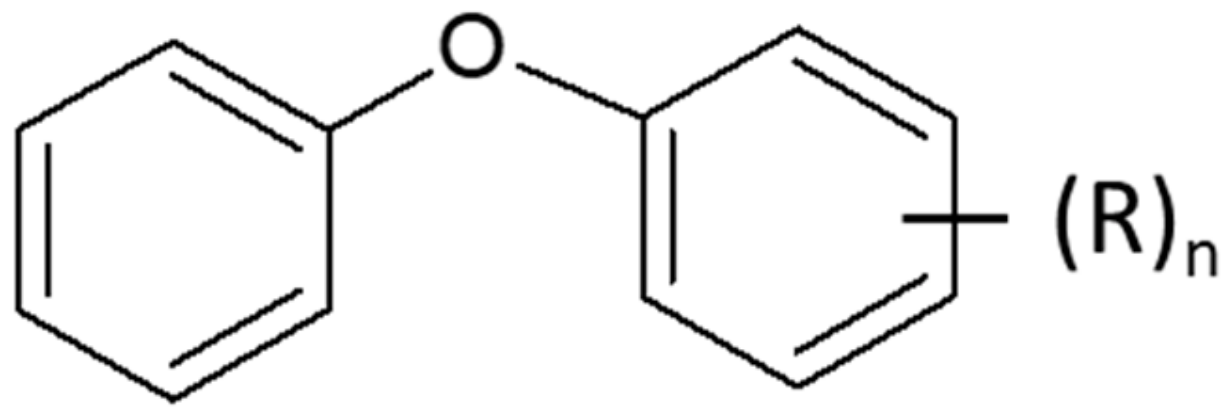
3.1. Tribological Properties of Alkyldiphenylethers under Reciprocating Sliding Condition
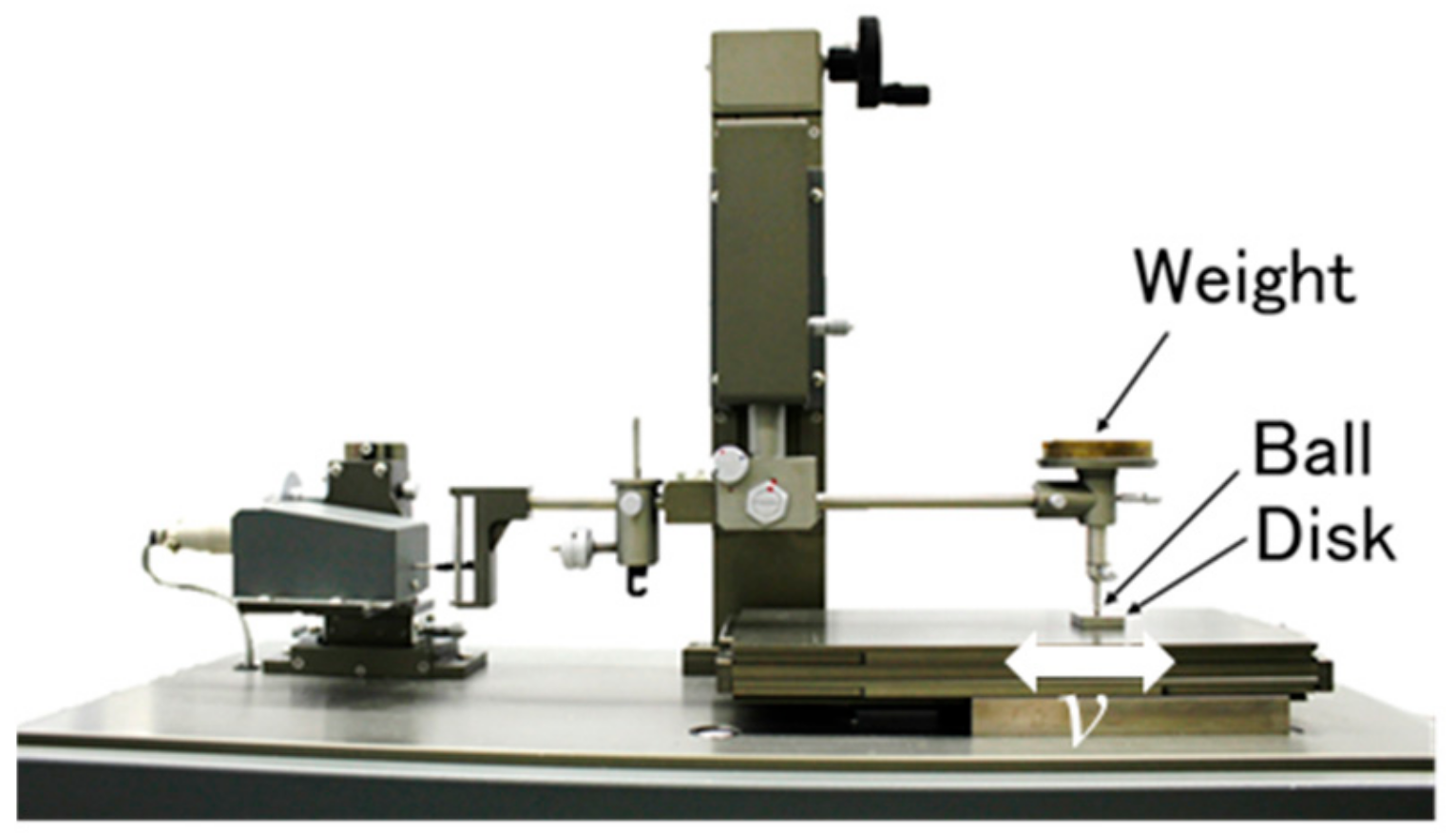
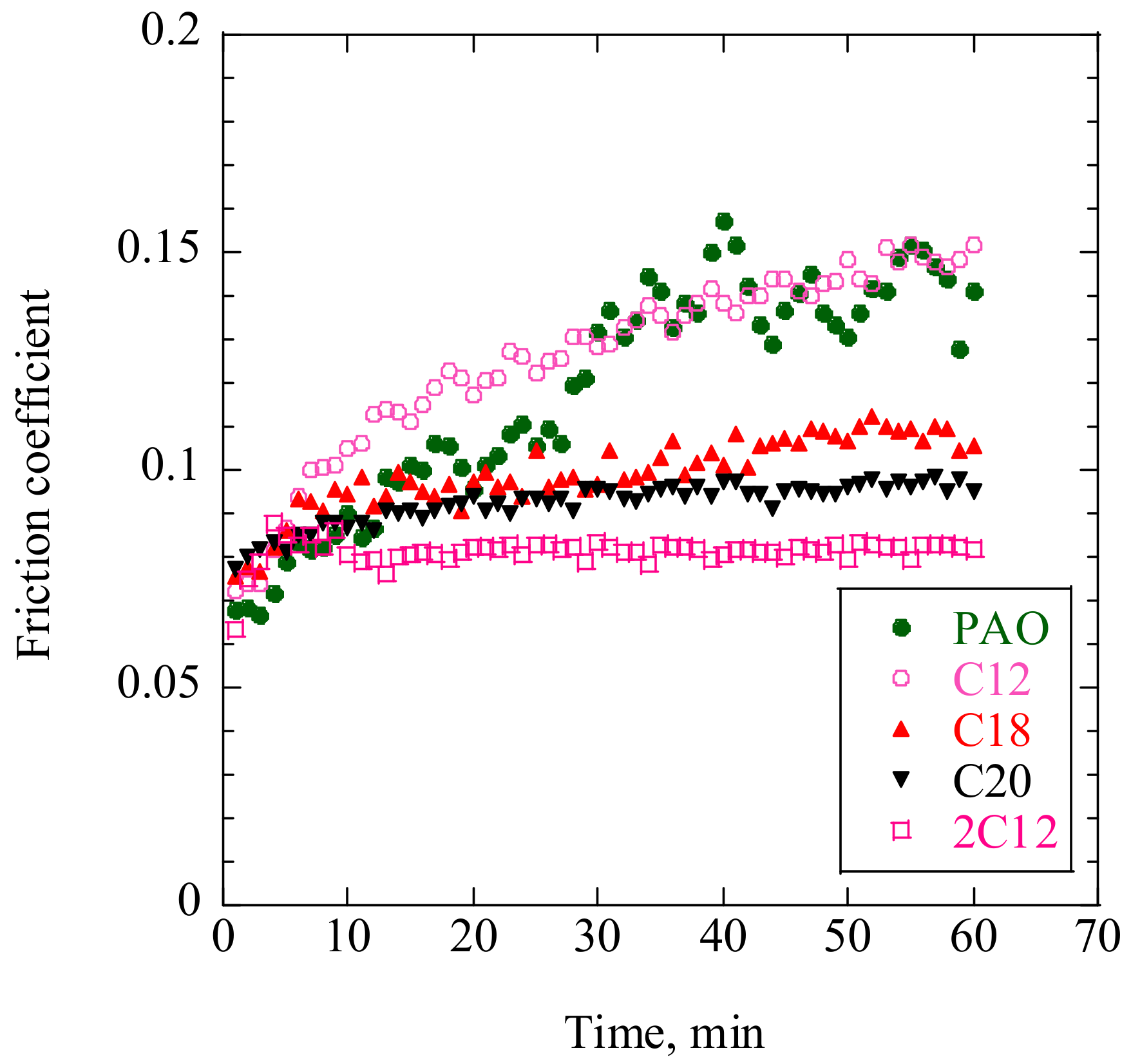
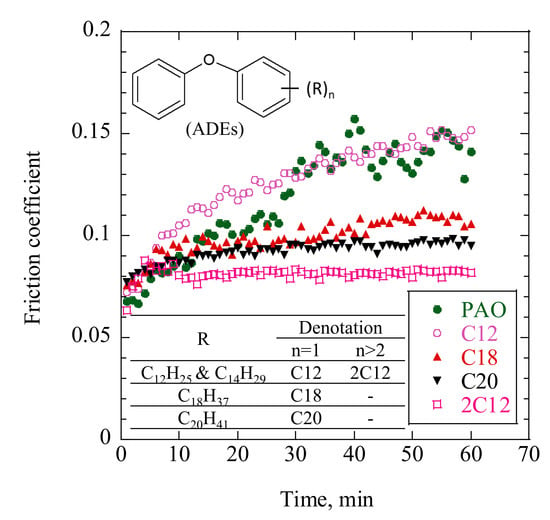
Typical friction curves, as measured using the reciprocating friction tester, are shown in
Figure 4. There was no obvious difference in friction coefficient during the first few minutes; however, that of C12 gradually increased with friction time, which was similar behavior to that of PAO30. The friction coefficients of C18, C20, and 2C12 stabilized within a few minutes. These results suggest that ADEs with longer alkyl chains or more attached alkyl chains preferred operating under more stable friction conditions.
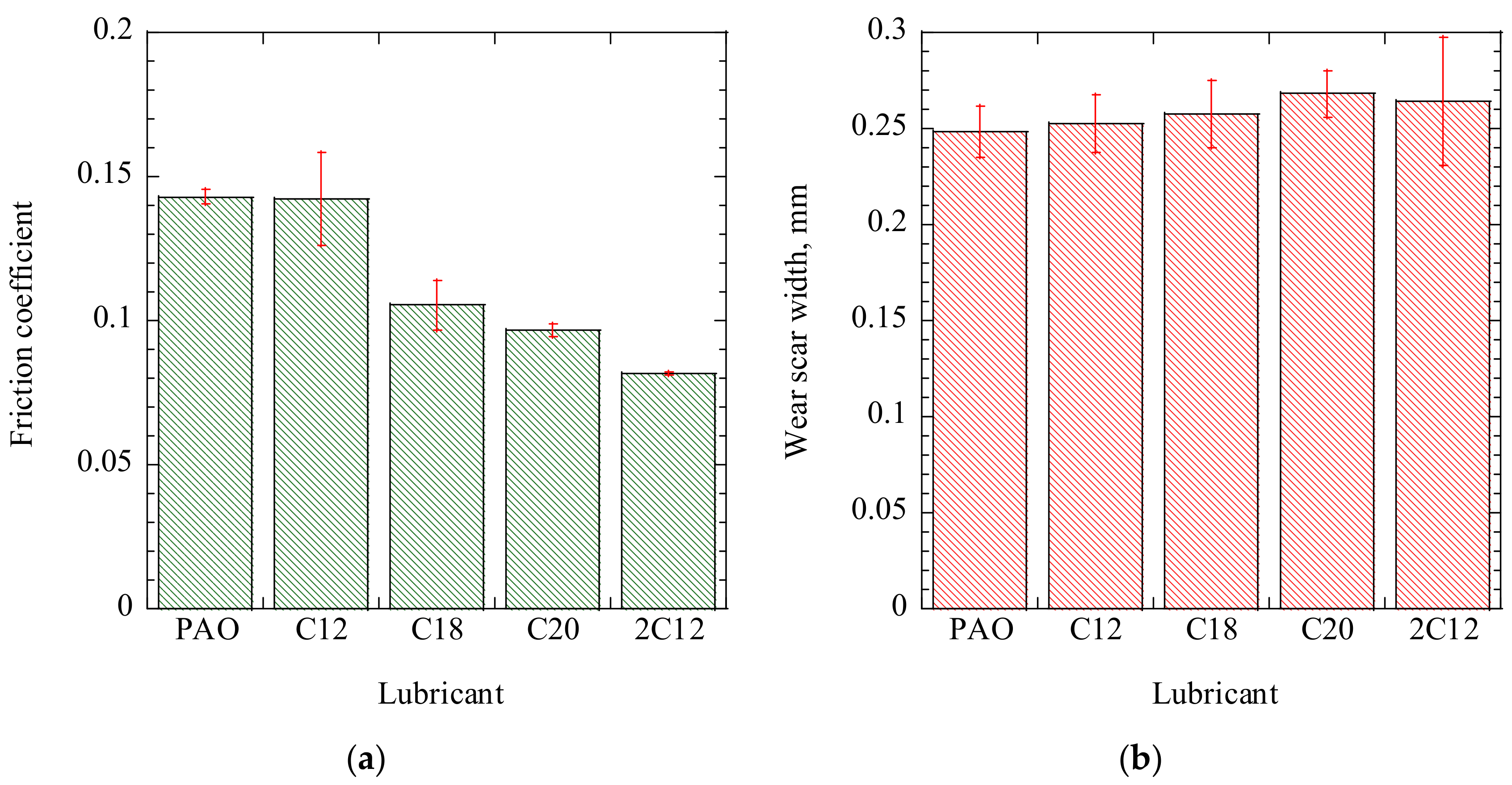
Figure 5a summarizes the averaged friction coefficients at steady state, as determined from the friction coefficient curves illustrated in
Figure 4. The C12 ADE with a short alkyl chain showed the highest friction coefficient of 0.142. When the alkyl chain length increased to C18, the friction coefficient decreased to 0.105. When the alkyl chain length further increased to C20, a much lower friction coefficient of 0.095 was observed, which was 33% lower than that of C12. These results showed that the friction coefficients of ADEs strongly depended on the alkyl chain length. Moreover, 2C12, which featured more than two alkyl chains, showed the lowest friction coefficient of 0.081. The friction coefficient decreased by 43% on increasing the number of attached alkyl chains, even if the alkyl chain length was short.
Figure 5b shows the wear scar width on the disks that were lubricated with the sample oils. Although the friction coefficients of ADEs depended on alkyl chain length, the wear scar width seemed to be independent of alkyl chain length and number of attached chains. The wear scar width was almost the same when PAO30 was used, so we think that reasons other than the molecular chemical structures induced this phenomenon.
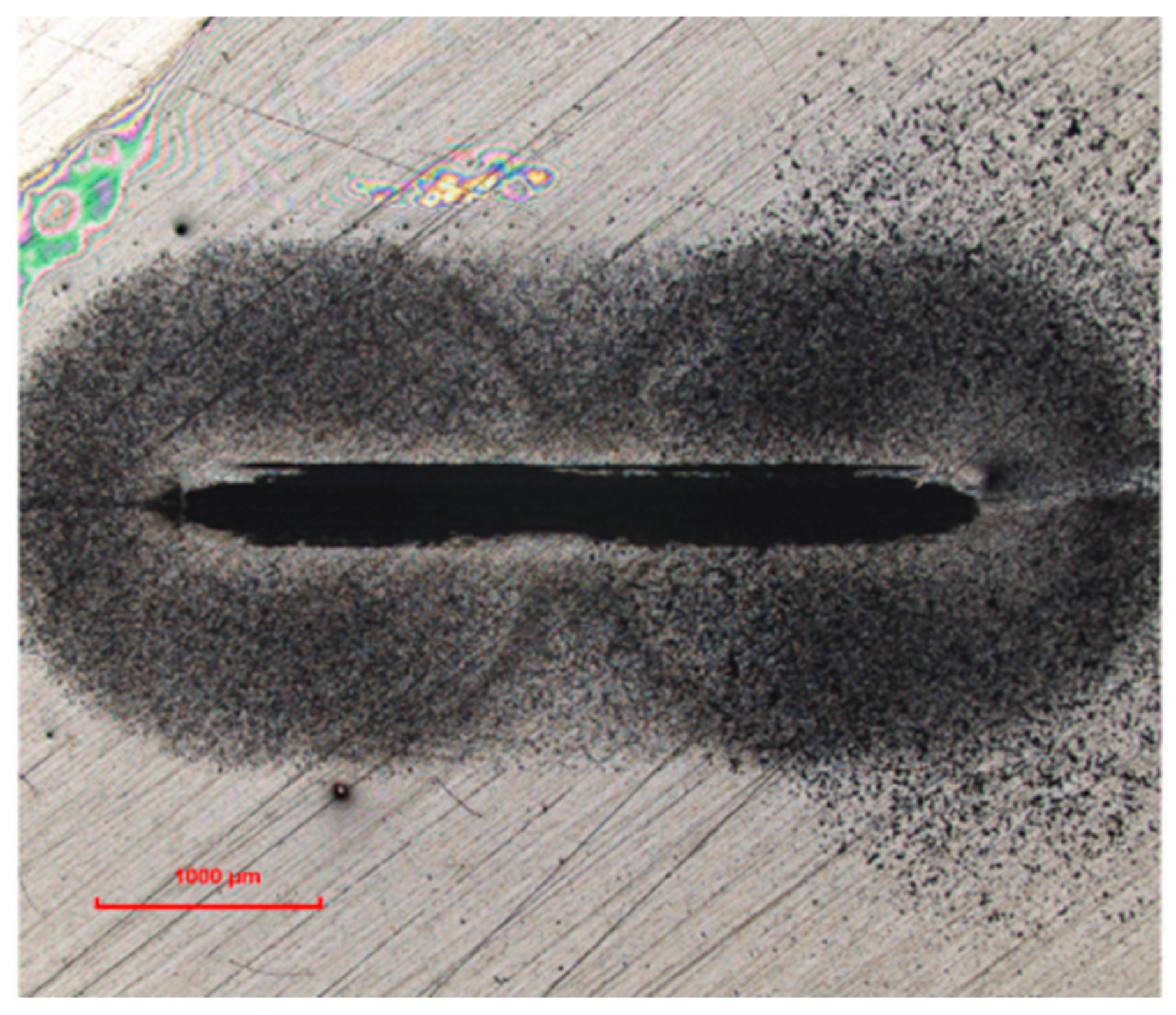
Figure 6 shows a typical photograph of the friction track on the disk under reciprocating sliding condition. We observed much debris, both inside and outside of the friction track. In this case, the speed decreased to zero at the end of a specimen traverse and then accelerated to a similar speed in the opposite direction, because the motion was reciprocating. Therefore, the debris could not be quickly removed from the friction area. Moreover, the short sliding stroke made the debris easily accumulate in friction track. Therefore, abrasion occurred [
12]. As a result, we observed severe wear on the disks for all of the sample oils.
3.2. Tribological Properties of Alkyldiphenylethers under Continuous Sliding Condition
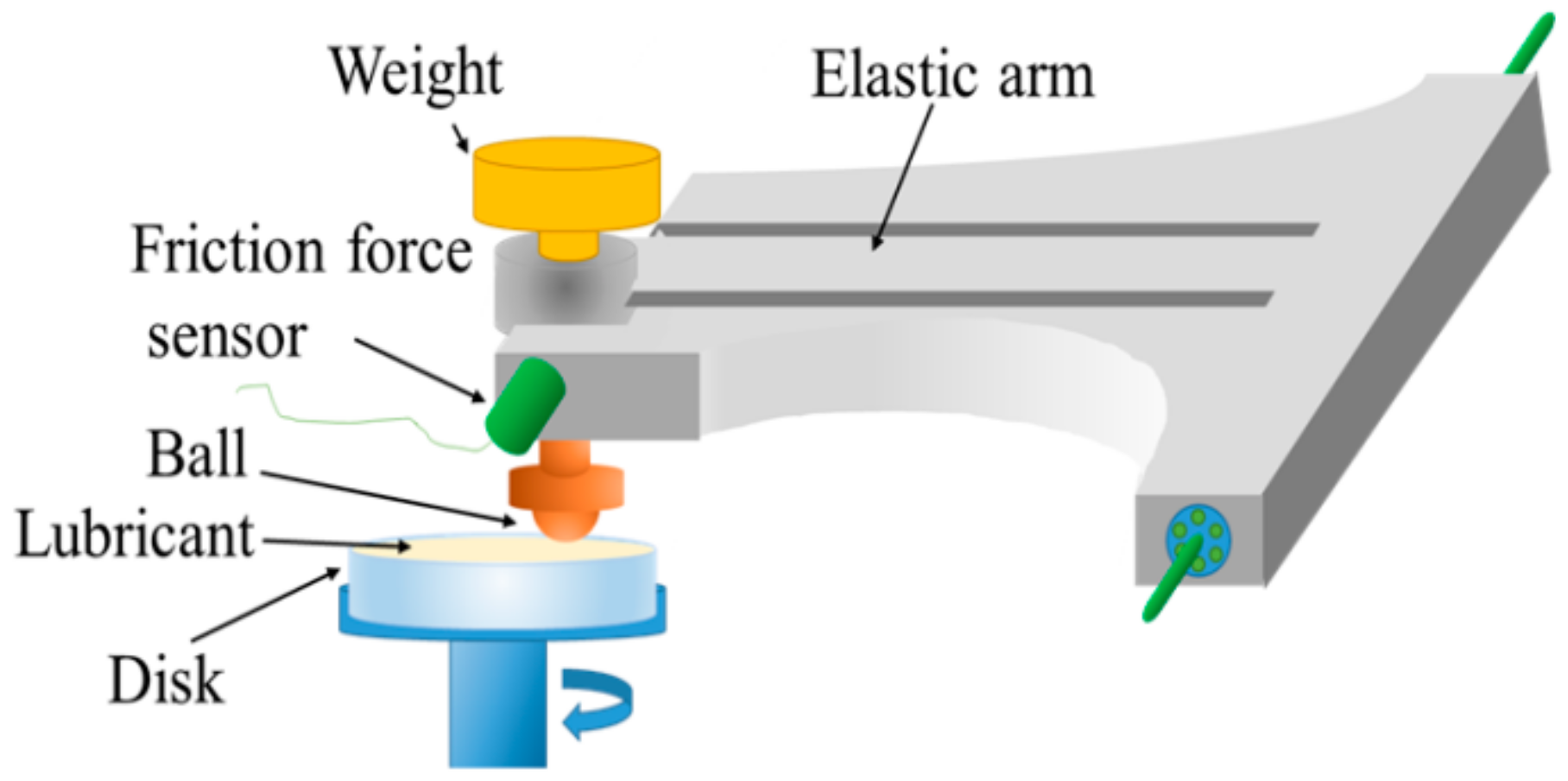
The friction coefficients of the ADEs were measured under continuous sliding conditions while using the ball-on-disk tribometer illustrated in
Figure 3.
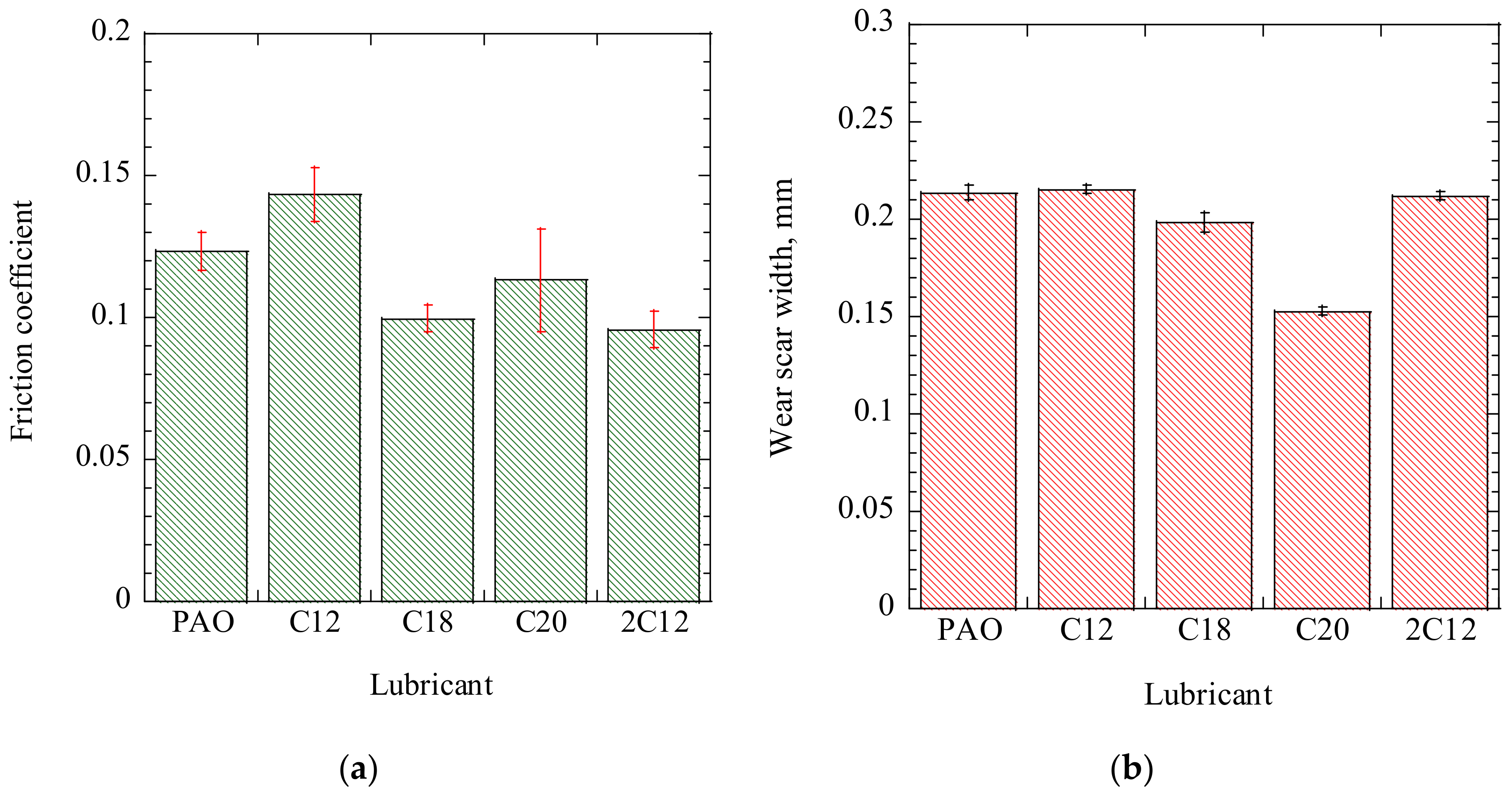
Figure 7a,b, respectively, show the friction coefficient of ADEs and wear scar width on the disks, which were tested at the same load and speed as those of the reciprocating sliding conditions. In this case, we also confirmed that the friction coefficient of the ADEs decreased with the number of attached alkyl chains. Although the averaged friction coefficient of C20 was higher than that of C18, both of them were lower than C12. Moreover, the wear scar width on the disk was 0.214 mm when lubricated with C12 in this case, but it decreased to 0.198 mm when C18 was used. The wear scar width further decreased to 0.155 mm when the longest alkyl chain, C20, was used. These results demonstrate that the increase in the alkyl chain length effectively improved the anti-wear properties. The wear decreased by 28% in the wear scar width when C20 was used. In contrast, the wear scar width was 0.210 mm when 2C12 was used, which was almost the same as that of C12. This indicated that the number of attached alkyl chains displayed less effect on the anti-wear properties than the alkyl chain length.
As shown in both
Figure 5 and
Figure 7, C12 showed almost the same friction coefficient and wear as the reference lubricant, PAO30; however, the friction coefficient of PAO30 decreased from 0.143 to 0.125 when the continuous sliding friction tests were carried out at a higher speed of 37.7 mm·s
−1, while the wear scar width on the disk increased from 0.212 to 0.268 mm, as shown in
Figure 8. For C12, the wear scar width decreased to 0.200 mm, although the friction coefficient was almost unchanged. In other words, C12 featured much lower wear on the disk when compared with PAO30. These results suggest that PAO and ADEs exhibited different friction mechanisms.
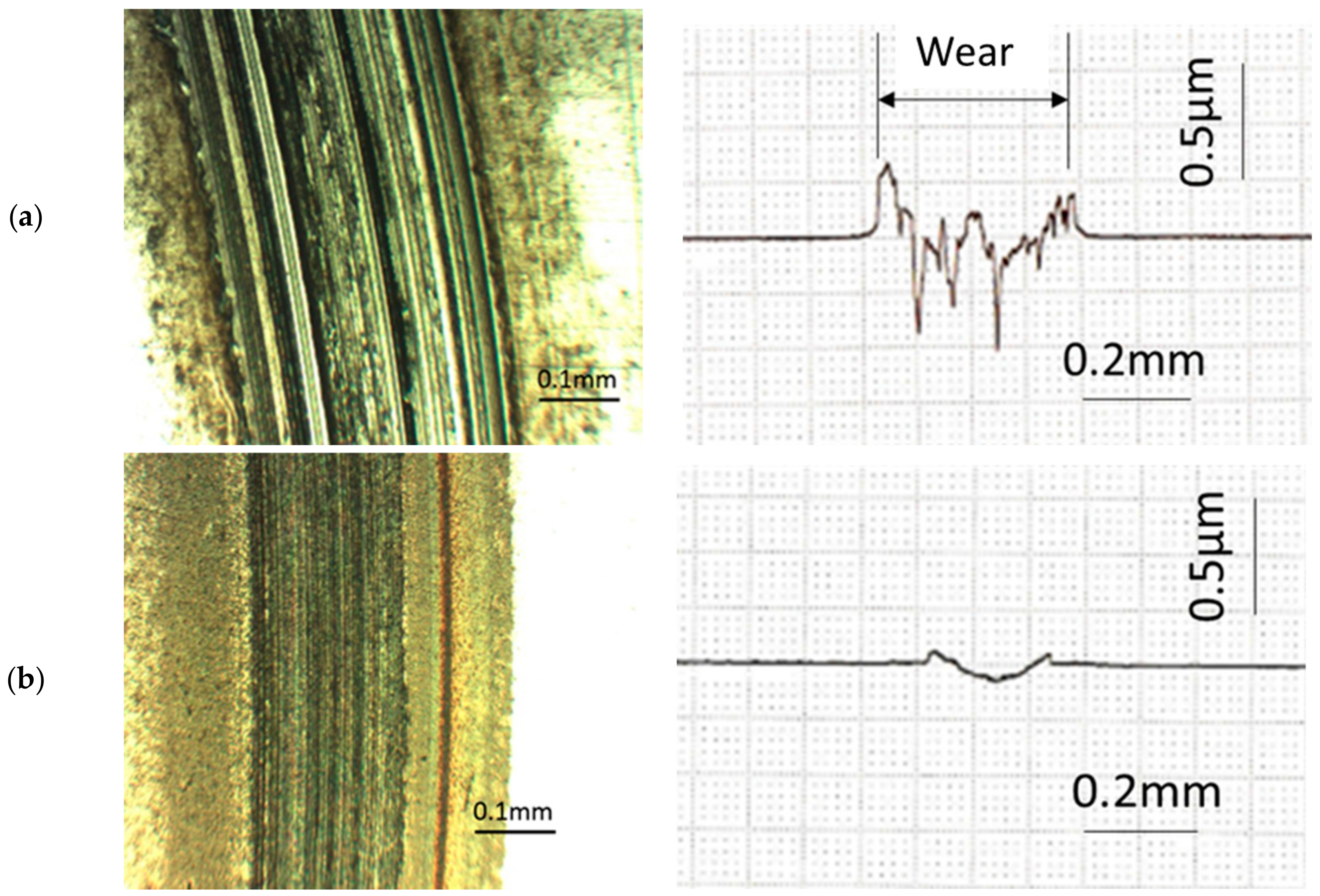
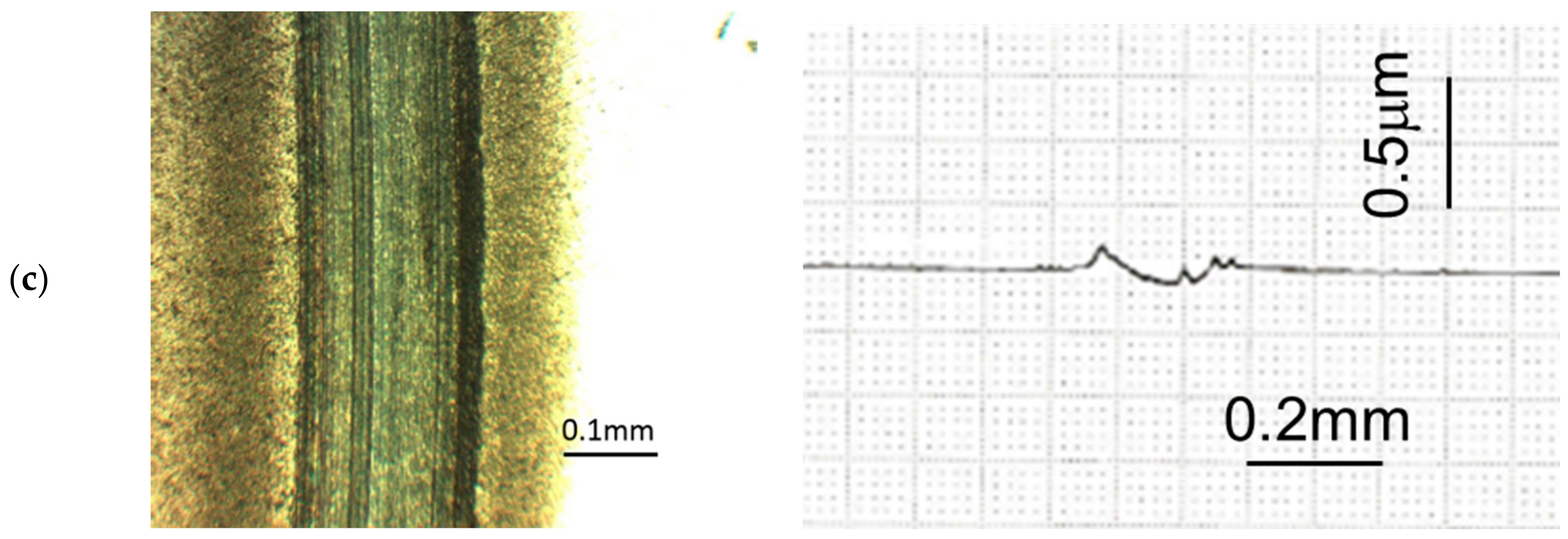
Figure 9 provides photographs and surface profiles of the wear scars lubricated with PAO30 and C12 to improve understanding of the mechanisms. A much rougher surface can be observed when PAO30 was used, with many grooves and deep furrows distributed in the friction track. In contrast, a relatively smooth surface was generated in the friction track when C12 was used. Moreover, the contact surface in the friction track became very smooth when only 1 wt % C12 was added into PAO30, as shown in
Figure 8. These data indicate that ADE effectively protected the surface from severe wear.
The question remained as to why ADEs showed these anti-wear properties. It was postulated that this might relate to their adsorption on the steel surface.
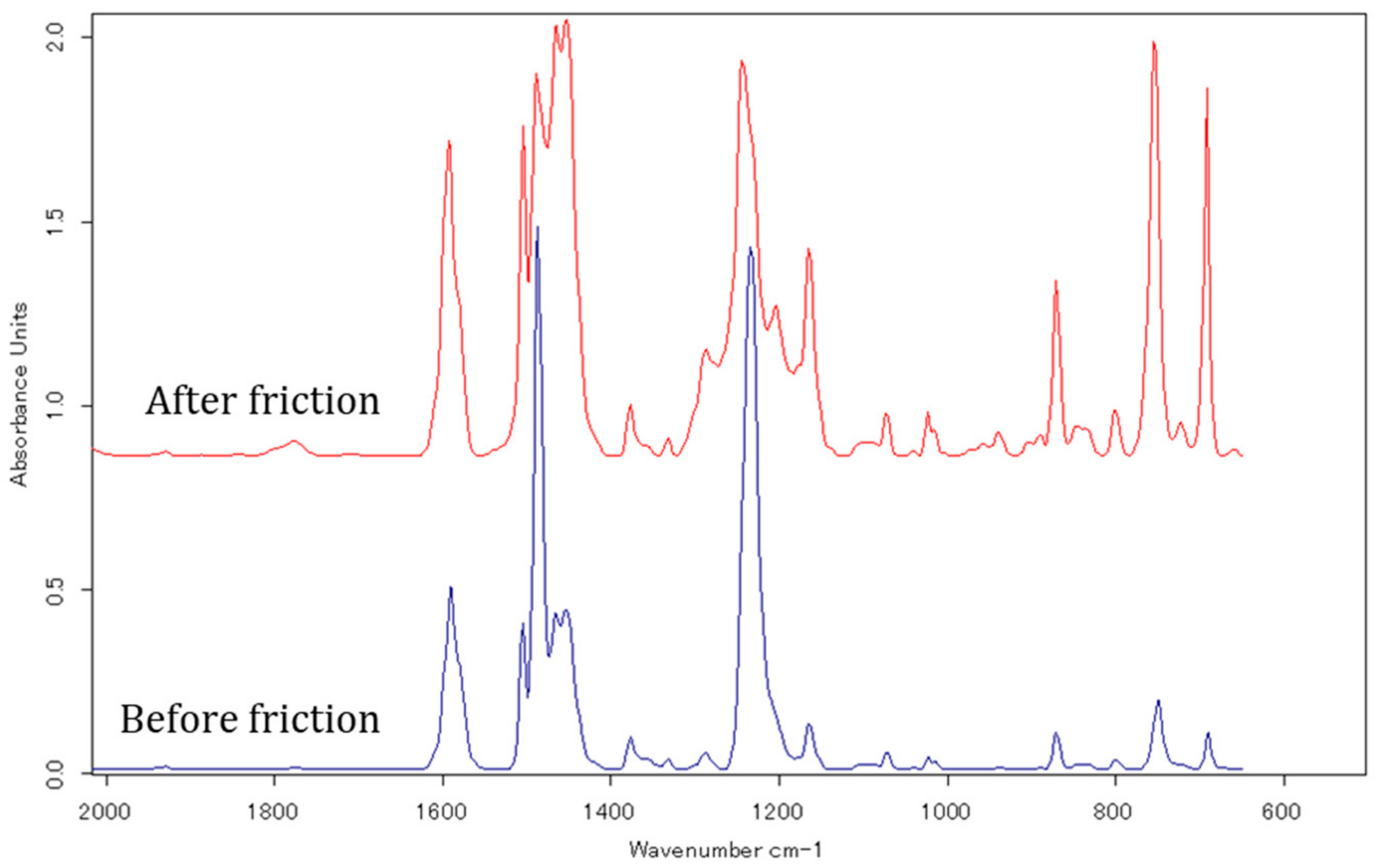
Figure 10 shows the FTIR spectra of C12. The peaks at 1350–1500 cm
−1 correspond to the deformation mode of the C–H vibration. The peak at 1594 cm
−1 is assigned to the aromatic C=C stretching vibration (ν), while that at 750 cm
−1 is attributed to the aromatic C–H out-of-plane deformation vibration (ω). As illustrated in
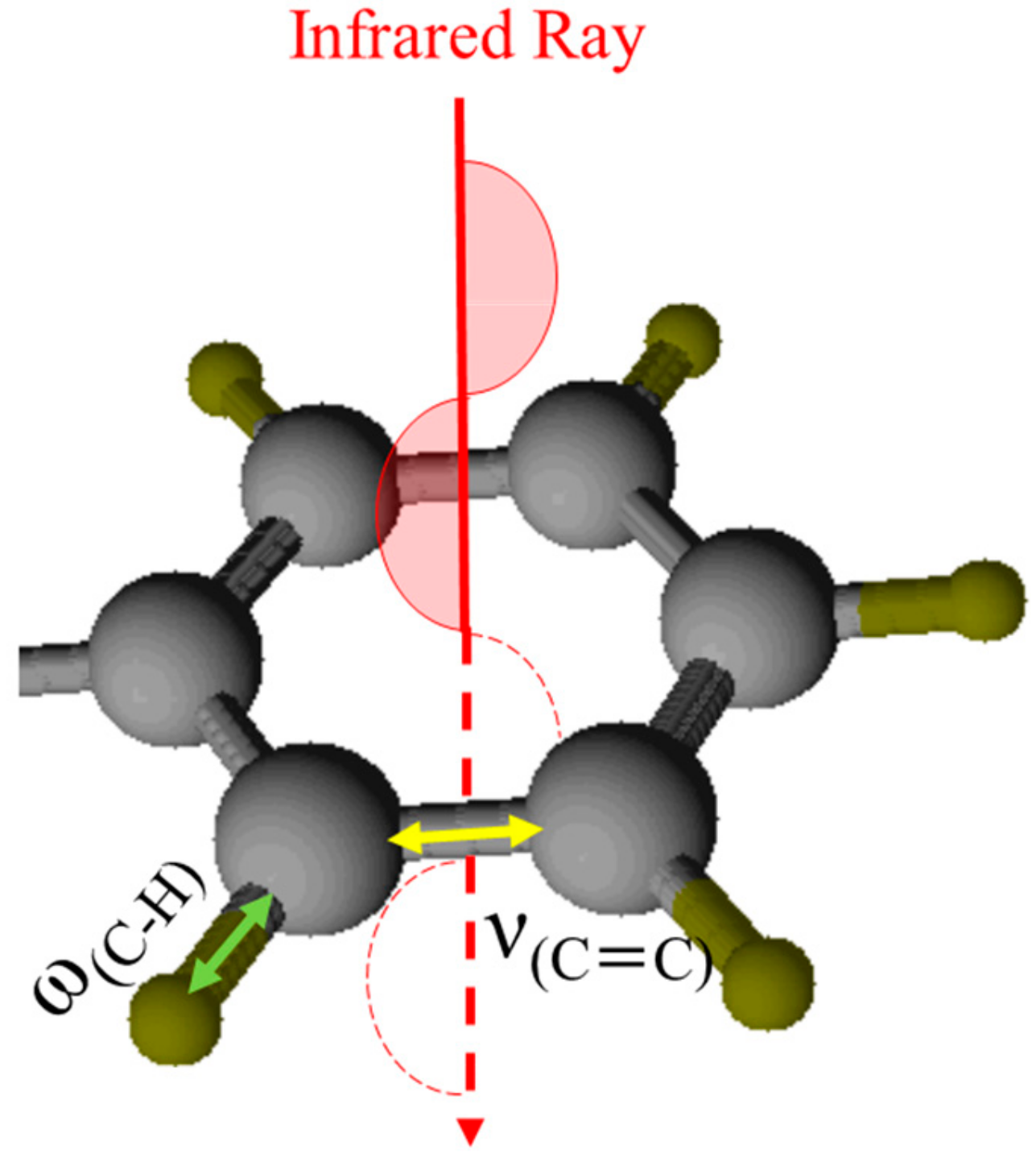
Figure 11, the observed IR absorption will be the largest for vibrations with in-plane dipole derivative vectors more parallel to the surface normal [
13,
14], while the lowest for vibration with out-of-plane dipole derivative vectors. The relative IR intensities of vibrations whose dipole derivatives are oriented along different molecular axes can be determine the orientation of the molecules. In this case, the observed IR absorption will be largest for aromatic C=C stretching vibrations and simultaneously smallest for the aromatic C–H out-of-plane deformation vibration when the aromatic ring is perpendicular to an infrared beam; therefore, the relative intensity of the aromatic C–C stretching vibration as compared with that of the aromatic C–H out-of-plane deformation vibration (
Iν
(C=C)/
Iω
(C–H)) can be used to represent the degree of orientation of the aromatic ring, i.e., the phenyl groups in ADEs. Based on the FTIR spectra, we found that
Iν(C=C)/Iω(C–H) was 4.0 before friction and decreased to 1.1 after friction. In other words, the phenyl group tended to adsorb parallel to the metal surface before friction, but perpendicularly to the worn metal surface. When the phenyl group adsorbed parallel to the metal surface, the attached alkyl chains simultaneously lay on surface due to the molecular conformation, leading to higher surface coverage and therefore protected the surface from wear. Once the surface was worn, the ether groups were attracted to the metal oxide surface, the phenyl groups became perpendicular, and the attached alkyl chains repelled other substances and prevented further wear of the surface. At the same time, weak bonds between the alkyl chains in adjacent molecules enhanced the durability of the lubricant film and reduced the friction, especially for longer alkyl chains.
3.3. Effect of Additives on Tribological Properties of Alkyldiphenylethers
We found that ADEs showed a lower friction coefficient and less wear, so we further sought means to promote their tribological properties. We hypothesized that the addition of traditional additives, such as ZnDTP, MoDTC, oleic acid, and stearic acid, would still show friction reduction or this anti-wear effect.
Here, we focused on C12. We measured the friction coefficients of the sample oil with these additives while using the ball-on-disk tribometer in a continuous sliding motion, as shown in
Figure 3. As shown in
Figure 12, additive-free C12 showed a high friction coefficient with an average value of 0.153. Fluctuations in the friction coefficient with friction time were also observed. When ZnDTP or MoDTC were used as the additive, the friction coefficient significantly decreased and then stabilized. The average friction coefficient of C12 containing ZnDTP/MoDTC was 0.112, which was 27% lower than that of additive-free C12. Oleic acid also decreased the friction coefficient by 27%. Moreover, the friction coefficient seemed to be more stable during the period of applied friction when oleic acid was used. For stearic acid, the friction coefficient decreased during the first 20 min., and then gradually increased to the value of additive-free lubricant.
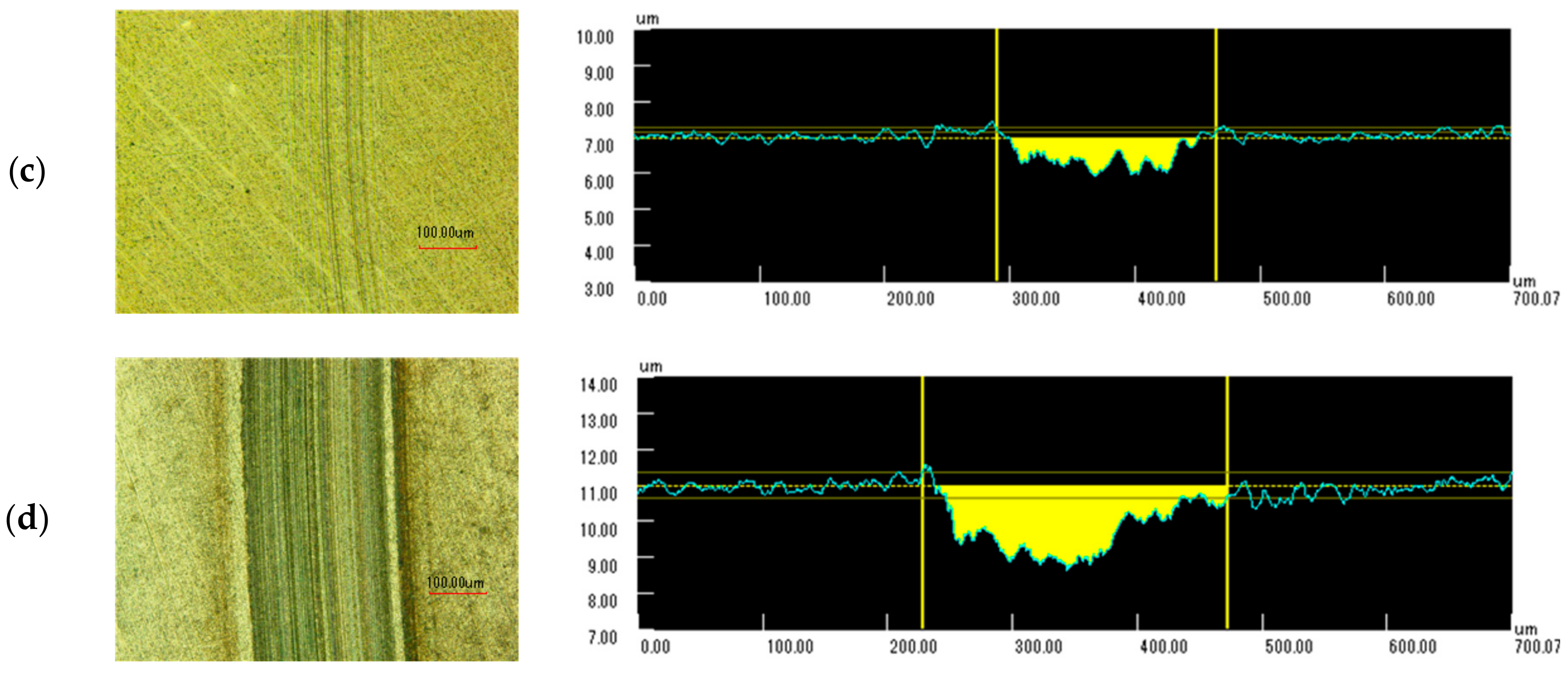
Figure 13 shows microphotographs of the wear scars and surface profiles measured while using a confocal laser scanning microscope (OLS5000, Olympus, Tokyo, Japan). When ZnDTP/MoDTC was added, the width and depth in the wear scar both obviously decreased when compared with the additive-free lubricant. Oleic acid provided the lowest wear in this case and a minor friction scratch was formed on the disk surface. Stearic acid presented less reduction in wear, which was possibly because of the increase in friction coefficient.
In a traditional lubricant, such as PAO, the addition of ZnDTP and MoDTC have proven effective in reducing the friction coefficient and wear due to tribofilm formation [
15]. In this case, we carried out XPS analysis after the friction tests when ZnDTP/MoDTC was used.
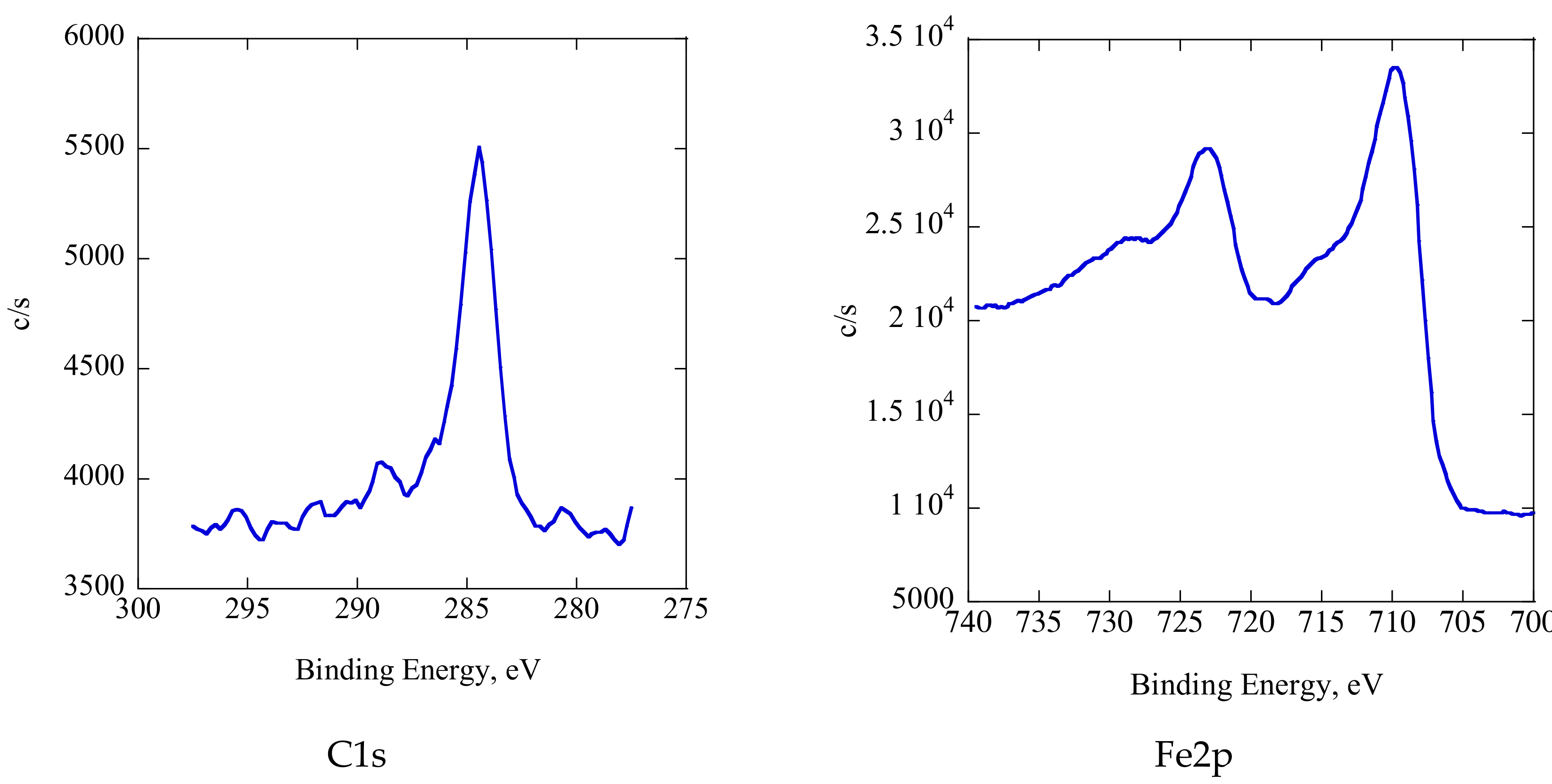
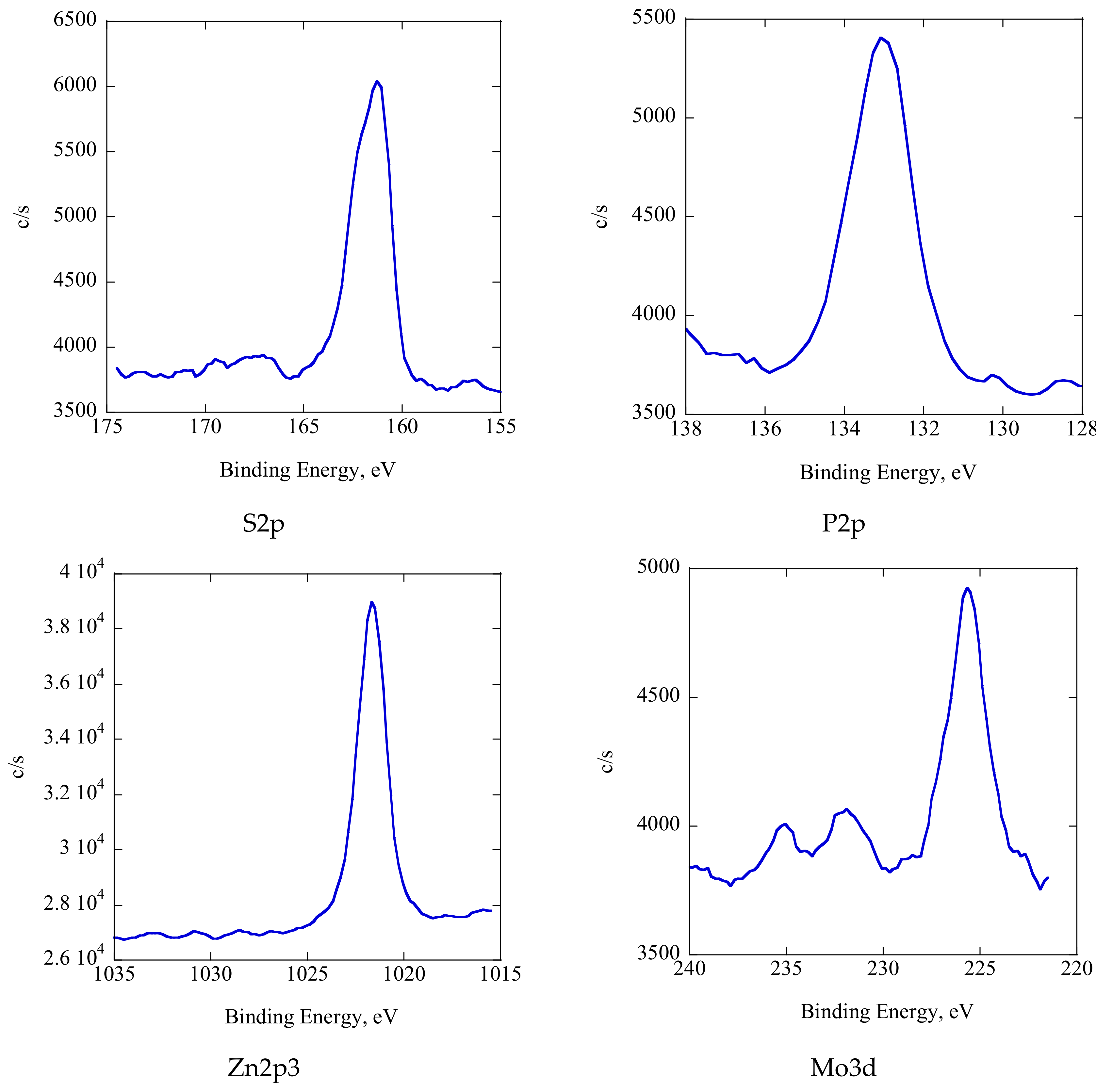
Figure 14 shows the XPS spectra of the tribofilm. The main S 2p peak at 161.28 eV indicated that the sulfur essentially appeared as a metal sulfide [
16]. The P 2p peak at 133.1 eV corresponded to the metal phosphate [
16]. The Zn 2p spectrum featured the main peak at 1021.7 eV, which suggested the formation of ZnS [
15,
16]. The Mo 3d spectrum shows a strong peak at 231.88 eV, indicating the presence of molybdenum oxide, while that at 229 eV suggests the formation of molybdenum disulfide, although the peak was very weak in this case [
15,
16]. We conclude that the tribofilm consisted of metal sulfide, metal phosphate, molybdenum oxide, molybdenum disulfide, and a carbon-rich phase. Therefore, the friction coefficient and wear decreased in comparison with additive-free C12 when ZnDTP/MoDTC was added. In other words, ZnDTP/MoDTC showed similarly good friction-reduction and anti-wear properties in ADEs as in traditional lubricants.
Oleic acid and stearic acid are generally used as friction modifiers to reduce the friction coefficient by the formation of a chemisorbed film. In this study, we confirmed that oleic acid effectively decreased the friction and wear of ADE; however, the friction coefficient increased after 20 min. when stearic acid was used. It was reported that stearic acid could reduce the friction coefficient of paraffinic oil to less than 0.1 at a concentration as low as 0.001 mol·L
−1 [
17]. In this case, the concentration of stearic acid in C12 was only 0.1 wt %, owing to its poor solubility in ADEs, which corresponds to 0.003 mol·L
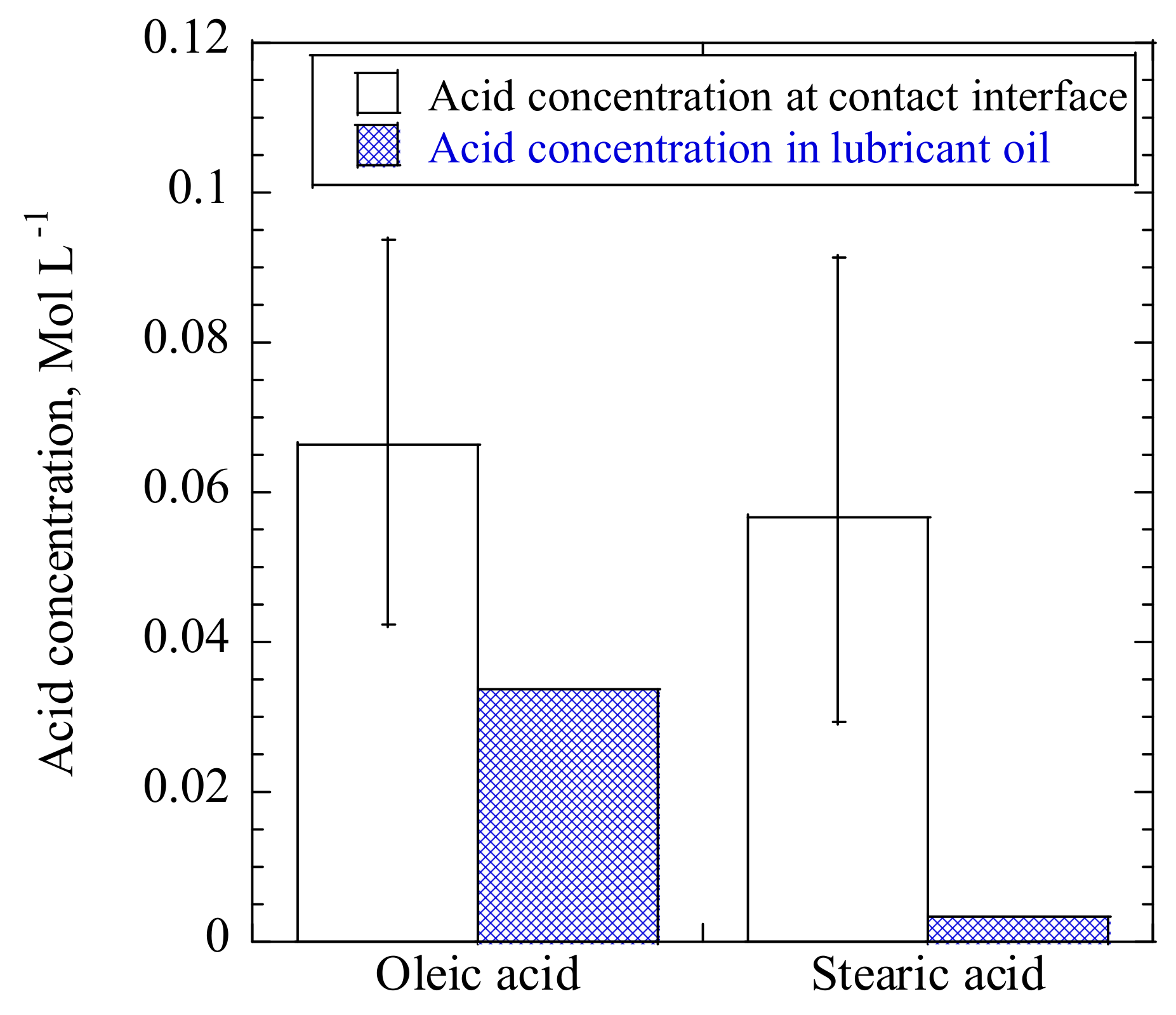
−1. Stearic acid reduced the friction coefficient of C12 for a short duration of applied friction. We analyzed the acid concentration at the contact interface while using an FTIR spectrometer in ATR mode. For both oleic and stearic acids, the acid concentration at the contact interface was found to be around 0.06 mol·L
−1, as shown in
Figure 15. The bulk concentration of oleic acid in the lubricants was 0.034 mol·L
−1, so enough oleic acid molecules were provided to adsorb on the contact surface, leading to low friction throughout the tests; in contrast, the concentration of stearic acid in the lubricants was significantly lower than that at the contact interface, so the supply was inadequate or not timeously available during friction. In other words, the surface coverage of stearic acid at the contact surface became insufficient, which resulted in the short duration of the period of low friction [
18].