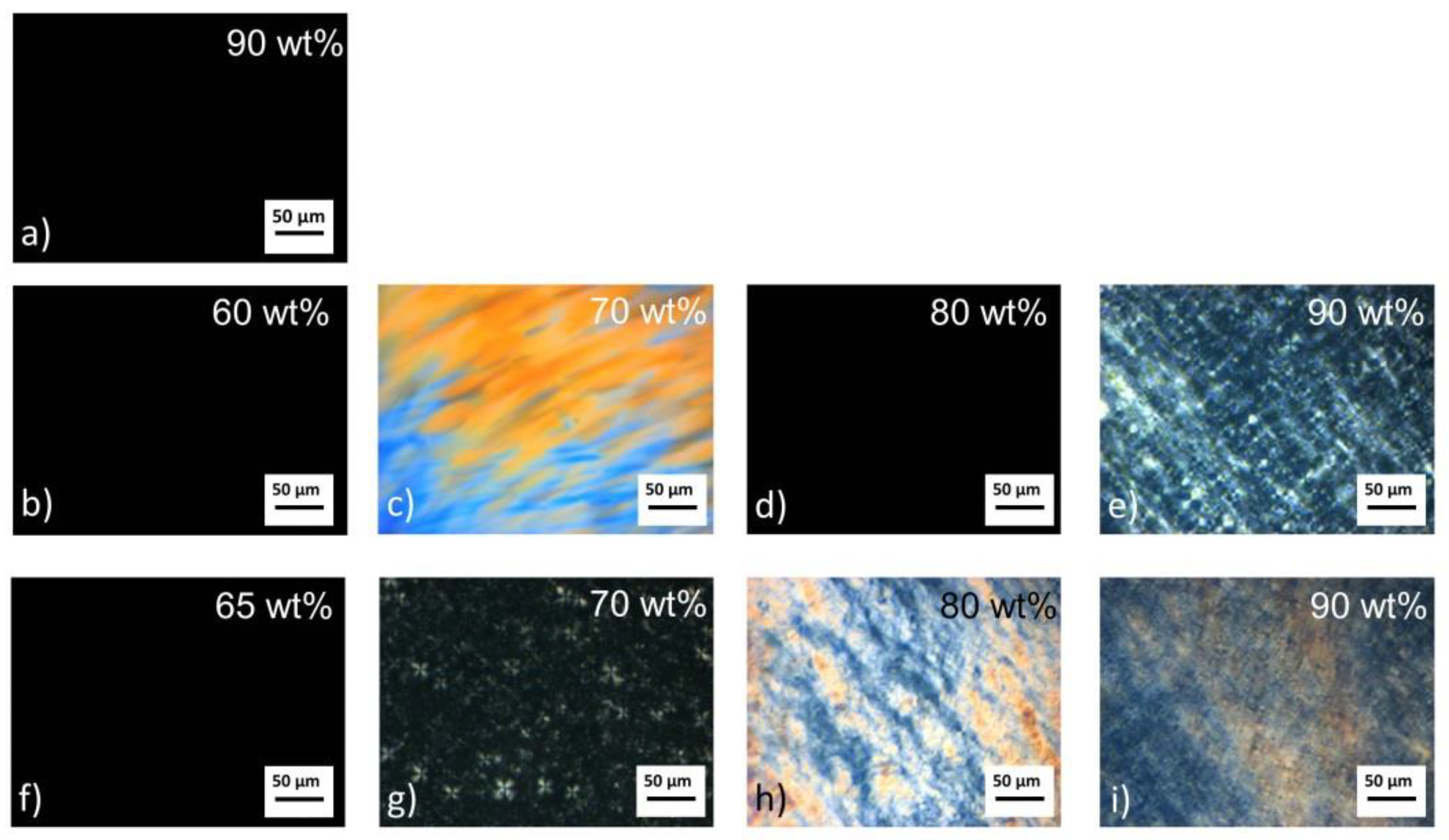
3.1. Physicochemical Properties
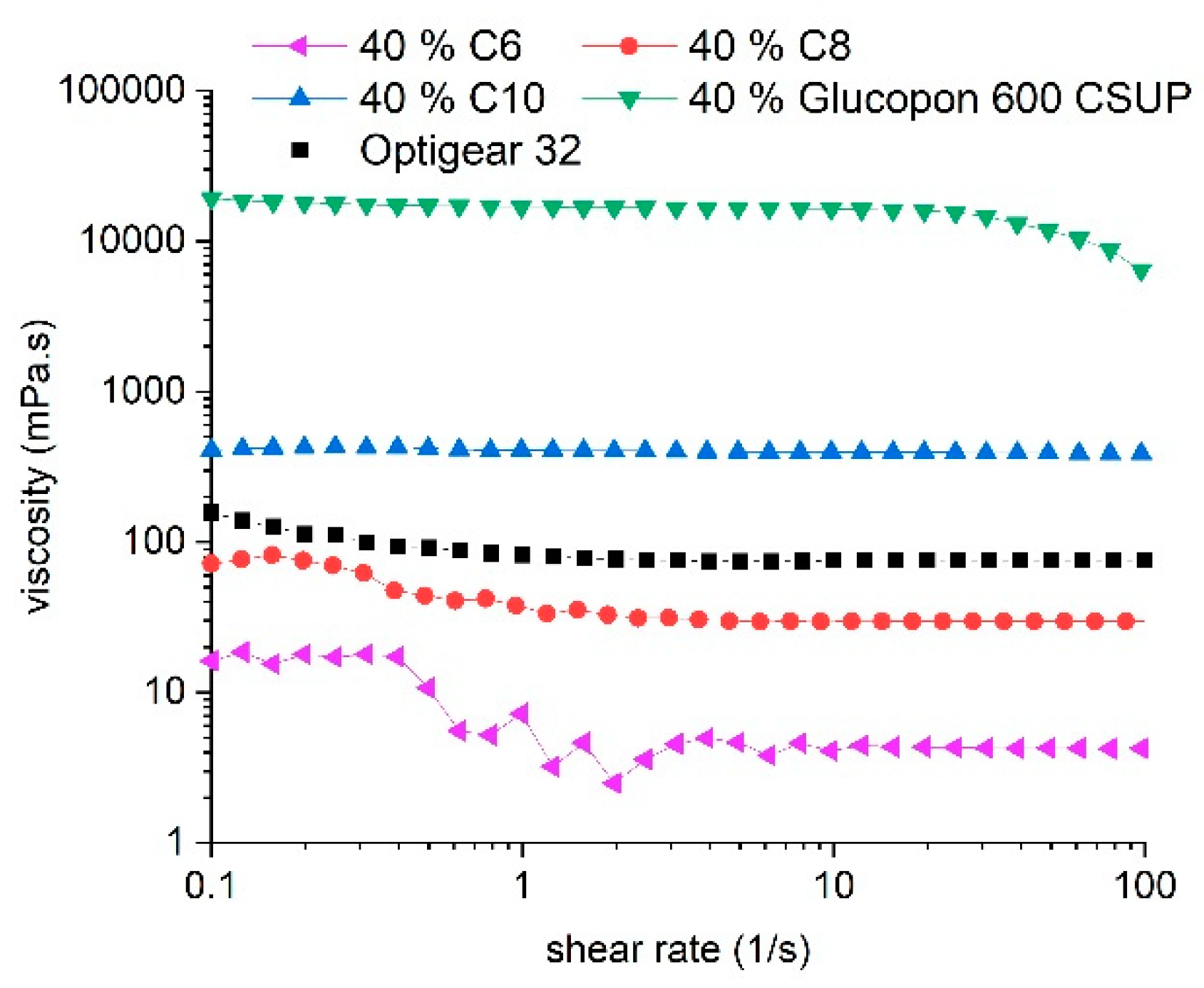
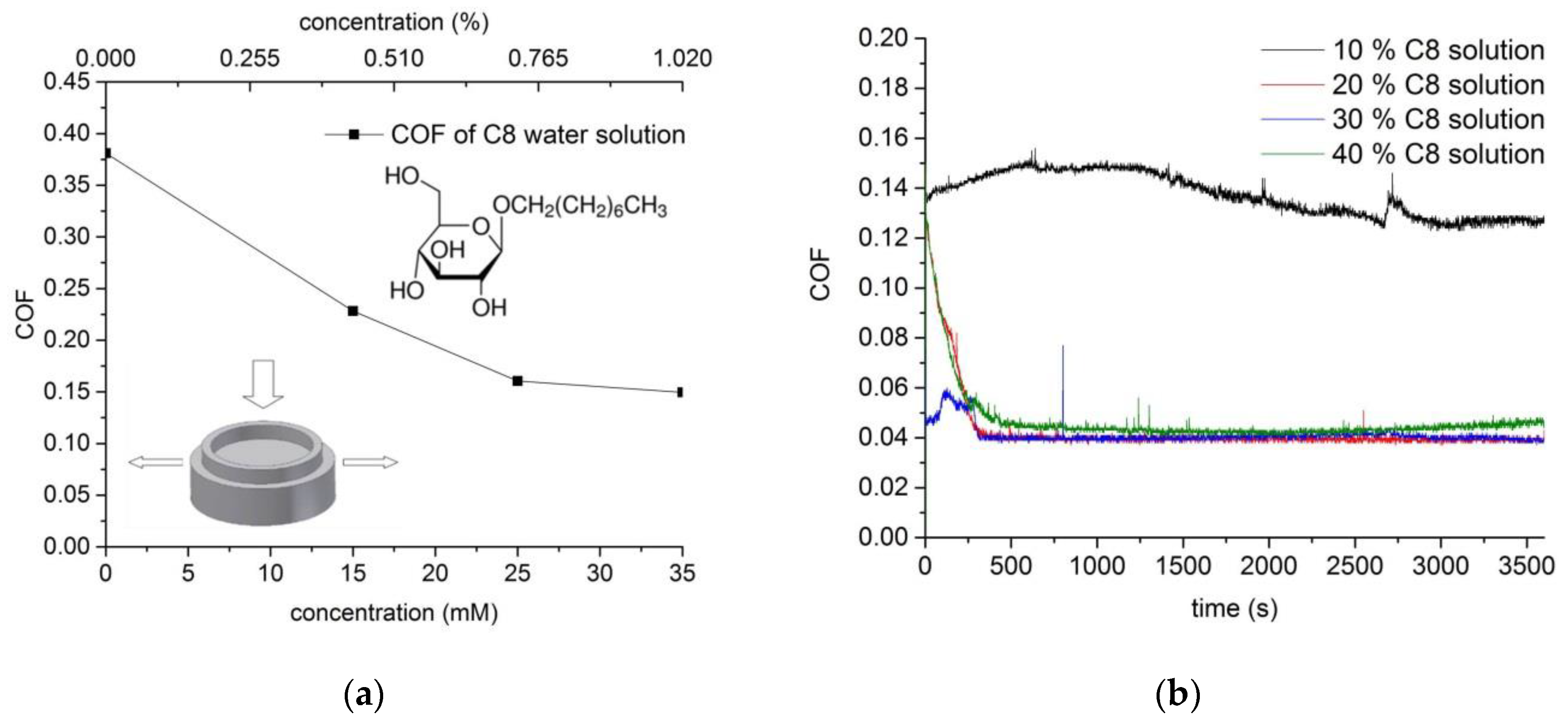
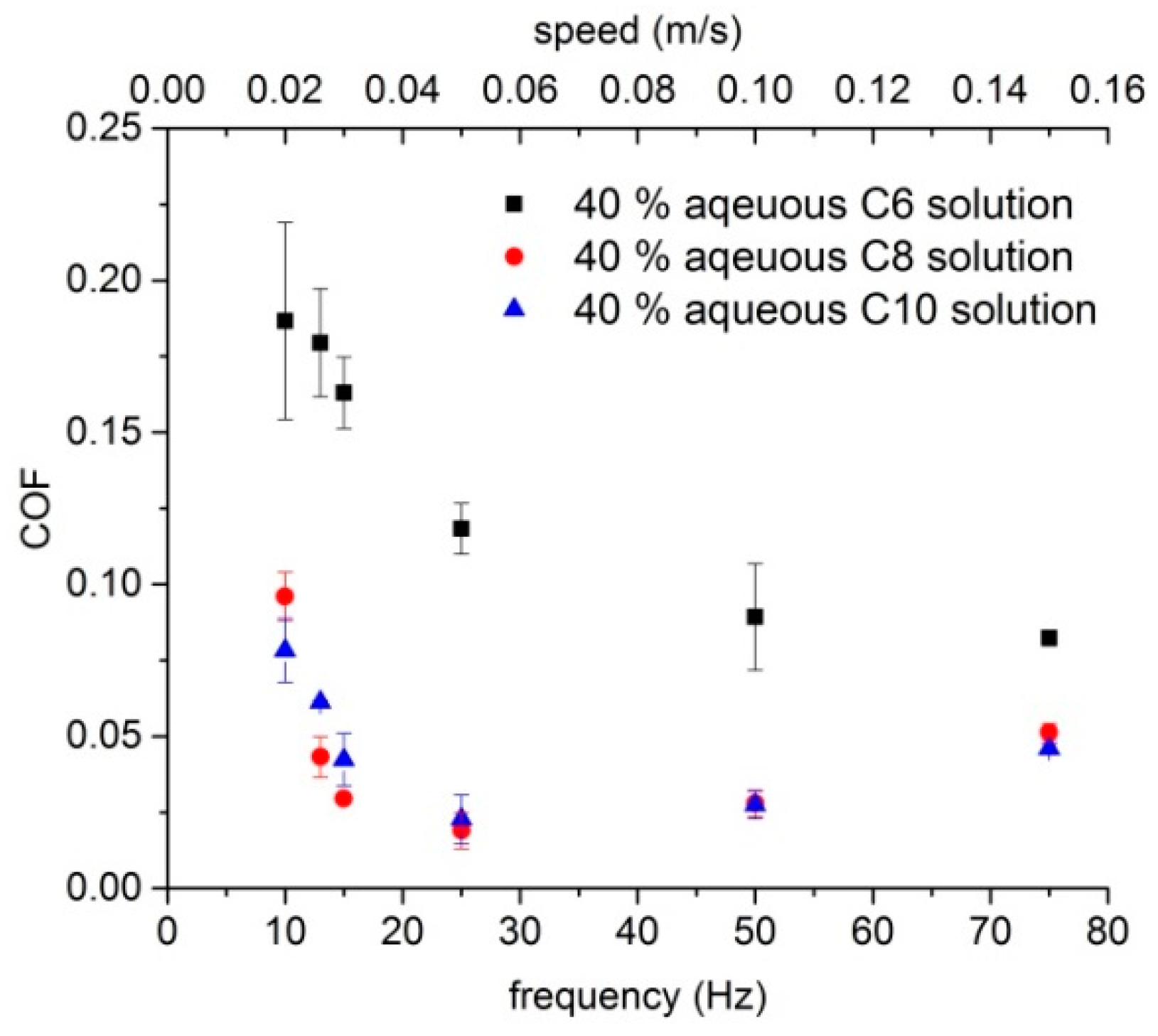
3.2. Friction Coefficient Behavior of Lubricants Based on Glucopyranoside
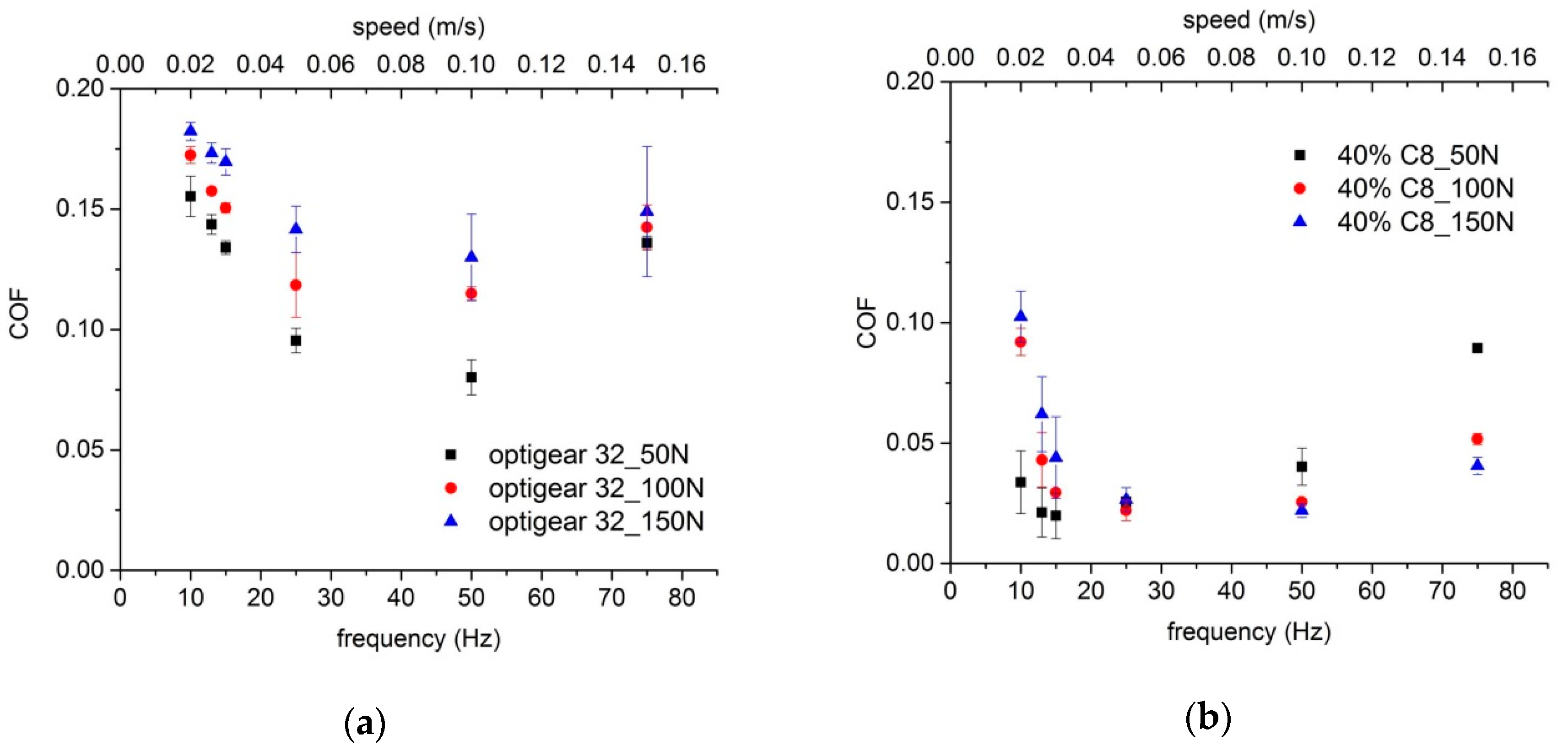
3.3. Comparison with a Standard Oil
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Holmberg, K.; Erdemir, A. Influence of tribology on global energy consumption, costs and emissions. Friction 2017, 5, 263–284. [Google Scholar] [CrossRef]
- Holmberg, K.; Kivikytö-Reponen, P.; Härkisaari, P.; Valtonen, K.; Erdemir, A. Global energy consumption due to friction and wear in the mining industry. Tribol. Int. 2017, 115, 116–139. [Google Scholar] [CrossRef]
- Holmberg, K.; Andersson, P.; Nylund, N.; Mäkelä, K.; Erdemir, A. Global energy consumption due to friction in trucks and buses. Tribol. Int. 2014, 78, 94–114. [Google Scholar] [CrossRef]
- Holmberg, K.; Andersson, P.; Erdemir, A. Global energy consumption due to friction in passenger cars. Tribol. Int. 2012, 47, 221–234. [Google Scholar] [CrossRef]
- Holmberg, K.; Siilasto, R.; Laitinen, T.; Andersson, P.; Jäsberg, A. Global energy consumption due to friction in paper machines. Tribol. Int. 2013, 62, 58–77. [Google Scholar] [CrossRef]
- Anand, A.; Irfan Ul Haq, M.; Vohra, K.; Raina, A.; Wani, M.F. Role of Green Tribology in Sustainability of Mechanical Systems: A State of the Art Survey. Mater. Today Proc. 2017, 4, 3659–3665. [Google Scholar] [CrossRef]
- Si-Wei, Z. Recent developments of green tribology. Surf. Topogr. Metrol. Prop. 2016, 4, 023004. [Google Scholar]
- Anand, A.; Irfan Ul Haq, M.; Raina, A.; Vohra, K.; Kumar, R.; Mohan Sharma, S. Natural Systems and Tribology—Analogies and Lessons. Mater. Today Proc. 2017, 4, 5228–5232. [Google Scholar] [CrossRef]
- Liang, S.; Shen, Z.; Yi, M.; Liu, L.; Zhang, X.; Ma, S. In-situ exfoliated graphene for high-performance water-based lubricants. Carbon 2016, 96, 1181–1190. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Jiang, L.; Chen, X.; Luo, J. Cationic Surfactant Micelles Lubricate Graphitic Surface in Water. Langmuir 2019, 35, 11108–11113. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.; Cheng, P.; Chen, X.; Wang, W.; Luo, J. AFM Studies on Liquid Superlubricity between Silica Surfaces Achieved with Surfactant Micelles. Langmuir 2016, 32, 5593–5599. [Google Scholar] [CrossRef] [PubMed]
- Raviv, U.; Giasson, S.; Kampf, N.; Gohy, J.; Jérôme, R.; Klein, J. Lubrication by charged polymers. Nature 2003, 425, 163–165. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Briscoe, W.; Armes, S.; Klein, J. Lubrication at Physiological Pressures by Polyzwitterionic Brushes. Science 2009, 323, 1698–1701. [Google Scholar] [CrossRef] [PubMed]
- Klein, J. Hydration lubrication. Friction 2013, 1, 1–23. [Google Scholar] [CrossRef]
- Chiñas-Castillo, F.; Lara-Romero, J.; Alonso-Núñez, G.; Barceinas-Sánchez, J.; Jiménez-Sandoval, S. MoS2 Films Formed by In-contact Decomposition of Water-soluble Tetraalkylammonium Thiomolybdates. Tribol. Lett. 2008, 29, 155–161. [Google Scholar] [CrossRef]
- Kinoshita, H.; Nishina, Y.; Alias, A.; Fujii, M. Tribological properties of monolayer graphene oxide sheets as water-based lubricant additives. Carbon 2014, 66, 720–723. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, S.; Song, S.; Yang, G.; Yu, L.; Wu, Z.; Li, X.; Zhang, P. Preparation and Tribological Properties of Surface-Capped Copper Nanoparticle as a Water-Based Lubricant Additive. Tribol. Lett. 2014, 54, 25–33. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, J.; Xia, W.; Cheng, X.; He, A.; Yun, J.; Wang, L.; Huang, H.; Jiao, S.; Huang, L.; et al. A study of the tribological behaviour of TiO2 nano-additive water-based lubricants. Tribol. Int. 2017, 109, 398–408. [Google Scholar] [CrossRef]
- Ye, X.; Ma, L.; Yang, Z.; Wang, J.; Wang, H.; Yang, S. Covalent Functionalization of Fluorinated Graphene and Subsequent Application as Water-based Lubricant Additive. ACS Appl. Mater. Interfaces 2016, 8, 7483–7488. [Google Scholar] [CrossRef]
- He, A.; Huang, S.; Yun, J.; Jiang, Z.; Stokes, J.; Jiao, S.; Wang, L.; Huang, H. Tribological Characteristics of Aqueous Graphene Oxide, Graphitic Carbon Nitride, and Their Mixed Suspensions. Tribol. Lett. 2018, 66, 42. [Google Scholar] [CrossRef]
- Zhang, C.; Zheng, M.; Dai, Y. Lubricating properties of polyalkylene glycol and organic phosphate ester mixed aqueous solutions. Sci. China Technol. Sci. 2013, 56, 2988–2993. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Q.; Cai, M.; Shi, L.; Zhou, F.; Liu, W. Ibuprofen-Based Ionic Liquids as Additives for Enhancing the Lubricity and Antiwear of Water–Ethylene Glycol Liquid. Tribol. Lett. 2017, 65, 55. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Q.; Ma, Z.; Huang, G.; Cai, M.; Zhou, F.; Liu, W. Significant enhancement of anti-friction capability of cationic surfactant by phosphonate functionality as additive in water. Tribol. Int. 2017, 112, 86–93. [Google Scholar] [CrossRef]
- Amann, T.; Dold, C.; Kailer, A. Potential Controlled Tribological Behavior of Water-Based Ionic Liquids. Key Eng. Mater. 2016, 674, 250–256. [Google Scholar] [CrossRef]
- Amann, T.; Gatti, F.; Oberle, N.; Kailer, A.; Rühe, J. Galvanically induced potentials to enable minimal tribochemical wear of stainless steel lubricated with sodium chloride and ionic liquid aqueous solution. Friction 2018, 6, 230–242. [Google Scholar] [CrossRef]
- Kurz, J.; Amann, T.; Kailer, A. Tribological Investigations of Silicon Nitride Lubricated by Ionic Liquid Aqueous Solutions. Tribol. Trans. 2019, 62, 295–303. [Google Scholar] [CrossRef]
- Amann, T.; Kailer, A.; Herrmann, M. Influence of Electrochemical Potentials on the Tribological Behavior of Silicon Carbide and Diamond-Coated Silicon Carbide. J. Bio Tribo Corros. 2015, 1, 30. [Google Scholar] [CrossRef]
- Amann, T.; Dold, C.; Kailer, A. Complex fluids in tribology to reduce friction: Mesogenic fluids, ionic liquids and ionic liquid crystals. Tribol. Int. 2013, 65, 3–12. [Google Scholar] [CrossRef]
- Avilés, M.; Sanchez, C.; Pamies, R.; Sanes, J.; Bermudez, M. Ionic Liquid Crystals in Tribology. Lubricants 2019, 7, 72. [Google Scholar] [CrossRef]
- Kupchinov, B.; Ermakov, S.; Rodnenkov, V.; Bobrysheva, S.; Beloenko, E. The effect of liquid crystals on joint lubrication. Wear 1994, 171, 7–12. [Google Scholar] [CrossRef]
- Cognard, J. Lubrication with Liquid Crystals. In Tribology and the Liquid-Crystalline State; American Chemical Society: Washington, DC, USA, 1990; pp. 1–47. [Google Scholar]
- Kupchinov, B.; Rodnenkov, V.; Ermakov, S.; Parkalov, V. A study of lubrication by liquid crystals. Tribol. Int. 1991, 24, 25–28. [Google Scholar] [CrossRef]
- Gribailo, P.; Kupreev, M.; Zamyatnin, V. Effect of liquid crystals on lubricating properties of mineral oils. Chem. Technol. Fuels Oils 1983, 19, 342–345. [Google Scholar] [CrossRef]
- Boschkova, K.; Elvesjo, J.; Kronberg, B. Frictional properties of lyotropic liquid crystalline mesophases at surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2000, 166, 67–77. [Google Scholar] [CrossRef]
- Sulek, M.; Wasilewski, T.; Kurzydłowski, K. The Effect of Concentration on Lubricating Properties of Aqueous Solutions of Sodium Lauryl Sulfate and Ethoxylated Sodium Lauryl Sulfate. Tribol. Lett. 2010, 40, 337–345. [Google Scholar] [CrossRef]
- Avilés, M.; Carrión, F.; Sanes, J.; Bermúdez, M. Effects of protic ionic liquid crystal additives on the water-lubricated sliding wear and friction of sapphire against stainless steel. Wear 2018, 408, 56–64. [Google Scholar] [CrossRef]
- Carrión, F.; Avilés, M.; Nakano, K.; Tadokoro, C.; Nagamine, T.; Bermúdez, M. Diprotic ammonium palmitate ionic liquid crystal and nanodiamonds in aqueous lubrication. Film thickness and influence of sliding speed. Wear 2019, 418, 241–252. [Google Scholar] [CrossRef]
- Pogodina, N.; Amann, T.; Dold, C.; Metwalli, E.; Müller-Buschbaum, P.; Kailer, A.; Friedrich, C. Triborheology and orientational dynamics of ionic liquid crystals. J. Mol. Liq. 2014, 192, 118–126. [Google Scholar] [CrossRef]
- Hiltrop, K. Lyotropic Liquid Crystals. In Liquid Crystals; Stegemeyer, H., Behret, H., Eds.; Steinkopff: Heidelberg, Germany, 1994; pp. 143–171. [Google Scholar]
- Carrion, F.; Martinez-Nicolas, G.; Iglesias, P.; Sanes, J.; Bermudez, M. Liquid Crystals in Tribology. Int. J. Mol. Sci. 2009, 10, 4102–4115. [Google Scholar] [CrossRef]
- Ma, C.; Li, C.; Shen, Q.; Hu, Y.; Liu, W.; Wang, H. Study of lyotropic liquid crystal in lubrication on aluminum alloy surfaces. J. Dispers. Sci. Technol. 1999, 20, 1025–1030. [Google Scholar] [CrossRef]
- Fuller, S.; Li, Y.; Tiddy, G.; Wyn-Jones, E.; Arnell, R. Formulation of Lyotropic Lamellar Phases of Surfactants as Novel Lubricants. Langmuir 1995, 11, 1980–1983. [Google Scholar] [CrossRef]
- Yang, H.; Yang, M.; Zhang, Y.; Chen, G. In situ Synthesis and Lubrication of PbS Nanoparticles in Lamellar Liquid Crystal. Colloid J. 2004, 66, 635–641. [Google Scholar] [CrossRef]
- Yang, H.; Yang, M.; Guo, R. Tribology Properties of CdS Nanoparticles in Situ Synthesized in Lamellar Liquid Crystal System of Triton X-100/C10H21OH/H2O. J. Dispers. Sci. Technol. 2005, 26, 477–482. [Google Scholar] [CrossRef]
- Yang, H.; Guo, R.; Wang, H. Lubrication of the mixed system of Triton X-100/n-C10H21OH/H2O lamellar liquid crystal and ZnS nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2001, 180, 243–251. [Google Scholar] [CrossRef]
- Ji, H.; Zhang, X.; Tan, T. Preparation of a Water-Based Lubricant from Lignocellulosic Biomass and Its Tribological Properties. Ind. Eng. Chem. Res. 2017, 56, 7858–7864. [Google Scholar] [CrossRef]
- Sulek, M.; Bak-Sowinska, A. New Ecological Lubricants on the Basis of Lyotropic Liquid Crystals Formed by Solutions of Maracuja Oil Ethoxylate. Ind. Eng. Chem. Res. 2013, 52, 16169–16174. [Google Scholar] [CrossRef]
- Sulek, M.; Ogorzalek, M.; Wasilewski, T.; Klimaszewska, E. Alkyl Polyglucosides as Components of Water Based Lubricants. J. Surfactants Deterg. 2013, 16, 369–375. [Google Scholar] [CrossRef]
- Sulek, M.; Wasilewski, T. Tribological properties of aqueous solutions of alkyl polyglucosides. Wear 2006, 260, 193–204. [Google Scholar] [CrossRef]
- Chen, W.; Amann, T.; Kailer, A.; Rühe, J. Thin-Film Lubrication in the Water/Octyl β-d-Glucopyranoside System: Macroscopic and Nanoscopic Tribological Behavior. Langmuir 2019, 35, 7136–7145. [Google Scholar] [CrossRef]
- Burlatsky, S.; Atrazhev, V.; Dmitriev, D.; Sultanov, V.; Timokhina, E.; Ugolkova, E.; Tulyani, S.; Vincitore, A. Surface tension model for surfactant solutions at the critical micelle concentration. J. Colloid Interface Sci. 2013, 393, 151–160. [Google Scholar] [CrossRef]
- Pool, R.; Bolhuis, P. Accurate Free Energies of Micelle Formation. J. Phys. Chem. B 2005, 109, 6650–6657. [Google Scholar] [CrossRef]
- Yan, Y. Tribology and tribo-corrosion testing and analysis of metallic biomaterials. In Metals for Biomedical Devices; Niinomi, M., Ed.; Woodhead Publishing: Cambridge, UK, 2010; pp. 178–201. [Google Scholar]
- Schreiber, P.; Schneider, J. Liquid superlubricity obtained for self-mated silicon carbide in nonaqueous low-viscosity fluid. Tribol. Int. 2019, 134, 7–14. [Google Scholar] [CrossRef]
- Matsson, M.; Kronberg, B.; Claesson, P. Adsorption of alkyl polyglucosides on the solid/water interface: Equilibrium effects of alkyl chain length and head group polymerization. Langmuir 2004, 20, 4051–4058. [Google Scholar] [CrossRef]
- Li, K.; Amann, T.; List, M.; Walter, M.; Moseler, M.; Kailer, A.; Rühe, J. Ultralow Friction of Steel Surfaces Using a 1,3-Diketone Lubricant in the Thin Film Lubrication Regime. Langmuir 2015, 31, 11033–11039. [Google Scholar] [CrossRef]
- Granick, S. Molecular Tribology of Fluids, in Fundamentals of Friction: Macroscopic and Microscopic Processes; Singer, I.L., Pollock, H.M., Eds.; Springer: Dordrecht, The Netherlands, 1992; pp. 387–401. [Google Scholar]
- Carson, G.; Hu, H.; Granick, S. Molecular Tribology of Fluid Lubrication—Shear Thinning. Tribol. Trans. 1992, 35, 405–410. [Google Scholar] [CrossRef]
- Luo, J.; Wen, S.; Huang, P. Thin film lubrication. Part I. Study on the transition between EHL and thin film lubrication using a relative optical interference intensity technique. Wear 1996, 194, 107–115. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Amann, T.; Kailer, A.; Rühe, J. Macroscopic Friction Studies of Alkylglucopyranosides as Additives for Water-Based Lubricants. Lubricants 2020, 8, 11. https://doi.org/10.3390/lubricants8010011
Chen W, Amann T, Kailer A, Rühe J. Macroscopic Friction Studies of Alkylglucopyranosides as Additives for Water-Based Lubricants. Lubricants. 2020; 8(1):11. https://doi.org/10.3390/lubricants8010011
Chicago/Turabian StyleChen, Wei, Tobias Amann, Andreas Kailer, and Jürgen Rühe. 2020. "Macroscopic Friction Studies of Alkylglucopyranosides as Additives for Water-Based Lubricants" Lubricants 8, no. 1: 11. https://doi.org/10.3390/lubricants8010011
APA StyleChen, W., Amann, T., Kailer, A., & Rühe, J. (2020). Macroscopic Friction Studies of Alkylglucopyranosides as Additives for Water-Based Lubricants. Lubricants, 8(1), 11. https://doi.org/10.3390/lubricants8010011




