3.1. Product Confirmation by 1H-NMR Spectroscopy
Epoxides and alkoxides were confirmed by
1H-NMR spectral analysis,
1H-NMR spectroscopy of the epoxides (ECO, ECB) and alkoxylated products are presented in
Figures S1 and S2 (Supplementary Materials). In
Figures S1a and S2a, the proton chemical shifts at δ 2.9 to δ 3.2 ppm and δ 2.9 to δ 3.1 ppm is the characteristic of epoxy protons indicated the formation of the epoxide from CO and CB respectively. Similarly, from the spectra (
Figure S1b,c; Figure S2b,c) it can be seen that the absence of epoxy protons at the aforementioned chemical shifts (δ 2.9 to δ 3.2 ppm and δ 2.9 to δ 3.1 ppm) for ECO, ECB indicated that during alkoxylation epoxy protons participated in the nucleophilic attack. Further, it can also be confirmed the nucleophilic attack on epoxy groups by the appearance of a singlet at δ 3.65 ppm (s, O
H of TBA) and multiple peaks at δ 0.88–0.9 ppm (m, C
H3 of TBA) (
Figures S1c and S2c) alkoxylation with TBA for ECO and ECB. Correspondingly, due to alkoxylation of ECO and ECB with 2-propanol, new singlet appears at δ 3.58 ppm (s, O
H of 2-propanol), multiple peaks at δ 1.24–1.4 ppm (m, C
H3 of 2-propanol) (
Figures S1b and S2b). Other characteristic shifts of the alkoxylated derivatives are as follows for oil derived alkoxides; δ 4.12–4.19 ppm (m, –C
H protons of glycerol), δ 4.29–4.34 ppm (q, –C
H2 protons of glycerol), δ 1.45–1.67 ppm (m, α-C
H2 protons of fatty acids), δ 0.88–0.98 ppm (m, terminal –C
H3 protons of fatty acids). Further, for the methyl ester derivatives absence of the glycerol protons was noticed, all the characteristic peaks agreed well with the earlier studies [
15,
16]. Further, the amount of protons at each chemical shift is addressed as a
Supplementary Materials in Table S1.
3.2. Pour Point
Finding the low-temperature properties such as pour point (PP) and cloud point (CP) enables a suitable application of the prepared alkoxides. At low temperature, plant seed oils have tendency to form macro crystalline structures through uniform stacking of the triglyceride backbone and they restrict the free flow due to the loss of kinetic energy of the individual molecules during self-stacking [
17]. Therefore, branching on the epoxidized fatty acid chains via ring opening reaction can disturb this stacking process, thereby low temperature properties can be enhanced. Thus, the same approach has been adopted in this study, the ester branching is attached at the mid- and end- sites on the fatty acid chains. The CP and PP of the prepared ECO and ECB alkoxides derivatives are reported in
Table 1, PPs of the alkoxides are found to be in the range of 2 to −21 °C. Higher PP alkoxides are desirable for higher temperature and radiation resistance applications [
18,
19]. PP of the BCBB is found to be favorable (−21 °C) and also meet the standard lubricant requirements of ISO VG32, VG 46, VG 68, VG 100 (−6 °C), SAE20W40 (−21 °C) and AG100 (−18 °C) specifications [
20]. PP of the PCBB satisfies (−7 °C) the ISO VG32, VG 46, VG 68, VG 100 (−6 °C) lubricant requirement. The relative excellent PP of the alkoxides derivatives is attributed to hydrogen bonding of the hydroxyl group present in the alkoxylation products [
21]. From this study, it was also noticed that alkoxylation with secondary alcohol (2-propanol, −21 °C) is more favorable to lower PP than tertiary alcohol (TBA, −7 °C). It was also observed that low-temperature properties of ECB derivatives were enhanced more than those of ECO derivatives. Due to structural modification (branching), PPs of the alkoxylated derivatives are enhanced (lower PP’s are noticed). During this study it was assumed that the presence of a branching group at the center of fatty acid chain creates a stearic barrier around the individual molecules there by crystallization can be inhibited, which results the lower pour and cloud points. Similar observations were also reported by other studies, lower PP alkoxides are applied for machine tool and hydraulic systems [
22].
3.3. Kinematic Viscosity and Viscosity Index
Kinematic viscosity (KV) and viscosity index (VI) of prepared alkoxides indicates the effect of temperature on the viscosity of alkoxides. A low VI intends a prominent change of viscosity with temperature. In other words, the alkoxide becomes very thin at high temperatures and very thick at low temperatures. On the other hand, high VI signifies relatively minor change in viscosity over a wide temperature range. Ideal alkoxide should maintain the constant viscosity during temperature changes. Kinematic viscosity (KV) of the formulated alkoxides derivatives are reported in
Table 1, ECO derived alkoxides have a higher viscosity as compared to ECB due to the presence of triacylglycerol in ECO derivatives. The KV of the formulated derivatives is found to be in the range of 18 to 1822 cSt at 40 °C and 4.2 to 102 cSt at 100 °C. Viscosities of the ECB derivatives are found to be lower than its derivatives due to the difference in molecular size. Viscosity attenuation was observed with an increase in chain length of the alkoxylated products due to branching, which tends to introduce spericity in the molecule, causing a decreasing trend in the viscosity [
23]. Likewise, viscosity index (VI) of the formulated derivatives are found to be in the range of 115 to 150 and these VI values are on par with ISO VG 32, 46; paraffin VG 95, 460; SAE20W40 and AG 100 grade lubricants in the market [
20]. ECB derived alkoxides exhibited higher VI values, which signifies that there is a minor change in viscosity over a wide temperature range. Further, mono-hydroxylated chemical structures was obtained for the end products in this study, which can be applied for various liquid lubricant applications depending on their physico-chemical properties. On the other hand, polyolesters or chemically more hydroxylated structured derivatives from vegetable oils were more useful in various polymer applications [
22,
23]. Depending on the PP and VI values of the formulated products, ECO/ECB derivatives match with the SAE20W40 and AG 100 lubricants. Therefore, the prepared ECO/ECB alkoxy derivatives can be a potential replacement for SAE20W40 and AG 100 lubricants in the market [
20].
3.4. Rheological Behavior
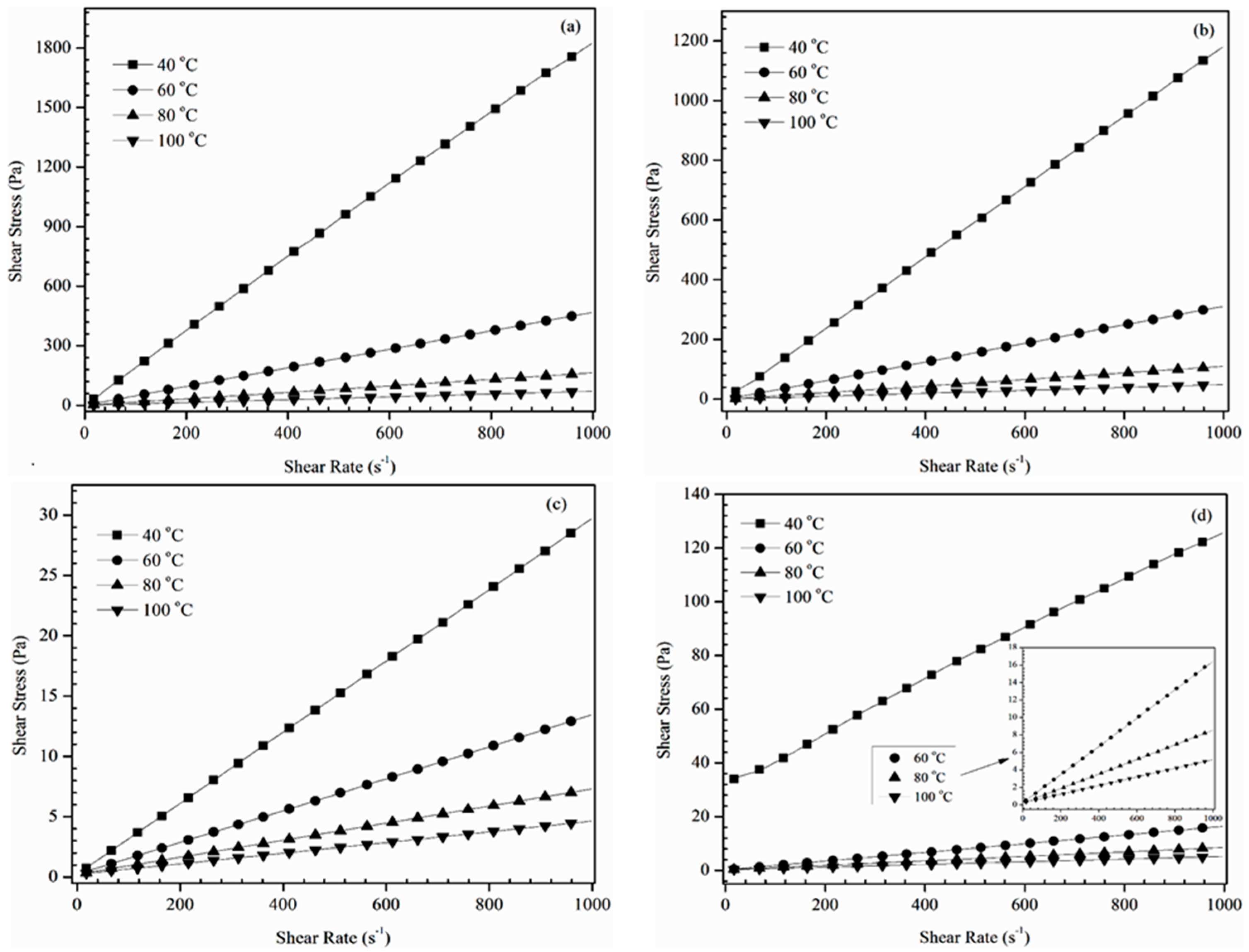
Shear stress versus shear rate flow curves of the prepared ECO/ECB alkoxy derivatives at 40, 60, 80 and 100 °C are shown in
Figure 1. These results revealed a linear relationship between shear stress and shear rate, in spite of the change in temperature; which signifies that all formulated alkoxy derivatives showed a typical Newtonian fluid behavior. It was also noticed that the average shear stress value decrease by increasing temperature and shear rate. Likewise,
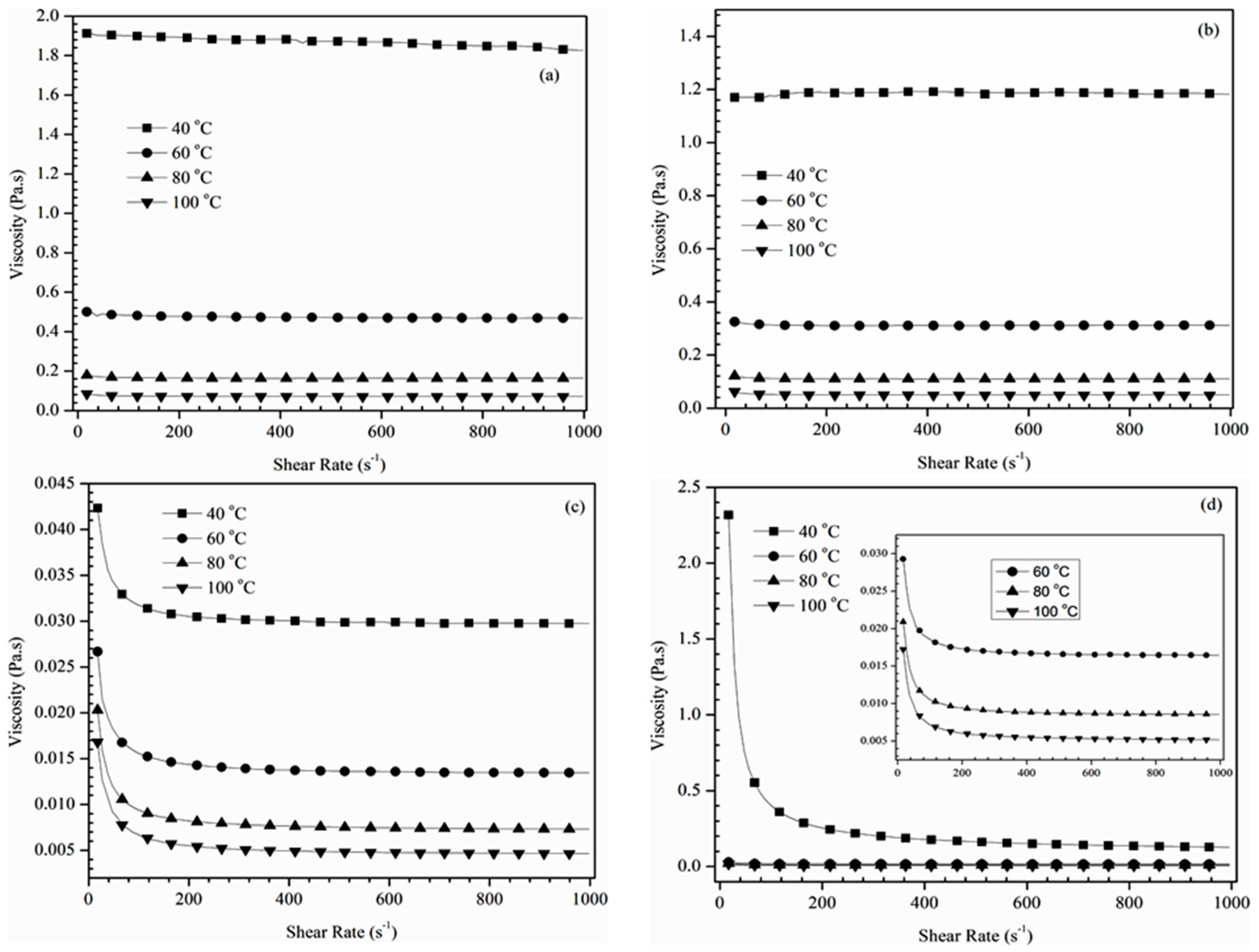
Figure 2 shows shear rate versus viscosity measurements for all the alkoxy derivatives. In spite of temperature variations, alkoxylation of ECB with 2-propanol and TBA showed (
Figure 2c,d) constant viscosity with shear rate, which also supports the Newtonian behavior. Among all the four formulated alkoxy derivatives, alkoxylation of ECB with 2-propanol and TBA showed (
Figure 2c,d) a shear thinning behavior at low shear rates (up to 100 s
−1) at all the tested temperatures. However, beyond the shear rate of 100 s
−1, it showed Newtonian behavior. This could be attributed to the alignment of anisotropic long carbon chains under shear which are present in the ECO and ECB [
24].
Similarly,
Figure 2 illustrates the change in viscosity as a function of temperature varying at 40, 60, 80 and 100 °C for the prepared alkoxy derivatives. Analyzing the influence of temperature on the viscosity of the alkoxy derivatives revealed an exponentially decreasing trend (
Figure 2;
Table 2), which is due to higher thermal moment among molecules. Similar results were reported by Borugadda and Goud during their study and an agreement was found with the other studies [
24,
25]. Therefore, based on the aforementioned rheological characteristics of the formulated derivatives, it could be predicted that the prepared alkoxides provide smooth performance during their usage without any operational difficulties.
3.5. Wear Scar Diameter (WSD)
The crucial property of the lubricants is to hold the stationary lubricating film at the metal contact zone; thereby static lubricant film can prevent the wear of metal surface. From the previous reports, it was noticed that renewable oils and their fatty acid esters are well-known to supply excellent lubricity due to their ester functionality [
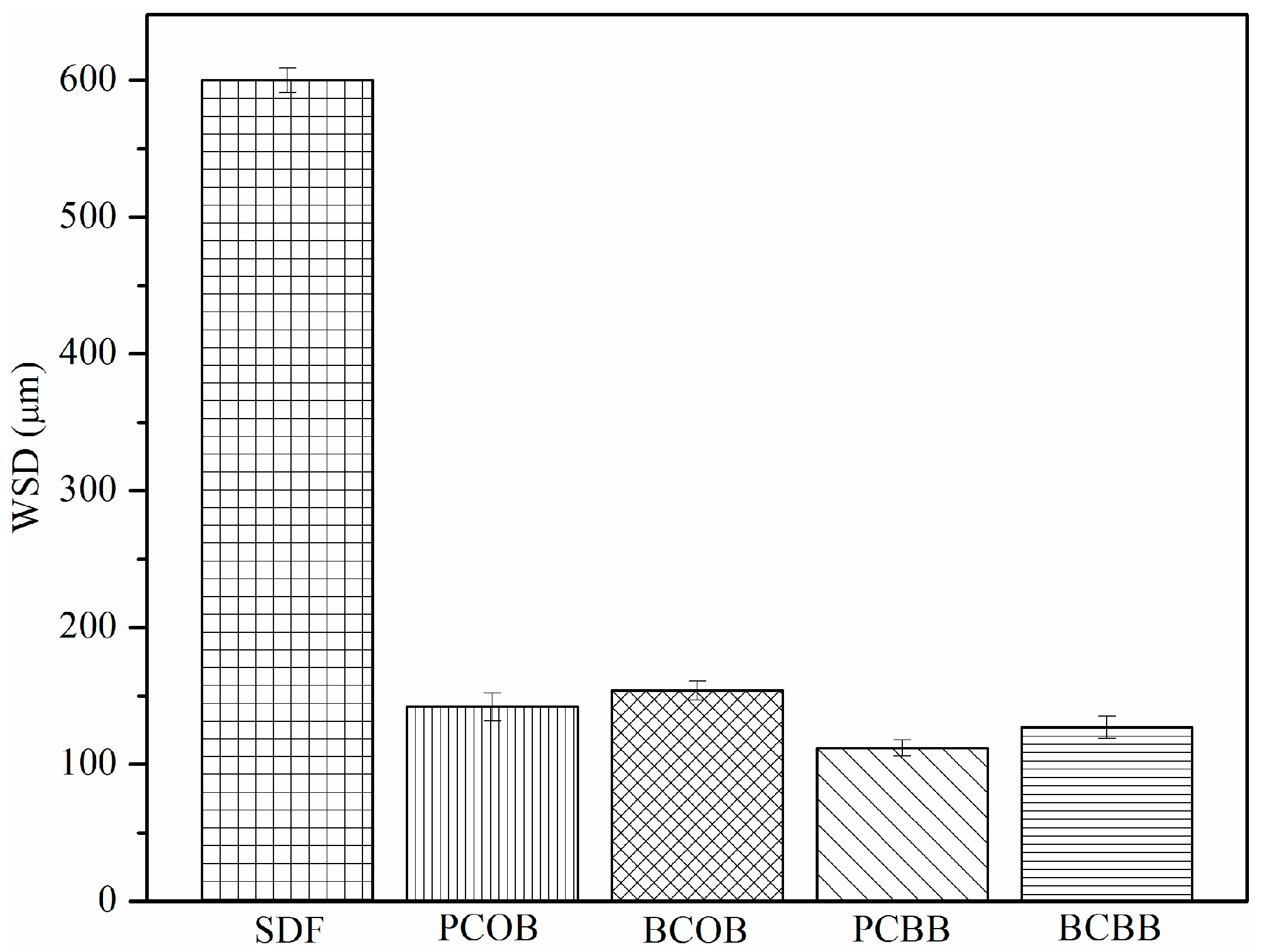
18]. Initially, WSD of the standard diesel fuel (SDF) sample was found to be 600 µm, after addition of 10% of formulated basestocks (PCOB, BCOB, PCBB, and BCBB) lubricity of the alkoxy derivatives were reduced significantly (
Figure 3;
Table 1). Thus, the addition of small amounts of prepared alkoxides enhanced the lubricity of SDF highly and reduced the WSD by more than 446 µm for all the formulated derivatives. The highest values of WSD was found for the PCOB and BCOB (142 and 154 µm) due to the intramolecular hydrogen bonding and stearic hindrances present in them, which enhances the viscosity and thus lowers the solubility in the SDF sample and led to more WSD.
Furthermore, lower WSD was found for PCBB and BCBB (112 and 127 µm), this difference owes to the lower number of aliphatic carbon atoms present in ECB alkoxy derivatives compared with ECO alkoxy derivatives, which makes less viscous and less non-polar and, hence, increases the solubility in SDF sample and results in less WSD. Also, the improved lubricity property of ECB alkoxy derivatives also justified by the presence of ester functionalities at 9, 10 positions on the fatty acid, which are free of hydrogen bonding and help the alkoxides adhere to the metal surface, develops the anti-frictional film and reduce friction [
26]. The observed difference in WSD values can also be explained and supported by the chemical composition of fatty acids and polar nature of the alkoxides. Alkoxylation increases the chain length via branching, and the ester shows improved lubricity performance. Another finding also reported that these alkoxides can exhibit satisfactory lubricant properties via strong chemical and physical adsorption of the metal with the contact surface. This can be attributed to the presence of polar functional groups of the triacylglycerol molecules which forms a non-polar molecular layer that separates the metal surface from friction [
18].
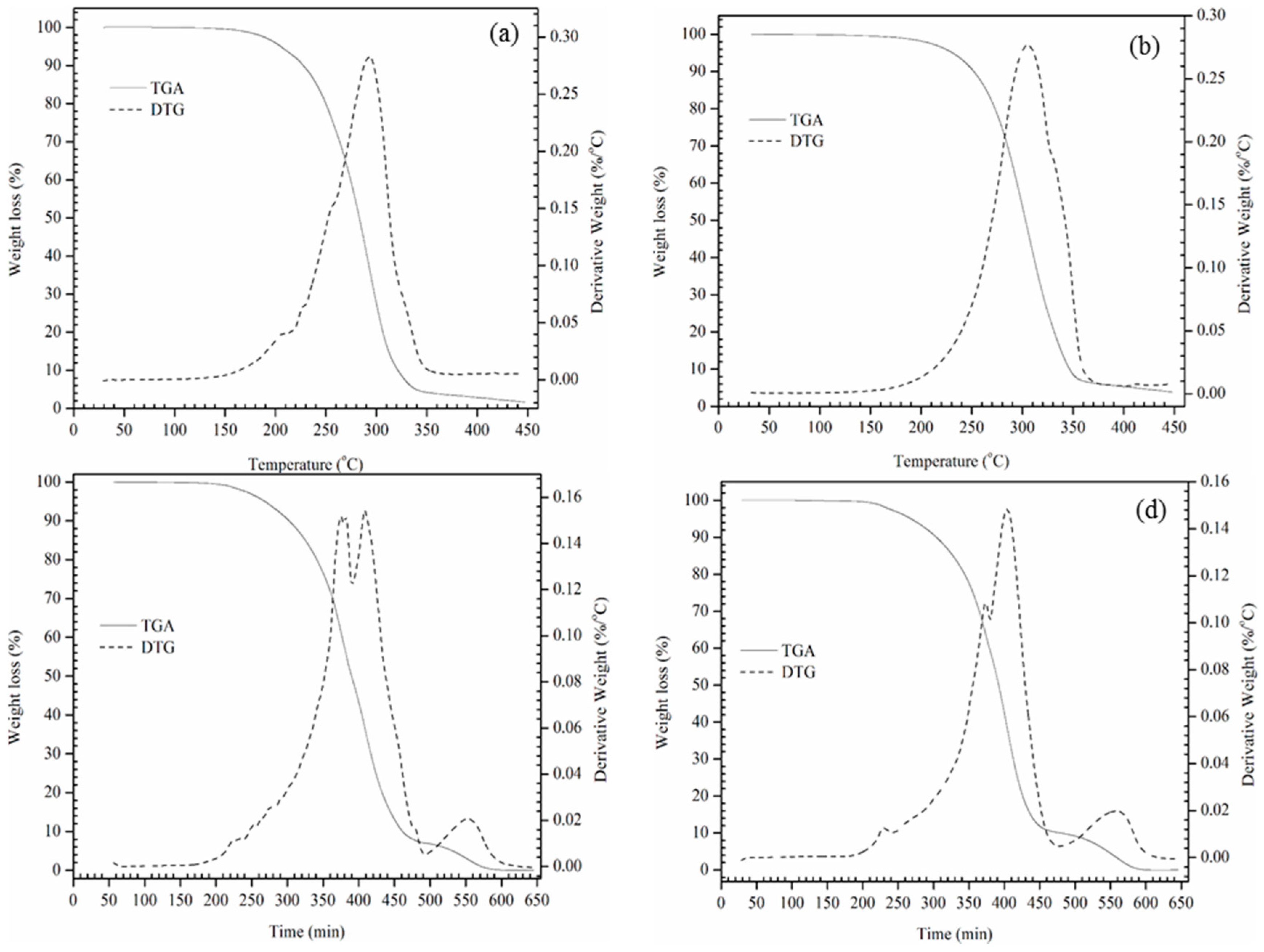
3.6. Thermal and Oxidative Stability by TGA
The ability of a prepared alkoxide to resist thermal and oxidative degradation is another significant factor for biolubricant application. The presence of bis-allylic protons in plant oils are susceptible to radical attach followed by oxidative degradation to form polar oxy compounds. This degradation leads to an increase in alkoxides viscosity and acidity [
25,
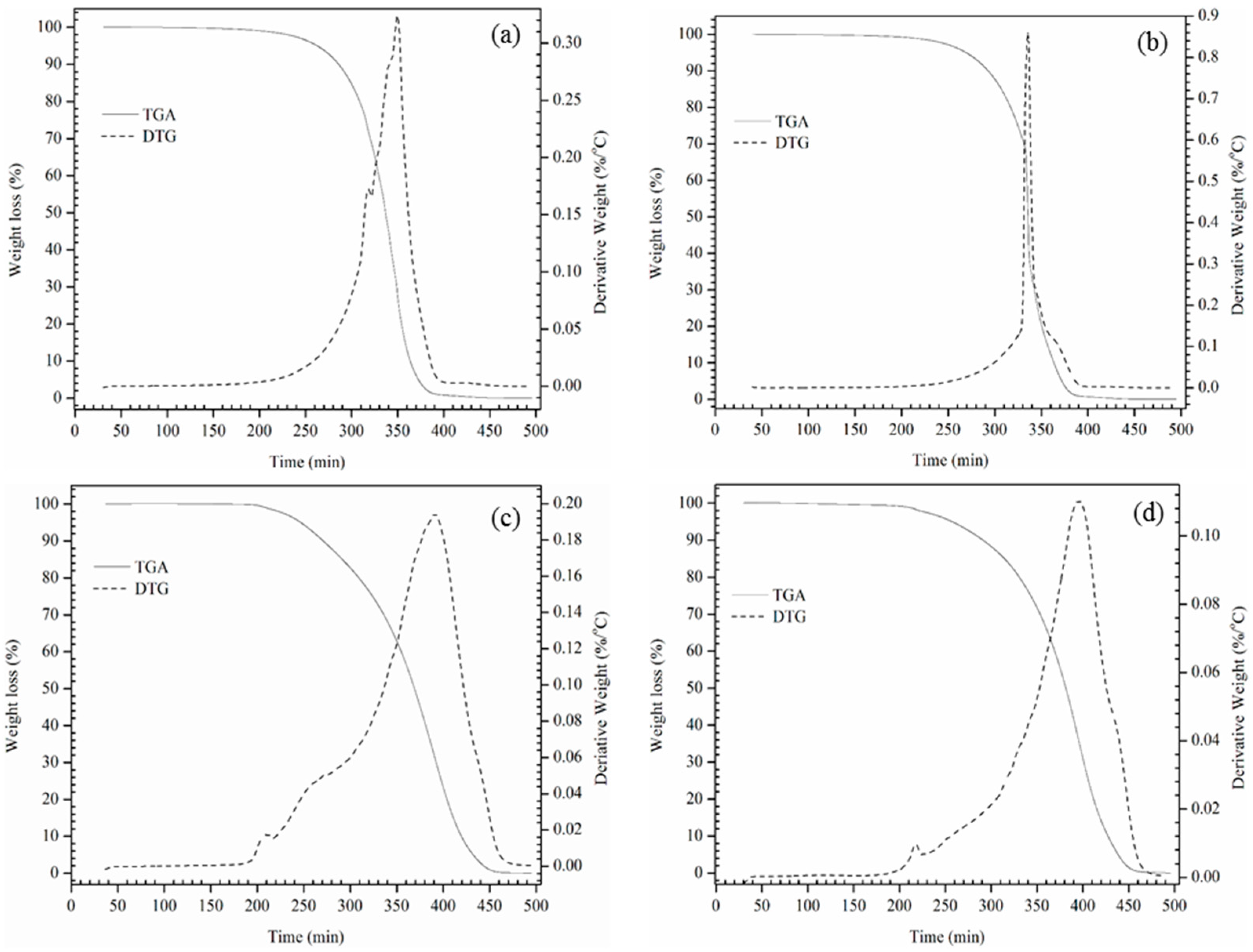
26]. Therefore, the prepared alkoxides were screened for thermo-oxidative stability by TGA. The onset temperature is defined as the temperature at which the weight loss begins up on heating the alkoxides at a constant heating rate. A high onset temperature would indicate high thermal and oxidation stability of the alkoxides, weight loss of the each alkoxide was calculated by considering the thermal degradation profile. TGA was used to understand the thermo-oxidative stability and degradation profile of the prepared alkoxides. From
Table 3 and
Table 4 to
Figure 4 and
Figure 5 show that the thermal and oxidative degradation of all the prepared alkoxides takes place in a single continuous step. Onset temperatures (in N
2) of the alkoxides revealed that they are stable up to 300–325 °C. Alkoxylation (BCOB, BCBB) with a tertiary alcohol (TBA) are found to be highly thermally stable (
Table 3) than secondary alcohol derivatives, weight loss profile showed the same outcomes. Similarly, oxidative stability of the alkoxides are determined as for quality-indicative parameter in an oxygen atmosphere to predict the actual service life at higher temperatures and pressures. Oxidative onset temperatures disclosed that ECB and ECO alkoxide derivatives are stable up to 248–255 °C and 310–325 °C respectively (
Table 4). Compared to ECB alkoxy derivatives, ECO alkoxy derivatives are highly stable in an oxygen atmosphere (
Table 4). Above all, ECO alkoxy derivatives exhibited higher thermo-oxidative stability due to the presence of glycerol in the ECO (
Table 3 and
Table 4). However, all the alkoxides prepared in this study can be used potentially up to their onset temperatures (
Table 3 and
Table 4) in the respective atmospheres.
3.7. Kinetic and Thermodynamic Studies
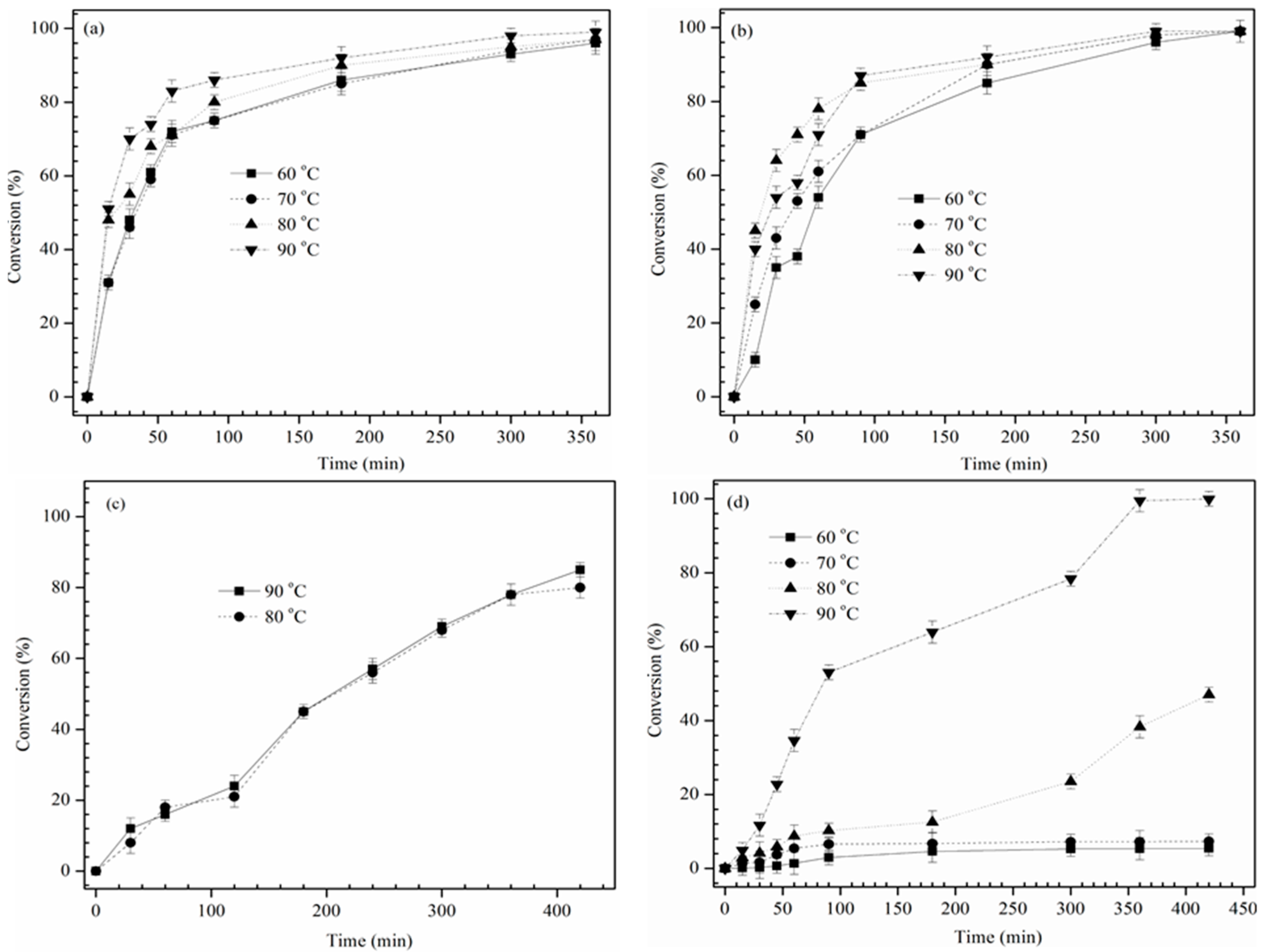
In the present investigation, the kinetic studies were carried out for the alkoxylation reaction at 60, 70, 80 and 90 °C; the other experimental conditions were according to the optimal conditions described in experimental section.
Figure 6 shows the effects of reaction temperature and time on conversion of epoxide into the alkoxy derivative and it was found that the reaction rate increased with the rise in temperature. Alkoxylation of ECB showed 70% (2-propanol,
Figure 6a), 60% (TBA,
Figure 6b) of the epoxy conversion during the first 90 min of reaction, after that the final conversion was similar for all reactions with TBA, 2-propanol (
Figure 6a,b). But, during the alkoxylation of ECO, at low temperatures (60 and 70 °C), reaction was slow and conversion almost remained constant for ECO with TBA (
Figure 6d). Therefore, in order to find the conversion at lower temperatures for longer reaction times, alkoxylation with TBA was continued for 20 h and conversion was found to be 38.3% at 60 °C and 98.5% at 70 °C. Similarly, alkoxylation of ECO with 2-propanol at lower temperatures showed identical conversion progress with respect to TBA. However, for both alkoxylation reagents, 90 °C was found to be favorable for 100% epoxide conversion. Therefore, due to unfavorable outcomes of alkoxylation of ECO with 2-propanol and TBA, the kinetics were calculated only for the alkoxylation of ECB with 2-propanol and TBA.
The alkoxylation reaction between oxirane groups of ECB and alkoxylation reagents 2-propanol, TBA takes place in a single step (see Equation (1)) and was considered to be rate limiting to form an alkoxide. Therefore, the rate (kinetic equation) of alkoxylation reaction can be given by Equation (2).
where
CECB denotes the concentration of epoxidized canola biodiesel;
CRR denotes the concentration of alkoxylation reagents.
During the test runs, 1:6 molar ratio of epoxide to alcohol ratio was found to be effective for best conversion. Therefore,
CRR,
0 = 6
CECB,0, where
CRR,
0 and
CECB,0 denotes initial concentrations of 2-propanol, TBA and ECB respectively. Also,
CRR =
CECB,0 (6 −
XECB),
CECB =
CECB,0 (1 −
XECB); therefore using aforementioned correlations, Equation (2) can be rewritten as:
where,
XECB is epoxide conversion,
CECB,0 is initial concentration of ECB.
Further, after integration Equation (5) is reduced to:
where C is a constant.
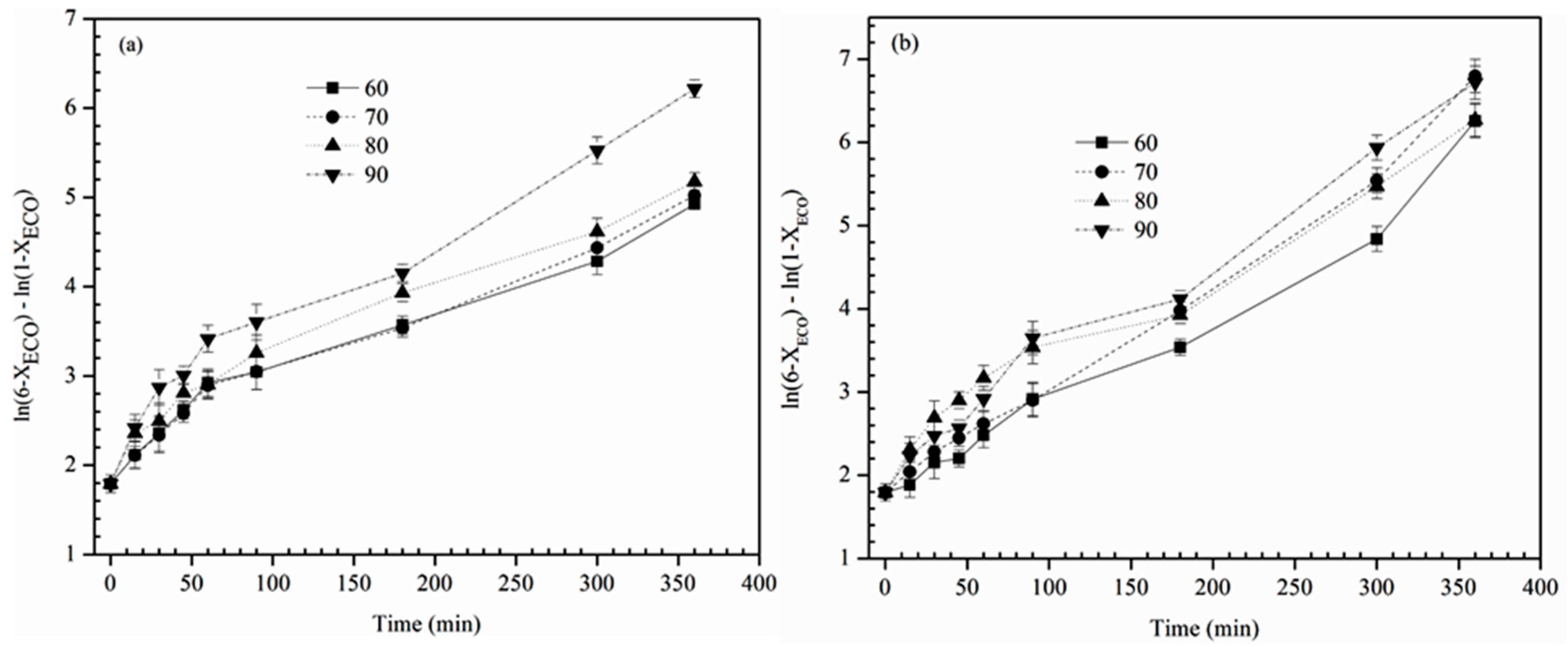
The rate constant (
k) value for each temperature was determined as the slope of plot between ln(6 −
XECB) − ln(1 −
XECB) vs. reaction time and the graphical representation of Equation (6) is shown in
Figure 7 at various reaction temperatures (60, 70, 80 and 90 °C). The results of the regression analysis of the data are plotted in
Figure 8. The kinetic constants (
k) and
R2 values are shown in
Table 5 and
Table 6. From these Tables, it was noticed that the rate constants linearly increased with increase in reaction temperature, which signifies that the higher temperature boosts the conversion rate. The results clearly show that the reaction rate constants depend strongly on temperature. The higher values of
R2 coefficient show that the data are a good fit to the kinetic model.
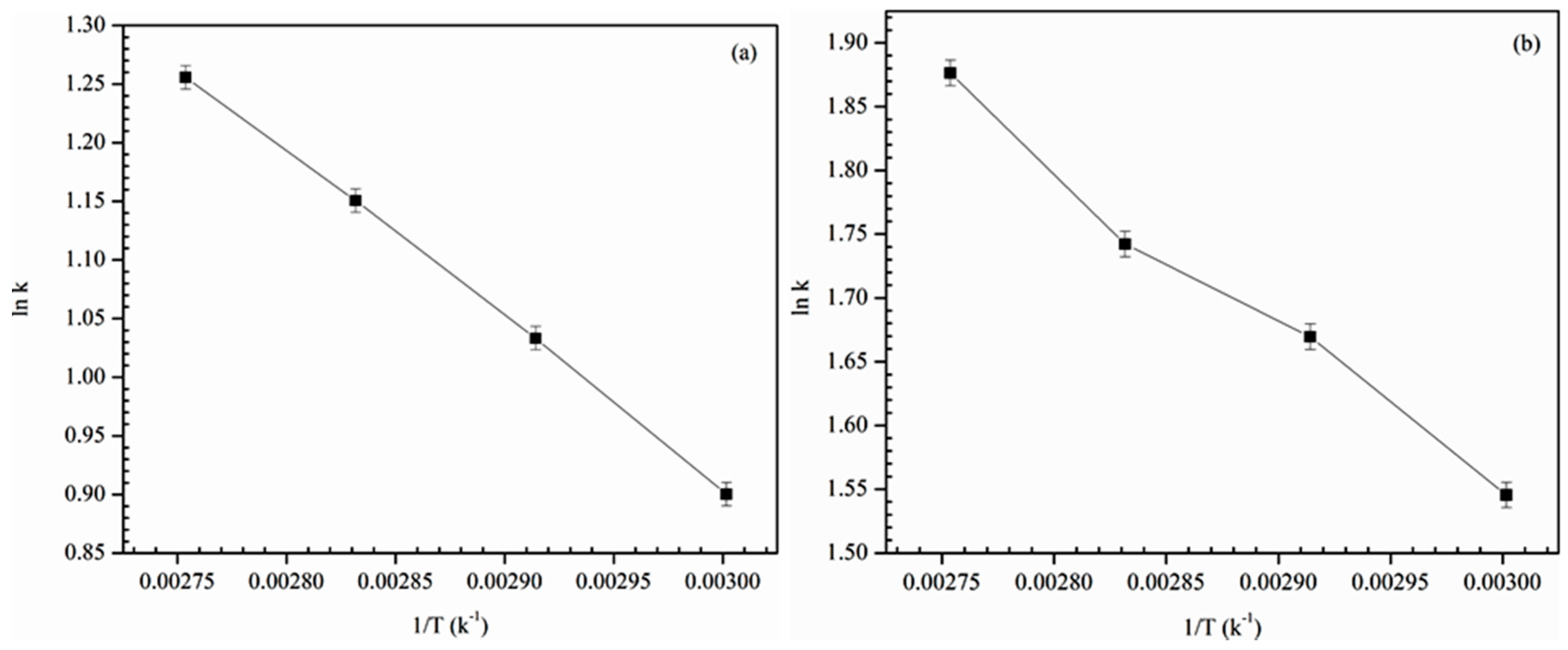
The activation energy (
Ea) was calculated from the Arrhenius equation (Equation (7)). The rate constant with reaction temperature was applied to the Arrhenius equation to compute the activation energy.
where
k is the rate constant, A is the frequency factor,
R is the universal gas constant and
T is the absolute temperature. In order to find out
Ea from the slope of the curve Equation (7) could be linearized as shown in
Figure 8 The value of
Ea was found to be 11.9 and 10.7 KJ mol
−1, respectively, for 2-propanol and TBA.
The enthalpy of activation, Δ
H, was calculated using the following equation:
The average energy of activation, Δ
S, was calculated using Eyring equation and the free energy of activation, Δ
F, was obtained from the following relationship:
The thermodynamic properties of the ring-opening of ECB with 2-propanol and TBA were calculated using aforementioned relationships and are shown in
Table 5 and
Table 6.
The positive values of Δ
H and Δ
G signify that the alkoxylation reactions are endergonic, non-spontaneous and endothermic in nature. The negative value of Δ
S indicates that the transition state has a higher degree of ordered geometry/alignment than the reactants in the ground state [
27]. The increased conversion was noticed during the rise in temperature signifying that heat input is required to bring the reactants to the transition state in order to form the products [
28].