Surface Energy and Tribology of Electrodeposited Ni and Ni–Graphene Coatings on Steel
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrodeposition
2.2. Surface Characterization
2.3. Friction and Wear Tests
2.4. Hardness Tests
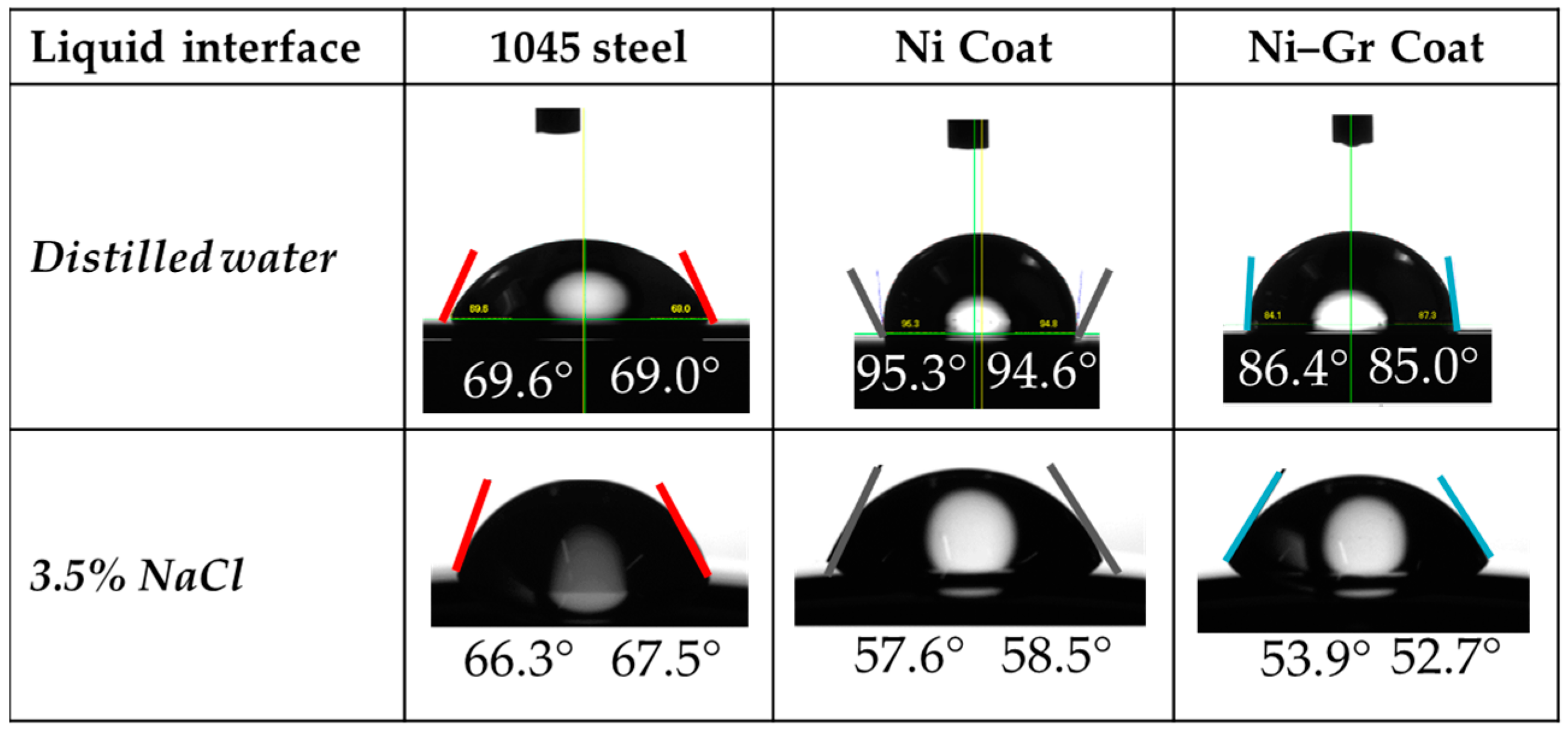
2.5. Contact Angle and Surface Energy Measurements
3. Results and Discussion
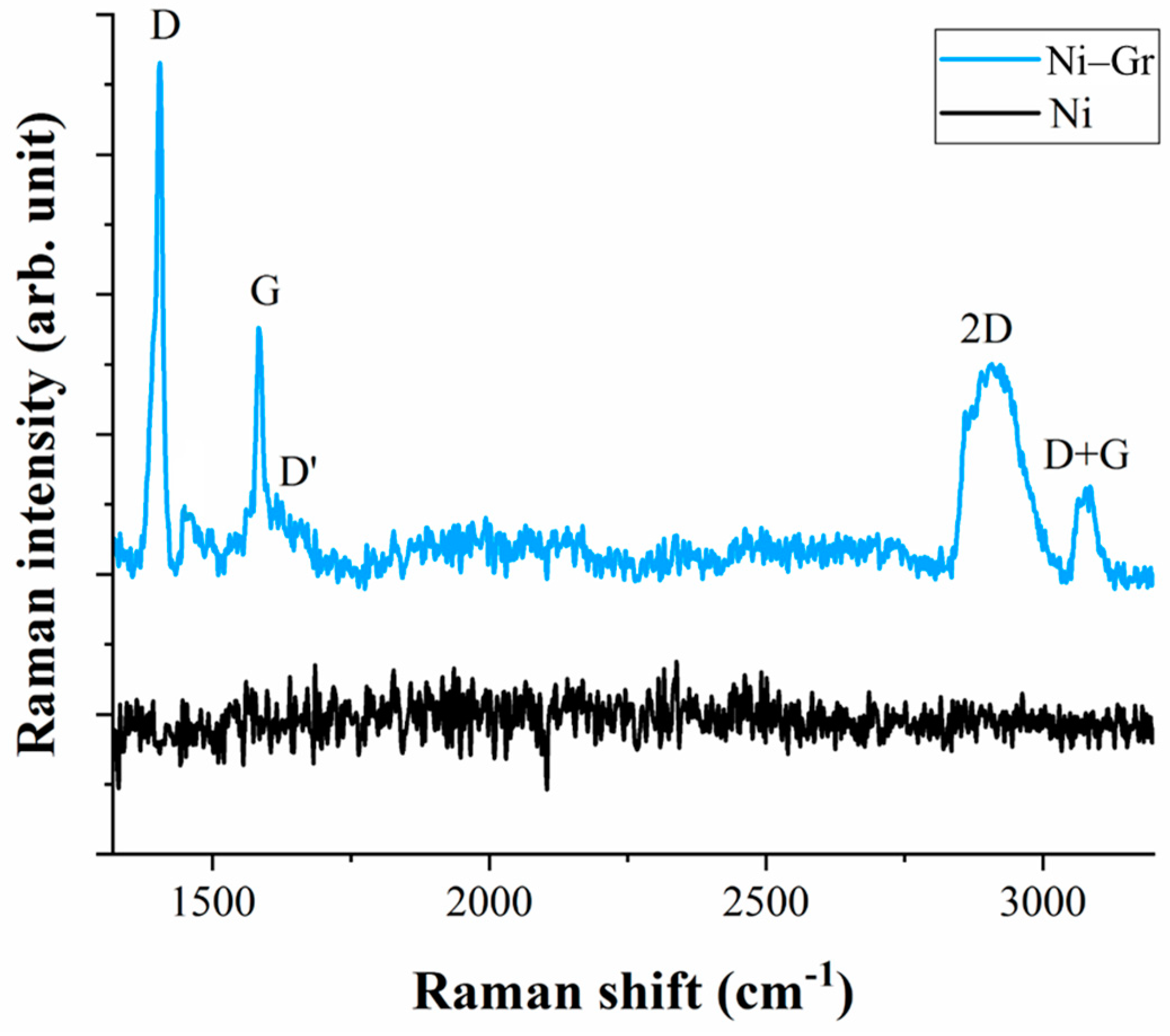
3.1. Raman Spectroscopy
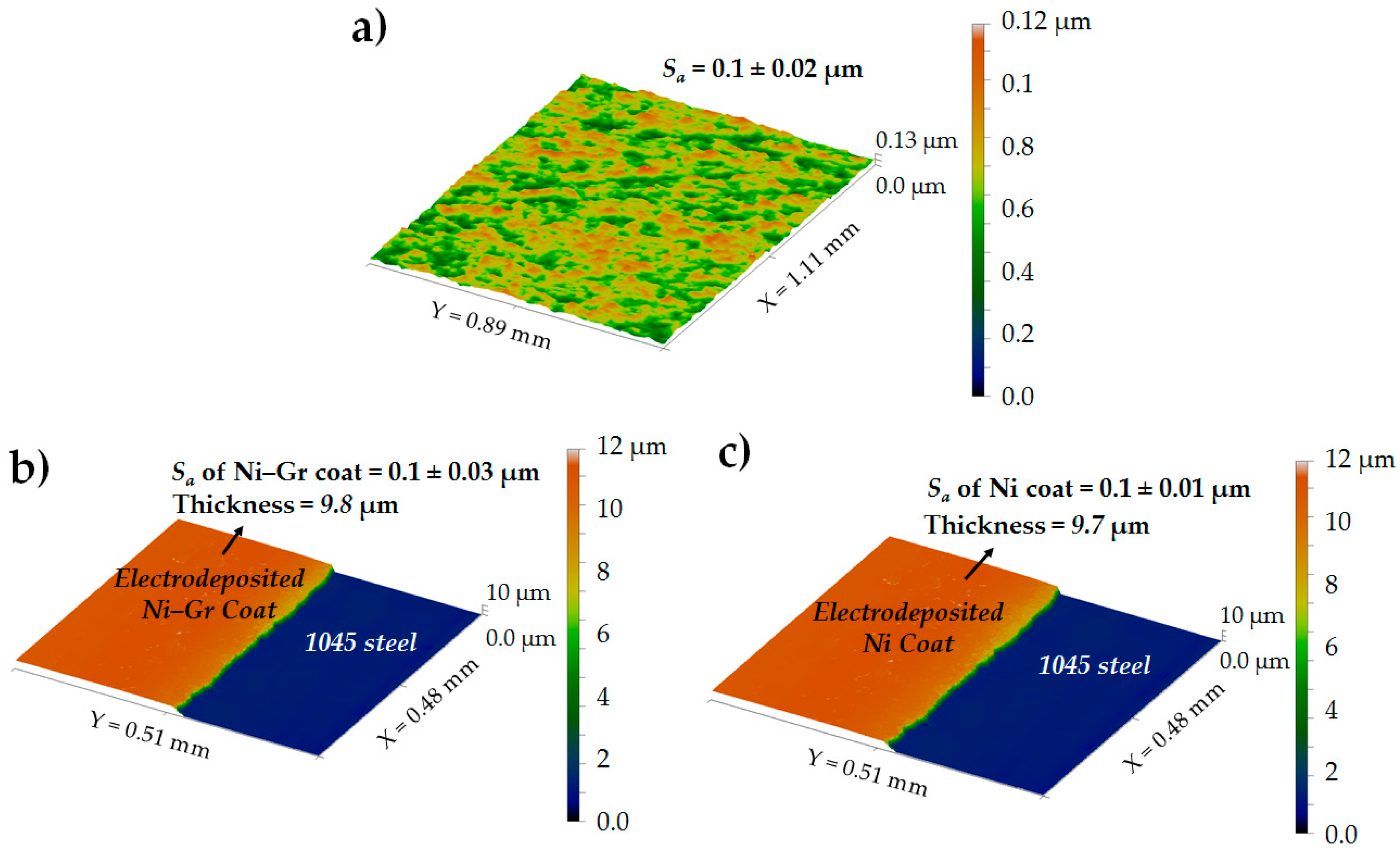
3.2. Surface Roughness
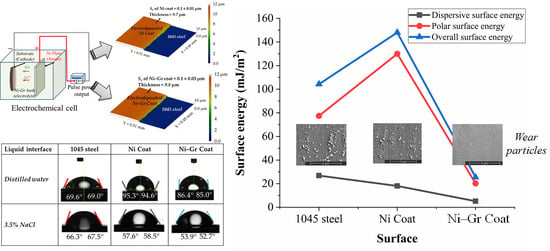
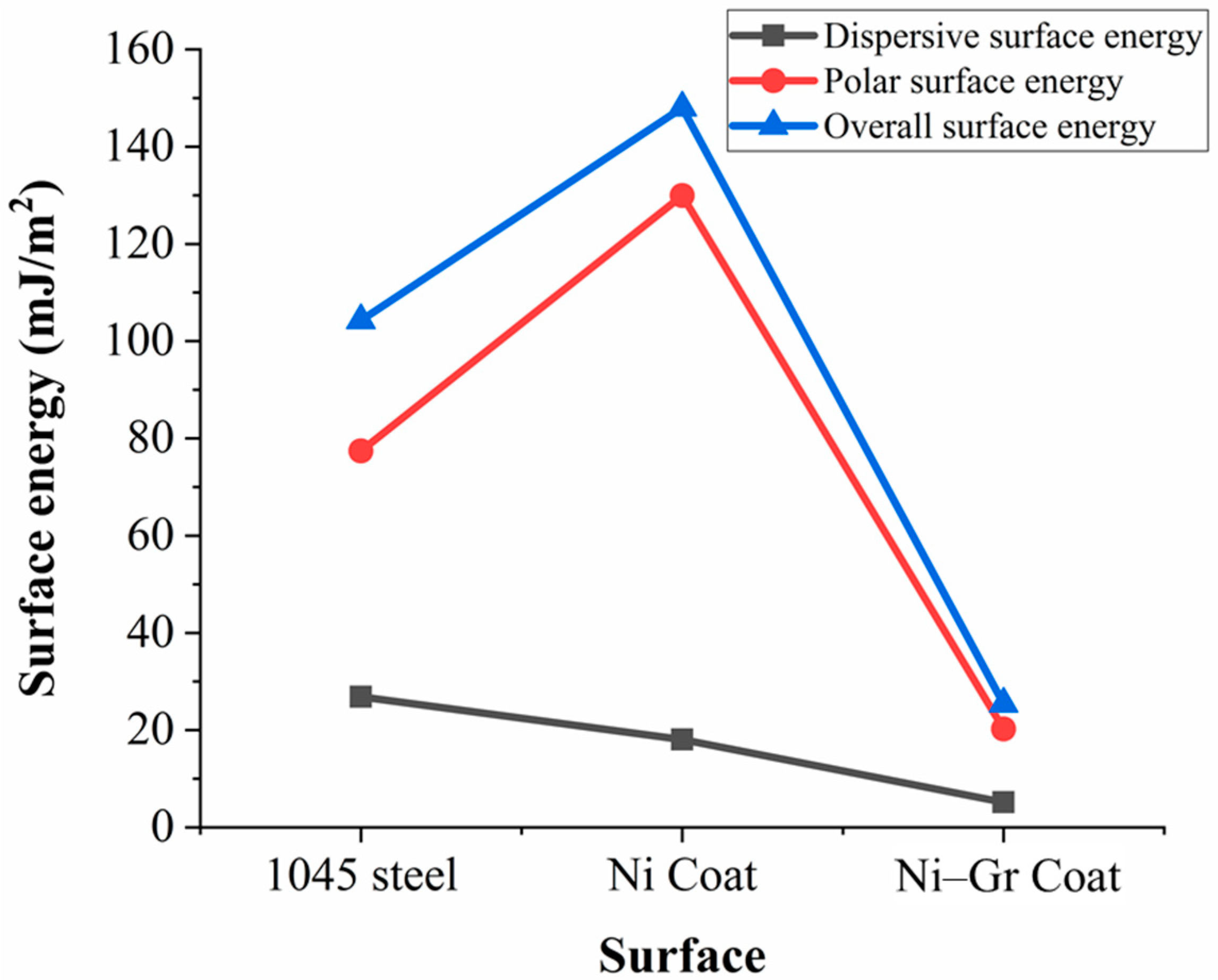
3.3. Surface Energy of the Coatings
3.4. Hardness
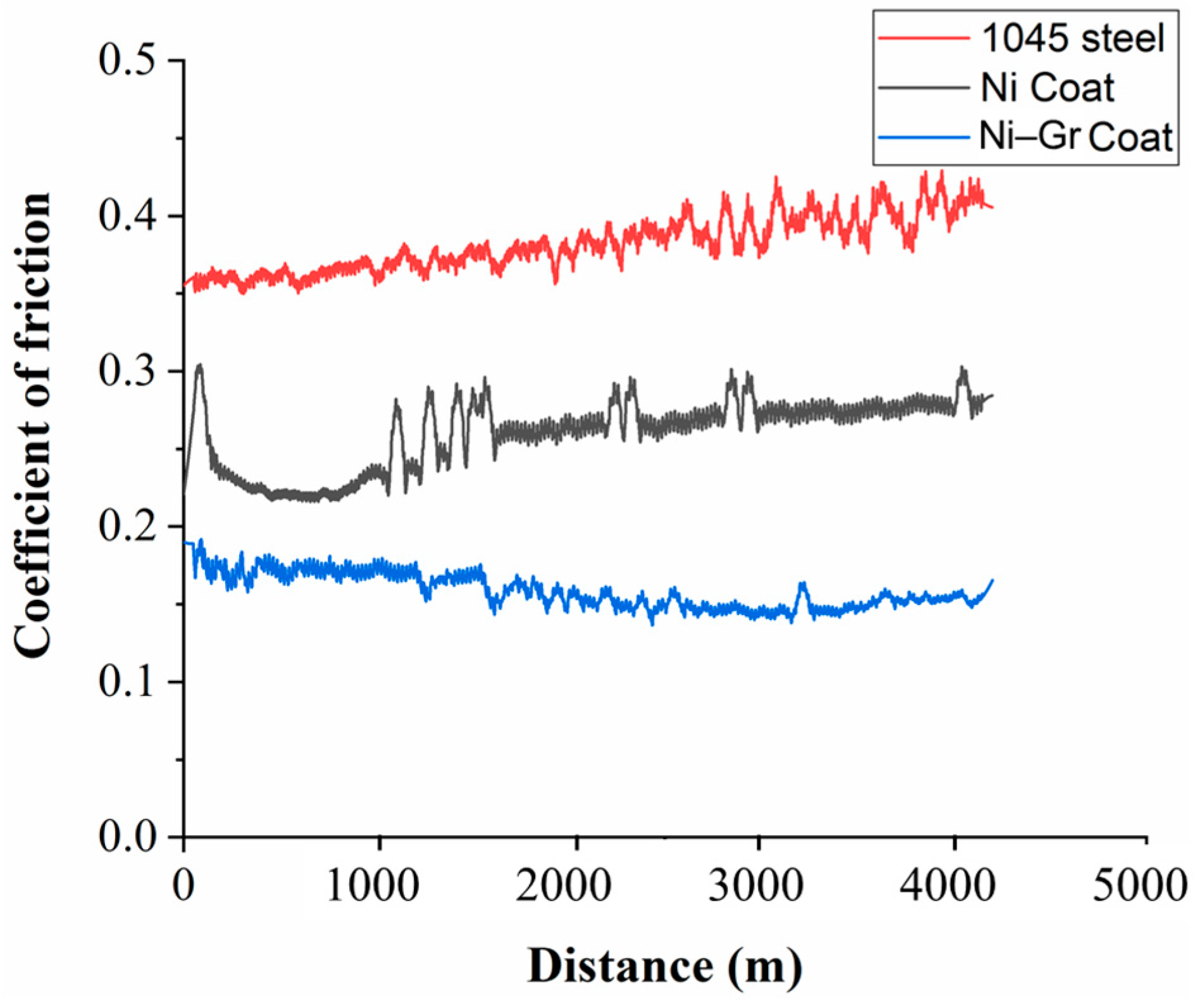
3.5. Friction and Wear
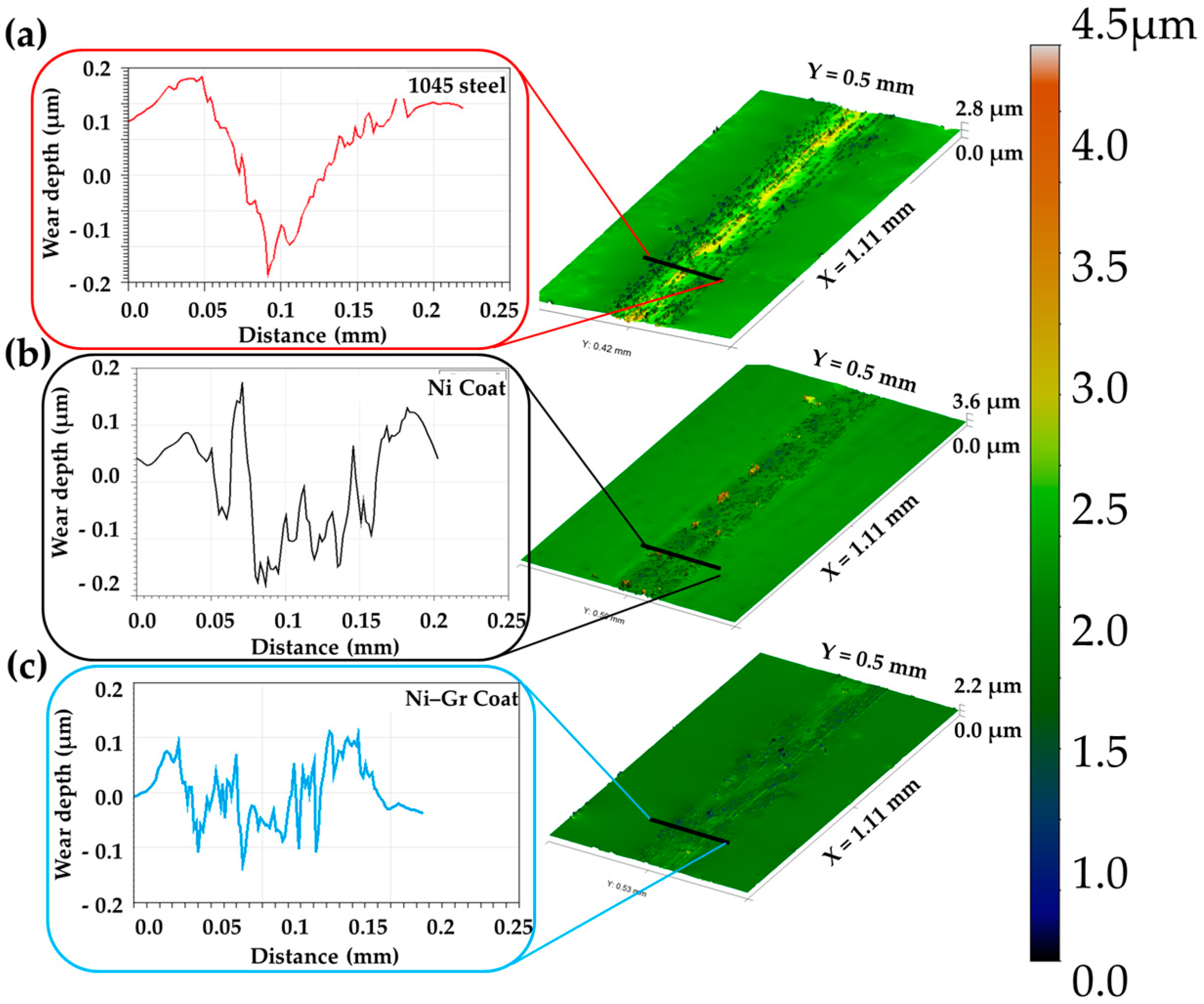
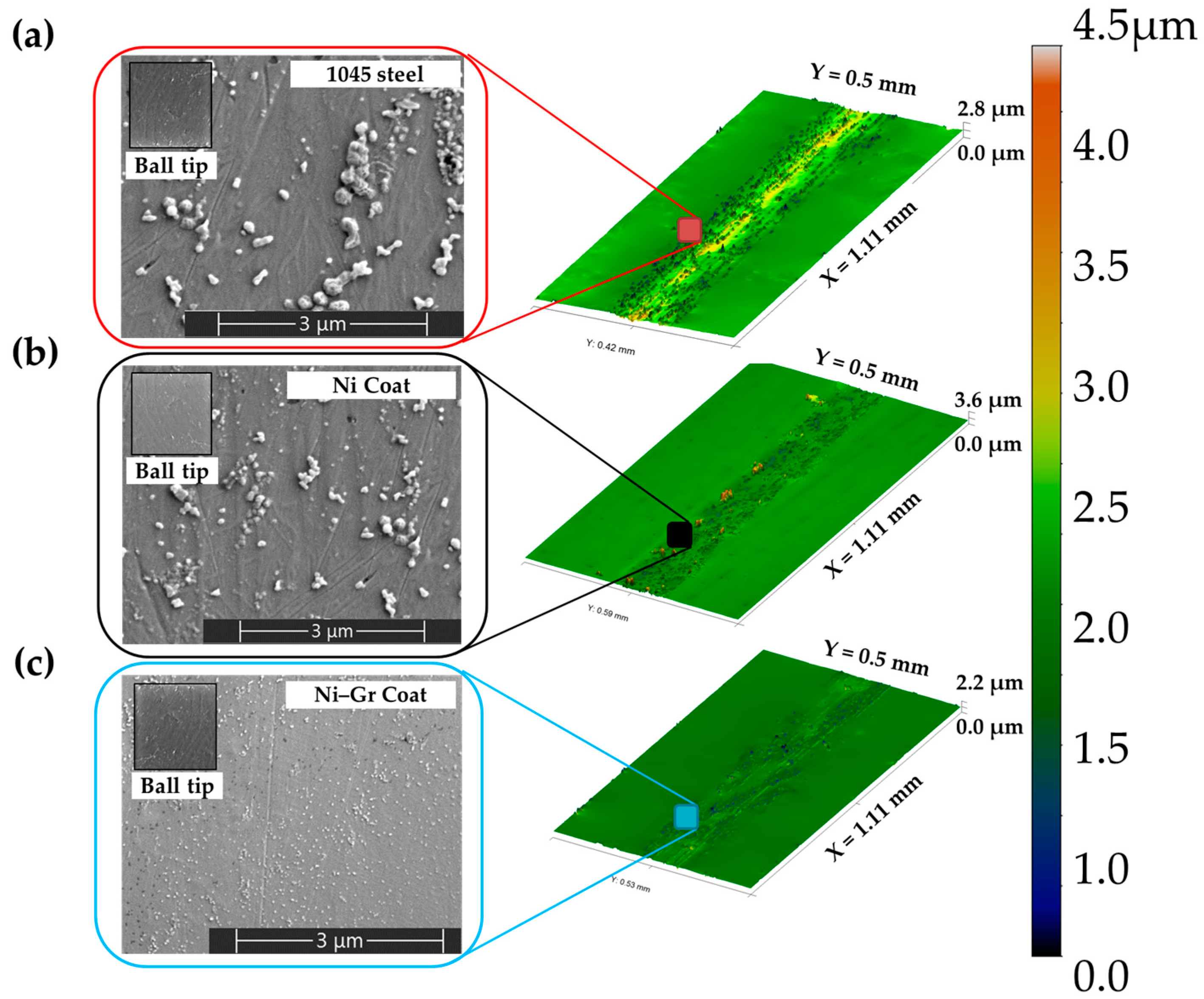
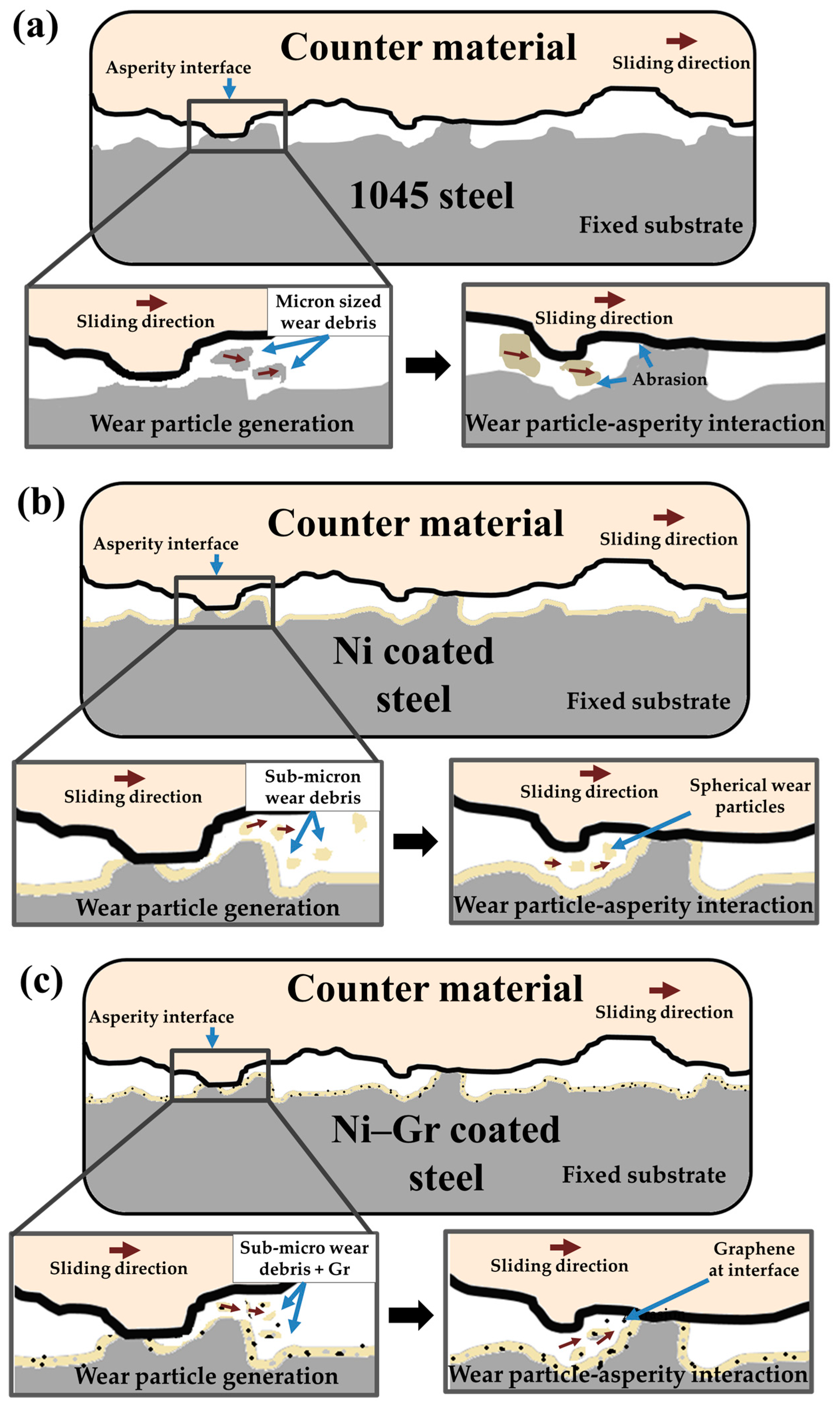
3.6. Wear Mechanisms
4. Conclusions
- The surface roughness and profile data indicated an excellent finish of electrodeposit coatings with a surface roughness that corresponded to the substrate surface.
- Ni–Gr coating had overall surface energy, which was about 82.84% less than the Ni coating, while 75.6% less than the steel substrate.
- The presence of graphene was found to decrease the polar component of surface energy significantly than the dispersive component.
- Fluids with high dispersion surface energy could wet the Ni–Gr coating, but the adhesion energy between the fluid and the surface could be low.
- The addition of Gr to Ni matrix was found to reduce the wear while providing low friction.
- The COF increased for 1045 steel (18% increase) and the Ni coated surface (12% increase), while it decreased by 11% for the Ni–Gr coating.
- Wear on Ni–Gr coated surface led to the exfoliation of graphene from the coating allowing the nanosized particles to enter the asperity contact and reduce the COF.
- The low surface energy of Ni–Gr coating was found to decrease the adhesive forces between the wear particles and surface.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Góral, A.; Lityńska-Dobrzyńska, L.; Kot, M. Effect of Surface Roughness and Structure Features on Tribological Properties of Electrodeposited Nanocrystalline Ni and Ni/Al2O3 Coatings. J. Mater. Eng. Perform. 2017, 26, 2118–2128. [Google Scholar] [CrossRef]
- Allahyarzadeh, M.H.; Aliofkhazraei, M.; Rezvanian, A.R.; Torabinejad, V.; Sabour Rouhaghdam, A.R. Ni-W electrodeposited coatings: Characterization, properties and applications. Surf. Coat. Technol. 2016, 307, 978–1010. [Google Scholar] [CrossRef]
- Ali, E. Solid Lubricants and Self-Lubricating Films. In Modern Tribology Handbook, Two Volume Set; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar] [CrossRef]
- Donnet, C.; Erdemir, A. Historical developments and new trends in tribological and solid lubricant coatings. Surf. Coat. Technol. 2004, 180–181, 76–84. [Google Scholar] [CrossRef]
- Sadeghinezhad, E.; Mehrali, M.; Saidur, R.; Mehrali, M.; Tahan Latibari, S.; Akhiani, A.R.; Metselaar, H.S.C. A comprehensive review on graphene nanofluids: Recent research, development and applications. Energy Convers. Manag. 2016, 111, 466–487. [Google Scholar] [CrossRef]
- Rasheed, A.K.; Khalid, M.; Rashmi, W.; Gupta, T.C.S.M.; Chan, A. Graphene based nanofluids and nanolubricants—Review of recent developments. Renew. Sustain. Energy Rev. 2016, 63, 346–362. [Google Scholar] [CrossRef]
- Saurín, N.; Sanes, J.; Bermúdez, M.-D. New Graphene/Ionic Liquid Nanolubricants. Mater. Today Proc. 2016, 3, S227–S232. [Google Scholar] [CrossRef]
- Gusain, R.; Mungse, H.P.; Kumar, N.; Ravindran, T.R.; Pandian, R.; Sugimura, H.; Khatri, O.P. Covalently attached graphene-ionic liquid hybrid nanomaterials: Synthesis, characterization and tribological application. J. Mater. Chem. A 2016, 4, 926–937. [Google Scholar] [CrossRef]
- Bandeira, P.; Monteiro, J.; Baptista, A.M.; Magalhães, F.D. Influence of oxidized graphene nanoplatelets and [DMIM][NTf2] ionic liquid on the tribological performance of an epoxy-PTFE coating. Tribol. Int. 2016, 97, 478–489. [Google Scholar] [CrossRef]
- Zhao, J.; He, Y.; Wang, Y.; Wang, W.; Yan, L.; Luo, J. An investigation on the tribological properties of multilayer graphene and MoS2 nanosheets as additives used in hydraulic applications. Tribol. Int. 2016, 97, 14–20. [Google Scholar] [CrossRef]
- Ramón-Raygoza, E.D.; Rivera-Solorio, C.I.; Giménez-Torres, E.; Maldonado-Cortés, D.; Cardenas-Alemán, E.; Cué-Sampedro, R. Development of nanolubricant based on impregnated multilayer graphene for automotive applications: Analysis of tribological properties. Powder Technol. 2016, 302, 363–371. [Google Scholar] [CrossRef]
- Blanco, D.; González, R.; Viesca, J.L.; Fernández-González, A.; Bartolomé, M.; Battez, A.H. Antifriction and antiwear properties of an ionic liquid with fluorine-containing anion used as lubricant additive. Tribol. Lett. 2017, 65, 66. [Google Scholar] [CrossRef]
- Dorri Moghadam, A.; Omrani, E.; Menezes, P.L.; Rohatgi, P.K. Mechanical and tribological properties of self-lubricating metal matrix nanocomposites reinforced by carbon nanotubes (CNTs) and graphene—A review. Compos. Part B Eng. 2015, 77, 402–420. [Google Scholar] [CrossRef]
- Tabandeh-Khorshid, M.; Omrani, E.; Menezes, P.L.; Rohatgi, P.K. Tribological performance of self-lubricating aluminum matrix nanocomposites: Role of graphene nanoplatelets. Eng. Sci. Technol. Int. J. 2016, 19, 463–469. [Google Scholar] [CrossRef]
- Kasar, A.K.; Xiong, G.; Menezes, P.L. Graphene-Reinforced Metal and Polymer Matrix Composites. JOM 2018, 70, 829–836. [Google Scholar] [CrossRef]
- Kasar, A.K.; Menezes, P.L. Synthesis and recent advances in tribological applications of graphene. Int. J. Adv. Manuf. Technol. 2018, 97, 3999–4019. [Google Scholar] [CrossRef]
- Omrani, E.; Menezes, L.P.; Rohatgi, K.P. Effect of Micro- and Nano-Sized Carbonous Solid Lubricants as Oil Additives in Nanofluid on Tribological Properties. Lubricants 2019, 7, 25. [Google Scholar] [CrossRef]
- Omrani, E.; Rohatgi, P.K.; Menezes, P.L. Tribology and Applications of Self-Lubricating Materials; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Kumar, C.M.P.; Venkatesha, T.V.; Shabadi, R. Preparation and corrosion behavior of Ni and Ni–graphene composite coatings. Mater. Res. Bull. 2013, 48, 1477–1483. [Google Scholar] [CrossRef]
- Algul, H.; Tokur, M.; Ozcan, S.; Uysal, M.; Cetinkaya, T.; Akbulut, H.; Alp, A. The effect of graphene content and sliding speed on the wear mechanism of nickel–graphene nanocomposites. Appl. Surf. Sci. 2015, 359, 340–348. [Google Scholar] [CrossRef]
- Berlia, R.; Punith Kumar, M.K.; Srivastava, C. Electrochemical behavior of Sn-graphene composite coating. RSC Adv. 2015, 5, 71413–71418. [Google Scholar] [CrossRef]
- Jiang, K.; Li, J.; Liu, J. Electrochemical codeposition of graphene platelets and nickel for improved corrosion resistant properties. RSC Adv. 2014, 4, 36245–36252. [Google Scholar] [CrossRef]
- Ren, Z.; Meng, N.; Shehzad, K.; Xu, Y.; Qu, S.; Yu, B.; Luo, J.K. Mechanical properties of nickel-graphene composites synthesized by electrochemical deposition. Nanotechnology 2015, 26, 065706. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; An, Z.; Wang, Z.; Toda, M.; Ono, T. Pulse-reverse electrodeposition and micromachining of graphene–nickel composite: An efficient strategy toward high-performance microsystem application. ACS Appl. Mater. Interfaces 2016, 8, 3969–3976. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Konishi, H.; Fehrenbacher, A.; Ma, C.; Xu, J.Q.; Choi, H.; Xu, H.F.; Pfefferkorn, F.E.; Li, X.C. Novel nanoprocessing route for bulk graphene nanoplatelets reinforced metal matrix nanocomposites. Scr. Mater. 2012, 67, 29–32. [Google Scholar] [CrossRef]
- Lee, S.-K.; Jang, H.Y.; Jang, S.; Choi, E.; Hong, B.H.; Lee, J.; Park, S.; Ahn, J.-H. All Graphene-Based Thin Film Transistors on Flexible Plastic Substrates. Nano Lett. 2012, 12, 3472–3476. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Brown, G.O.; Burris, D.L.; Korley, L.T.J.; Epps, T.H. Coating Architects: Manipulating Multiscale Structures To Optimize Interfacial Properties for Coating Applications. ACS Appl. Polym. Mater. 2019. [Google Scholar] [CrossRef]
- Jokari-Sheshdeh, M.; Mahboubi, F.; Dehghani, K. Structure and tribological behavior of diamond-like carbon coatings deposited on the martensitic stainless steel: The influence of gas composition and temperature. Diam. Relat. Mater. 2018, 81, 77–88. [Google Scholar] [CrossRef]
- Tysoe, W.T. 17 Surface Chemistry at the Tribological Interface. Surfactants Tribol. 2016, 2, 437. [Google Scholar]
- Emerson, J.A.; Garabedian, N.T.; Burris, D.L.; Furst, E.M.; Epps, T.H. Exploiting Feedstock Diversity To Tune the Chemical and Tribological Properties of Lignin-Inspired Polymer Coatings. ACS Sustain. Chem. Eng. 2018, 6, 6856–6866. [Google Scholar] [CrossRef]
- Tripathi, M.; Awaja, F.; Paolicelli, G.; Bartali, R.; Iacob, E.; Valeri, S.; Ryu, S.; Signetti, S.; Speranza, G.; Pugno, N.M. Tribological characteristics of few-layer graphene over Ni grain and interface boundaries. Nanoscale 2016, 8, 6646–6658. [Google Scholar] [CrossRef]
- Low, C.T.J.; Wills, R.G.A.; Walsh, F.C. Electrodeposition of composite coatings containing nanoparticles in a metal deposit. Surf. Coat. Technol. 2006, 201, 371–383. [Google Scholar] [CrossRef]
- Szeptycka, B.; Gajewska-Midzialek, A.; Babul, T. Electrodeposition and corrosion resistance of Ni–Graphene composite coatings. J. Mater. Eng. Perform. 2016, 25, 3134–3138. [Google Scholar] [CrossRef]
- Pagnanelli, F.; Altimari, P.; Bellagamba, M.; Granata, G.; Moscardini, E.; Schiavi, P.G.; Toro, L. Pulsed electrodeposition of cobalt nanoparticles on copper: Influence of the operating parameters on size distribution and morphology. Electrochim. Acta 2015, 155, 228–235. [Google Scholar] [CrossRef]
- Topolovec, S.; Krenn, H.; Würschum, R. Enhanced magnetic moment of ultrathin Co films measured by in situ electrodeposition in a SQUID. J. Magn. Magn. Mater. 2016, 397, 96–100. [Google Scholar] [CrossRef]
- Ibrahim, M.A.M.; Al Radadi, R.M. Noncrystalline cobalt coatings on copper substrates by electrodeposition from complexing acidic glycine baths. Mater. Chem. Phys. 2015, 151, 222–232. [Google Scholar] [CrossRef]
- Abou-Krisha, M.M. Influence of Ni2+ concentration and deposition potential on the characterization of thin electrodeposited Zn–Ni–Co coatings. Mater. Chem. Phys. 2011, 125, 621–627. [Google Scholar] [CrossRef]
- Jabbar, A.; Yasin, G.; Khan, W.Q.; Anwar, M.Y.; Korai, R.M.; Nizam, M.N.; Muhyodin, G. Electrochemical deposition of nickel graphene composite coatings: Effect of deposition temperature on its surface morphology and corrosion resistance. RSC Adv. 2017, 7, 31100–31109. [Google Scholar] [CrossRef]
- Yaghoubinezhad, Y.; Afshar, A. Design of Experiments for Pulse Reverse Electrodeposition of Graphene Oxide toward Hydrogen Evolution Reaction. ECS J. Solid State Sci. Technol. 2015, 4, M7–M17. [Google Scholar] [CrossRef]
- Tao, S.; Li, D.Y. Tribological, mechanical and electrochemical properties of nanocrystalline copper deposits produced by pulse electrodeposition. Nanotechnology 2006, 17, 65. [Google Scholar] [CrossRef]
- Abou-Krisha, M.M. Electrochemical studies of zinc–nickel codeposition in sulphate bath. Appl. Surf. Sci. 2005, 252, 1035–1048. [Google Scholar] [CrossRef]
- Nazir, M.H.; Khan, Z.A.; Saeed, A.; Siddaiah, A.; Menezes, P.L. Synergistic wear-corrosion analysis and modelling of nanocomposite coatings. Tribol. Int. 2018, 121, 30–44. [Google Scholar] [CrossRef]
- Rapp, B.E. Chapter 22—Measuring Surface Tension and Free Surface Energy. In Microfluidics: Modelling, Mechanics and Mathematics; Rapp, B.E., Ed.; Elsevier: Oxford, UK, 2017; pp. 453–465. [Google Scholar]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cançado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1290. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Chen, G.; Joshi, P.; Tadigadapa, S.; Eklund. Raman Scattering from High-Frequency Phonons in Supported n-Graphene Layer Films. Nano Lett. 2006, 6, 2667–2673. [Google Scholar] [CrossRef] [PubMed]
- Childres, I.; Jauregui, L.A.; Park, W.; Cao, H.; Chen, Y.P. Raman spectroscopy of graphene and related materials. New Dev. Photon Mater. Res. 2013, 19, 1–20. [Google Scholar]
- Yu, Q.; Jauregui, L.A.; Wu, W.; Colby, R.; Tian, J.; Su, Z.; Cao, H.; Liu, Z.; Pandey, D.; Wei, D. Control and characterization of individual grains and grain boundaries in graphene grown by chemical vapour deposition. Nat. Mater. 2011, 10, 443. [Google Scholar] [CrossRef] [PubMed]
- Kong, A.N.G.; Gong, B.-K.; Wang, G.; Cui, H.-W. Influence of surface roughness of substrate on the properties of Ni–Co–Fe electrodeposition coating on copper. Surf. Rev. Lett. 2017, 25, 1850120. [Google Scholar] [CrossRef]
- Nazir, M.H.; Khan, Z. Maximising the interfacial toughness of thin coatings and substrate through optimisation of defined parameters. Int. J. Comput. Methods Exp. Meas. 2015, 3, 316–328. [Google Scholar] [CrossRef]
- Fowkes, F.M. Attractive forces at interfaces. Ind. Eng. Chem. 1964, 56, 40–52. [Google Scholar] [CrossRef]
- Busscher, H.J.; Van Pelt, A.W.J.; De Boer, P.; De Jong, H.P.; Arends, J. The effect of surface roughening of polymers on measured contact angles of liquids. Colloids Surf. 1984, 9, 319–331. [Google Scholar] [CrossRef]
- Lin, J.; Wang, L.; Chen, G. Modification of Graphene Platelets and their Tribological Properties as a Lubricant Additive. Tribol. Lett. 2011, 41, 209–215. [Google Scholar] [CrossRef]
- Shin, Y.J.; Wang, Y.; Huang, H.; Kalon, G.; Wee, A.T.S.; Shen, Z.; Bhatia, C.S.; Yang, H. Surface-Energy Engineering of Graphene. Langmuir 2010, 26, 3798–3802. [Google Scholar] [CrossRef] [PubMed]
- French, R.H. Origins and applications of London dispersion forces and Hamaker constants in ceramics. J. Am. Ceram. Soc. 2000, 83, 2117–2146. [Google Scholar] [CrossRef]
- Puttock, M.J.; Thwaite, E.G. Elastic compression of spheres and cylinders at point and line contact. In Elastic Compression of Spheres and Cylinders at Point and Line Contact; Puttock, M.J., Thwaite, E.G., Eds.; Commonwealth Scientific and Industrial Research Organization: Melbourne, Australia, 1969; p. 64. [Google Scholar]
- Coronado, J.J. Abrasive Size Effect on Friction Coefficient of AISI 1045 Steel and 6061-T6 Aluminium Alloy in Two-Body Abrasive Wear. Tribol. Lett. 2015, 60, 40. [Google Scholar] [CrossRef]
- Hurricks, P.L. The mechanism of fretting—A review. Wear 1970, 15, 389–409. [Google Scholar] [CrossRef]
- Siddaiah, A.; Kasar, K.A.; Khosla, V.; Menezes, L.P. In-Situ Fretting Wear Analysis of Electrical Connectors for Real System Applications. J. Manuf. Mater. Process. 2019, 3, 47. [Google Scholar] [CrossRef]
- Reeves, C.J.; Menezes, P.L.; Lovell, M.R.; Jen, T.-C. The Size Effect of Boron Nitride Particles on the Tribological Performance of Biolubricants for Energy Conservation and Sustainability. Tribol. Lett. 2013, 51, 437–452. [Google Scholar] [CrossRef]
- Siddaiah, A.; Khan, A.Z.; Ramachandran, R.; Menezes, L.P. Performance Analysis of Retrofitted Tribo-Corrosion Test Rig for Monitoring In Situ Oil Conditions. Materials 2017, 10, 1145. [Google Scholar] [CrossRef]
- Menezes, P.L.; Ingole, S.P.; Nosonovsky, M.; Kailas, S.V.; Lovell, M.R. Tribology for scientists and engineers. In Tribology for Scientists and Engineers; Springer: Berlin, Germany, 2013; pp. 9–28. [Google Scholar]
| Properties | 1045 Medium Carbon Steel (ASTM A108) | Properties | 1045 Medium Carbon Steel (ASTM A108) | |
|---|---|---|---|---|
| Material Composition | Iron | 98.21%–98.85% | Yield Strength | 530 MPa |
| Carbon | 0.43%–0.50% | Hardness | 190HV0.5 | |
| Manganese | 0.60%–0.90% | Hardness rating | Medium | |
| Phosphorus | 0%–0.04% | Melting Point | 1427 °C | |
| Silicon | 0.15%–0.30% | Elongation | 19% | |
| Sulfur | 0%–0.05% | |||
| Plating bath Composition | Quantity (g/L) |
|---|---|
| NiSO4·7H2O | 26.26 |
| Na2SO4 | 56.81 |
| H3BO4 | 18.54 |
| Graphene (Gr) | 0.1 |
| Surface | Contact Angle (θ) | Surface Energy (mJ/m2) | Overall Surface Energy (mJ/m2) | Adhesive Energy (mJ/m2) | |||
|---|---|---|---|---|---|---|---|
| Distilled Water | 3.5% NaCl | Dispersive Component | Polar Component | Distilled Water | 3.5% NaCl | ||
| 1045 steel | 70.6 | 66.3 | 26.83 (26%) | 77.44 (74%) | 104.27 (100%) | 2.97 | 2.58 |
| Ni Coat | 95 | 58.5 | 18.06 (12%) | 129.96 (88%) | 148.02 (100%) | 13.52 | 12.75 |
| Ni–Gr Coat | 86 | 52.9 | 5.15 (20%) | 20.25 (80%) | 25.4 (100%) | 11.92 | 12.53 |
| Surface | H (GPa) | Std. Dev. |
|---|---|---|
| 1045 steel | 4.632 | 0.443 |
| Ni Coat | 6.606 | 0.949 |
| Ni–Gr Coat | 6.769 | 0.425 |
| SiN ball | 10.82 | 0.396 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddaiah, A.; Kumar, P.; Henderson, A.; Misra, M.; Menezes, P.L. Surface Energy and Tribology of Electrodeposited Ni and Ni–Graphene Coatings on Steel. Lubricants 2019, 7, 87. https://doi.org/10.3390/lubricants7100087
Siddaiah A, Kumar P, Henderson A, Misra M, Menezes PL. Surface Energy and Tribology of Electrodeposited Ni and Ni–Graphene Coatings on Steel. Lubricants. 2019; 7(10):87. https://doi.org/10.3390/lubricants7100087
Chicago/Turabian StyleSiddaiah, Arpith, Pankaj Kumar, Artie Henderson, Manoranjan Misra, and Pradeep L. Menezes. 2019. "Surface Energy and Tribology of Electrodeposited Ni and Ni–Graphene Coatings on Steel" Lubricants 7, no. 10: 87. https://doi.org/10.3390/lubricants7100087
APA StyleSiddaiah, A., Kumar, P., Henderson, A., Misra, M., & Menezes, P. L. (2019). Surface Energy and Tribology of Electrodeposited Ni and Ni–Graphene Coatings on Steel. Lubricants, 7(10), 87. https://doi.org/10.3390/lubricants7100087