1. Introduction
Total joint replacement is a relatively common and generally very successful procedure. Data from the largest joint registry in the world, the National Joint Registry for England, Wales and Northern Ireland, reports that in the last year for which data is available, 2012–13, over 80,000 hip prostheses and 85,000 knee prostheses were implanted in these countries [
1]. The registry also states that, at 10 years follow up, the revision rate for cemented hips and cemented knees was only 3.20% and 3.33% respectively, thus indicating the long term success of the vast preponderance of these implants [
1]. The majority of these hip and knee prostheses consist of a hard metal or ceramic component which articulates against a polyethylene counterface. However, a small number of these implants do need to be revised and in the majority of cases this is due to wear induced osteolysis [
2,
3]. Here the polyethylene wear debris provokes a negative cascade of events within the body eventually leading to osteolysis and a revision operation. Therefore the issue of wear is a long-term problem in joint replacements.
As such it is essential both to understand and to minimize the wear processes taking place, and tribological studies have been widely undertaken to study the wear of polyethylene and other orthopedic biopolymers
in vitro. A key element in such testing has been the appropriate choice of lubricant [
4].
Dilute bovine serum is currently recommended as the lubricant for wear testing orthopedic biopolymers [
5,
6,
7]. This is because: it results in clinically relevant wear rates; it prevents the formation of a transfer film (and such transfer films are not seen on explanted joints); and it results in wear debris which matches the size and shape of polyethylene wear debris seen
in vivo [
4,
8]. However there are recognized issues with this lubricant including batch to batch variation, cost and safety [
4]. As a biological material it also needs to be changed regularly and this will likely remove wear particles which can influence the tribological performance. For these and other reasons, comparing wear results between different labs can be problematic. Moreover, while it can be fascinating from a tribological view to investigate the constituents of bovine serum and their effect on wear performance, it must also be accepted that bovine serum lacks key elements which exist within synovial fluid and are known to influence the tribology of joints. Likewise it should be self-evident that it is not bovine serum but synovial fluid in which artificial joints must operate [
4]. The ideal would be to have a biolubricant which is safe, relatively inexpensive, mimics the properties of synovial fluid and which does not need to be replaced at frequent intervals. The current paper is one contribution towards this overall ideal.
For all of these reasons alternative lubricants have been sought and tested [
9]. To add to this body of data a new lubricant was investigated which has been shown to mimic certain rheological properties of synovial fluid [
10]. Wear tests were undertaken in a screening wear tester which had previously been shown to produce clinically relevant wear factors for orthopedic biopolymers [
11,
12,
13,
14,
15,
16,
17]. Details of the new lubricant, alongside comparable properties of bovine serum and human synovial fluid, are given in
Table 1. It should be noted that the characteristics of synovial fluid, as a biological fluid, will cover a spectrum and will vary depending on the individual as well as any arthritic disease that may be present [
4].
Table 1.
Comparative table of lubricant properties. * Data taken from [
4].
† Data taken from [
10].
Table 1.
Comparative table of lubricant properties. * Data taken from [4]. † Data taken from [10].
| | Bovine Serum | Human Synovial Fluid | New Lubricant |
|---|
| Protein | Yes (60 g/L) * | Yes (17 g/L) * | No |
| Polysaccharide | None * | Hyaluronic acid (3.2 g/L) * | Sodium alginate 2% w/w and Gellan gum 0.75% w/w |
| Phospholipids | None * | Yes (0.13–1.15 g/L) * | None † |
| Viscosity across Shear rates 0.1–10 (s−1) | 0.1–0.005 Pas † | 5–0.05 Pas † | 1–0.05 Pas † |
| Elastic Modulus (at 1 rad−1) | ~0.01 Pa † | ~0.5 Pa † | ~0.75 Pa † |
2. Results and Discussion
A polysaccharide solution consisting of a 50:50 mix of 2%
w/
w alginate and 0.75%
w/
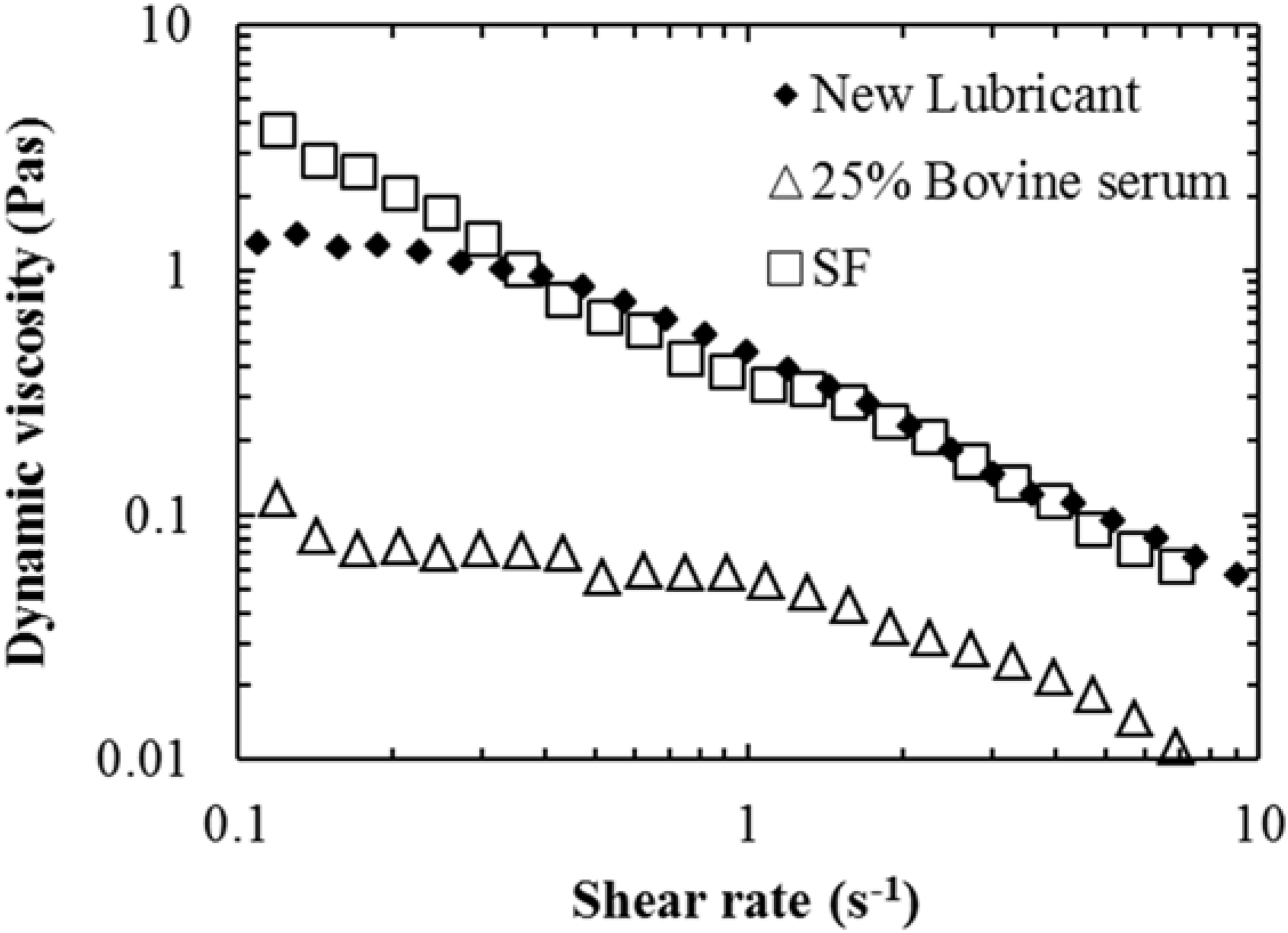
w gellan gum was prepared and investigated as a lubricant for wear testing orthopedic implant materials. This lubricant was shown to have the non-Newtonian characteristics similar to that of aspirated synovial fluid with a reduction in dynamic viscosity with increasing shear rate (
Figure 1). Furthermore, the viscosity of both the synovial fluid and the new lubricant was a factor of 10 greater than the bovine serum across all the shear rates measured.
Figure 1.
Dynamic viscosity
vs. sheer rate for: aspirated synovial fluid (SF) (open squares); 25%
w/
v bovine serum (open triangles); and the new lubricant (50:50 mix of 2%
w/
w alginate and 0.75%
w/
w gellan gum) (filled diamonds); measurements undertaken at 37 °C. Data adapted from [
10].
Figure 1.
Dynamic viscosity
vs. sheer rate for: aspirated synovial fluid (SF) (open squares); 25%
w/
v bovine serum (open triangles); and the new lubricant (50:50 mix of 2%
w/
w alginate and 0.75%
w/
w gellan gum) (filled diamonds); measurements undertaken at 37 °C. Data adapted from [
10].
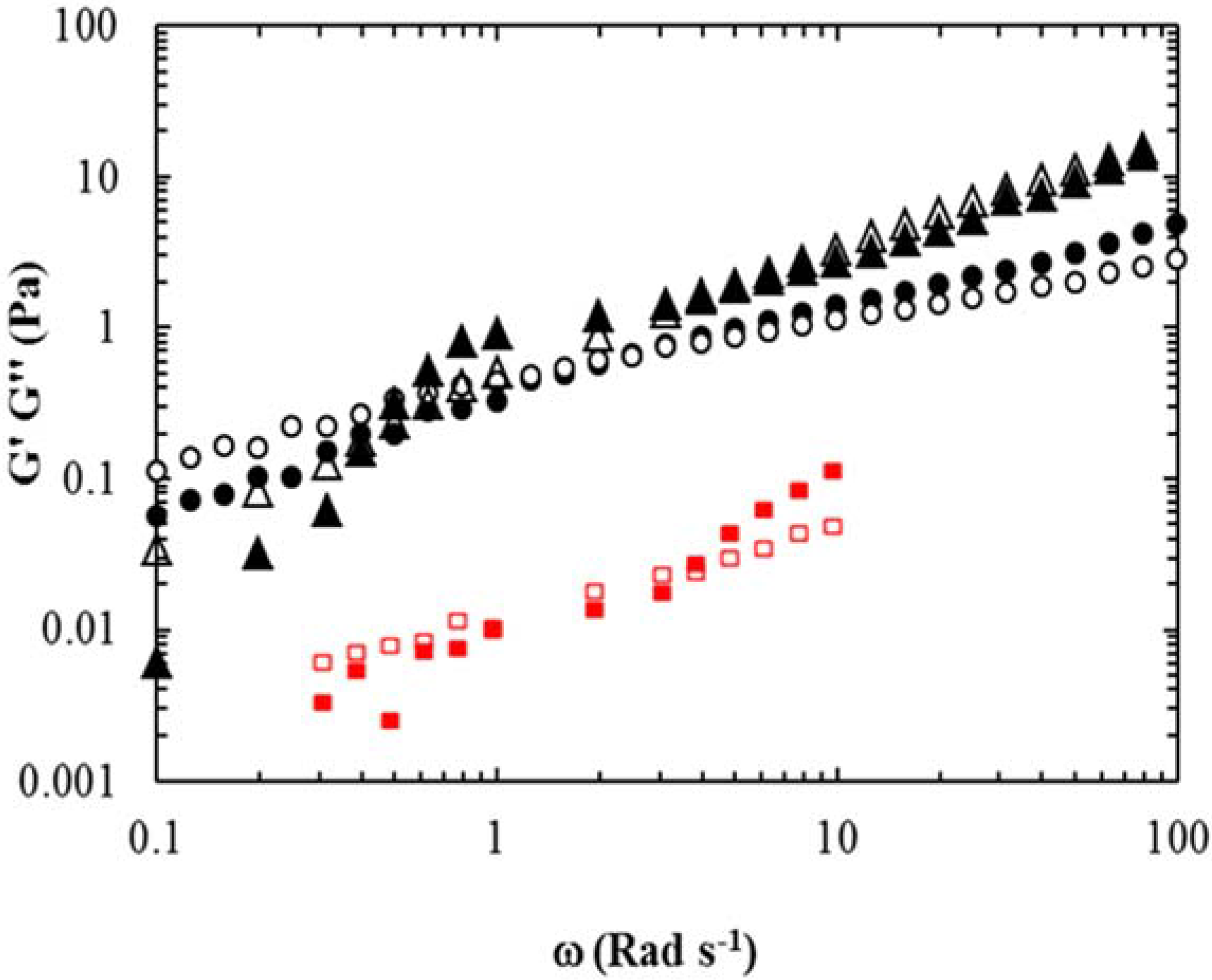
The rheological disparity of bovine serum, and similarity of the new lubricant to that of synovial fluid, is further highlighted in
Figure 2. Synovial fluid has a mechanical spectra characteristic of a concentrated entangled biopolymer solution meaning the storage modulus (G′) and loss modulus (G″) (G″ > G′ at low frequencies of oscillation and G′ > G″ at high frequencies) are mimicked by the new lubricant. Moreover, the moduli measured in the synovial fluid and the new lubricant samples were over an order of magnitude greater than that of the bovine serum. It is thought that the viscoelastic behavior of the alginate/gellan mixture is due to the alginate providing the viscous response at low frequencies and the gellan contributing to the elastic response at high frequencies [
10].
Figure 2.
Mechanical spectra (2% strain; 37 °C) for: synovial fluid (G′ filled circles and G″ open circles); the new lubricant (G′ filled triangles and G″ open triangles); and dilute bovine serum 25 g/L protein (G′ filled squares and G″ open squares). Data adapted from [
10].
Figure 2.
Mechanical spectra (2% strain; 37 °C) for: synovial fluid (G′ filled circles and G″ open circles); the new lubricant (G′ filled triangles and G″ open triangles); and dilute bovine serum 25 g/L protein (G′ filled squares and G″ open squares). Data adapted from [
10].
After 2.4 million cycles (125 km) of sliding, the mean volumetric wear rate of the ultra high molecular weight polyethylene (UHMWPE) test pins were 0.45 mm
3/million cycles. This was equivalent to a mean wear factor of 0.099 × 10
−6 mm
3/Nm. Weight changes were measured for each pin at 12 intervals during the 125 km of testing. Wear factors for each test pin, corrected for the control pins, are shown in
Table 2. The control pins increased in weight, and this increase fluctuated in magnitude over the duration of testing, but at the end of testing an increase of 110 µg was measured. In comparison, at the end of testing, the four test pins showed a mean weight loss of 115 µg. Plate surface roughness values changed from a mean of 0.015 µm Rq, prior to the test, to 0.029 µm Rq at the end of testing; as shown in
Table 3. Rq is the root mean square roughness. No noticeable changes in the characteristics of the new testing fluid over the duration of the test were observed.
Table 2.
Mean wear factors of the four ultra high molecular weight polyethylene (UHMWPE) test pins after 125 km of sliding; also the final roughness values.
Table 2.
Mean wear factors of the four ultra high molecular weight polyethylene (UHMWPE) test pins after 125 km of sliding; also the final roughness values.
| Pin No. | Wear Factor (k) (×10−6 mm3/Nm) | Standard Deviation (×10−6 mm3/Nm) | Mean Rq after Test |
|---|
| 1 | 0.120 | | 2.188 µm |
| 2 | 0.133 | | 1.303 µm |
| 3 | 0.078 | | 2.279 µm |
| 4 | 0.063 | | 2.430 µm |
| Average | 0.099 | 0.034 | 2.050 µm |
Table 3.
Mean roughness values of the wear tracks of the four test plates before testing and at the end of testing.
Table 3.
Mean roughness values of the wear tracks of the four test plates before testing and at the end of testing.
| Plate No. | Mean Rq before Test | Mean Rq after Test |
|---|
| 1 | 0.012 µm | 0.019 µm |
| 2 | 0.015 µm | 0.036 µm |
| 3 | 0.018 µm | 0.031 µm |
| 4 | 0.014 µm | 0.027 µm |
| Average | 0.015 µm | 0.028 µm |
When the same biomaterials were tested in the same rig using a lubricant of dilute bovine serum a mean wear factor of 1.6 × 10
−6 mm
3/Nm was measured [
18]. This is close to the reported mean wear factor of 2.1 × 10
−6 mm
3/Nm for explanted Charnley hips which also used the same biomaterials of stainless steel and UHMWPE [
19]. With the new lubricant, the average mean wear factor was 0.099 × 10
−6 mm
3/Nm and therefore over an order of magnitude lower than with dilute bovine serum.
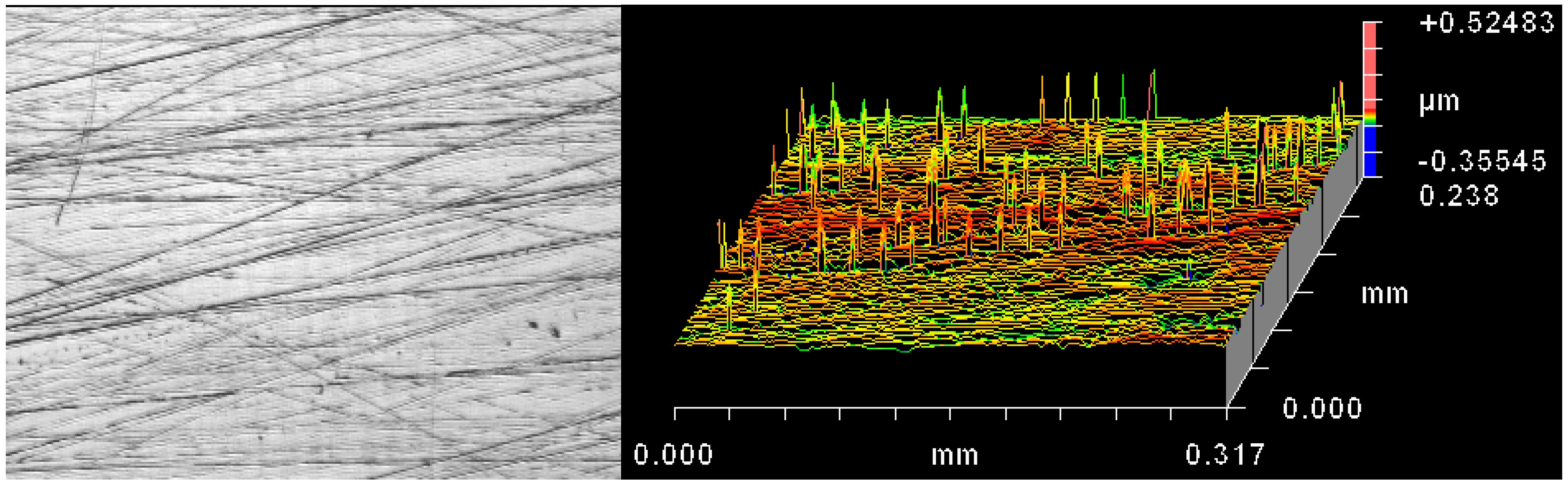
The plate surface roughness values at the end of the test were higher than at the beginning of the test and this may indicate the presence of a transfer film. From the non-contacting profilometer measurements, the key feature was multi-directional scratching (
Figure 3). In addition surface adhesions were seen on the wear tracks of the test plates (
Figure 3) and these adhesions could have originated from the polyethylene pins. Such a transfer film is formed when a hard material, such as a metal, moves against a softer material, such as a polymer, and shears off and picks up a coating of polymer [
20]. If the transfer film is stable, then wear rates may be reduced after an initial high wear interval during film formation [
20]. Previous work with bovine serum as a lubricant for wear testing UHMWPE pins against a metal counterface has indicated no change in roughness of the metal counterface at the end of testing, at a minimum of 2.5 million cycles, and no transfer film [
21,
22]. In addition, transfer films of UHMWPE are not seen clinically with such implants [
23]. Previously it has been shown that the addition of hyaluronic acid to serum to increase its viscosity had little effect on wear of UHMWPE [
18,
24]. It may be that, as the sliding velocity is relatively low, so viscosity is not the principal issue in the wear of UHMWPE. Instead, the action of animal-based proteins in boundary lubrication seems to be of high importance as, when animal-based proteins are absent, a transfer film occurs. This has been known for some time with lubricants of distilled water and Ringer solution [
25,
26] but has also been shown to occur when other novel lubricants (DPPC (dipalmitoylphosphatidylcholine) and soy protein) have been used [
27]. A more recent study which wear tested UHMWPE pins against CoCr plates in the presence of 13 different lubricants [
9] found that only an egg white based lubricant gave wear factors which were statistically similar to those given by dilute bovine serum. It has been argued for some time that bovine serum serves as a boundary lubricant to prevent a transfer film being formed [
28]. In turn it is felt that the proteins within bovine serum allow boundary lubrication without transfer film [
27]. As shown by our results, polysaccharides are unable to facilitate boundary lubrication in this application.
Figure 3.
Left hand side image shows an optical image of the worn plate; note the multi-directional scratches; Right hand side image shows the equivalent “oblique plot” produced by the ZYGO non-contacting profilometer. Note the peaks which indicate attached material; note too that the horizontal scale is over one thousand times larger than the vertical so that the peaks are not as “severe” as they appear.
Figure 3.
Left hand side image shows an optical image of the worn plate; note the multi-directional scratches; Right hand side image shows the equivalent “oblique plot” produced by the ZYGO non-contacting profilometer. Note the peaks which indicate attached material; note too that the horizontal scale is over one thousand times larger than the vertical so that the peaks are not as “severe” as they appear.
For the UHMWPE pins, the mean pre-test roughness was 2.143 µm Rq. While the Rq values at the end of the test were numerically similar, it was noted that the initial concentric machining marks on the pins had largely been removed.
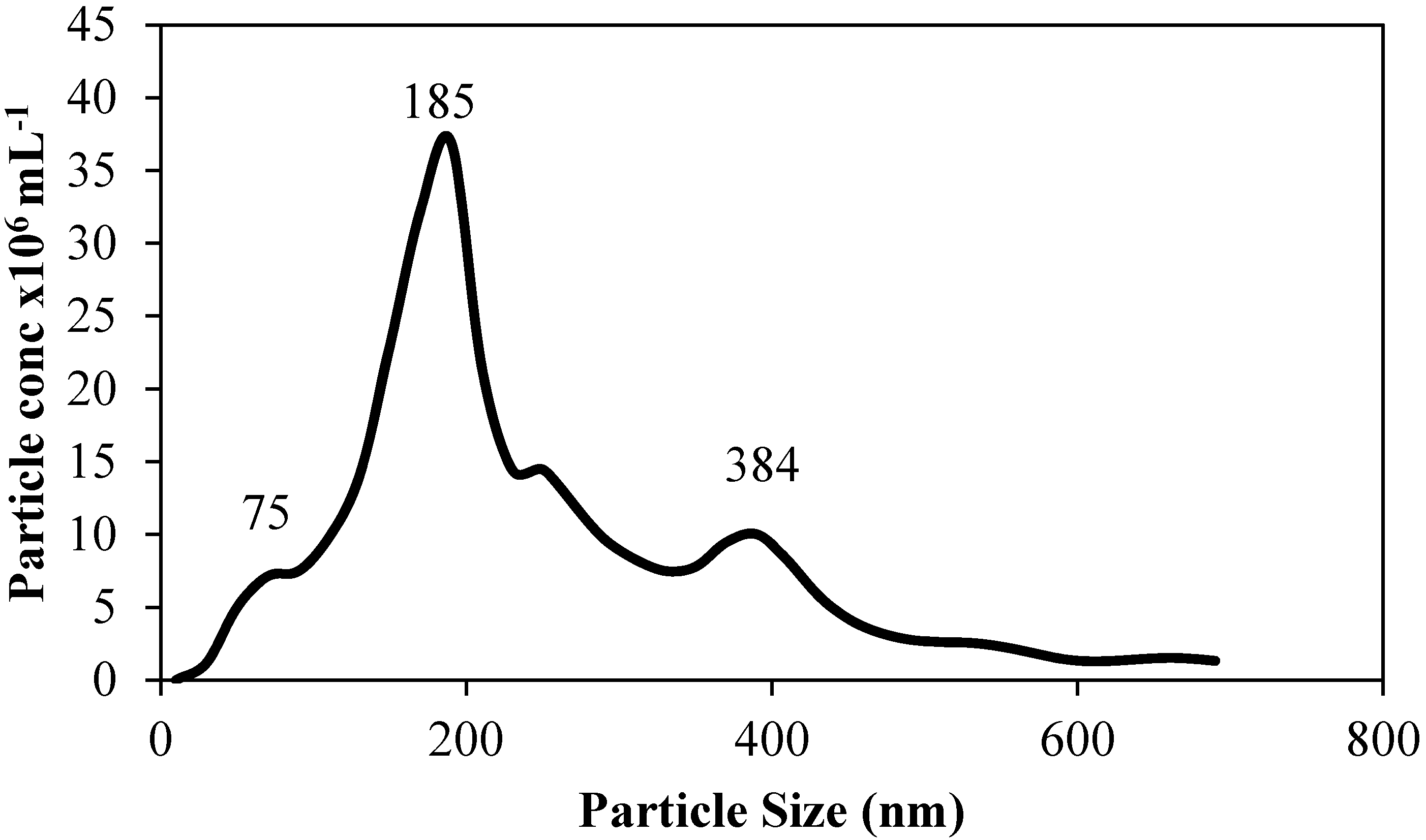
Analysis of the wear debris in the new lubricant revealed particle sizes ranging from ~50 to 400 nm (
Figure 4). These nanoparticles are of a similar size range to wear debris found in failed total knee arthroplasties (low contact stress mobile bearing prostheses) [
29].
Figure 4.
Particle size distribution of wear debris in the new lubricant post test (50:50 mix of 2% w/w alginate and 0.75% w/w gellan gum).
Figure 4.
Particle size distribution of wear debris in the new lubricant post test (50:50 mix of 2% w/w alginate and 0.75% w/w gellan gum).
There were a number of limitations. Given that the influence of the controls was so important on the overall wear, we could perhaps have employed control pins which were subject to the same axial load as our test pins. However we would point out that: we employed three control pins to try and minimize the effect of lubricant uptake on the overall wear values; unloaded control pins allowed a direct comparison with our previous work which is compared to in the text [
18]; and also that it is usual to employ unloaded control pins in such screening wear tests [
30,
31,
32]. For future work we will look to employing statically loaded control pins. Another limitation was the small test sample size, however such a sample size was in line with previous work [
18,
31] and the sample size was sufficient to indicate that the new lubricant was unable to match wear factors associated with the use of bovine serum as a lubricant.
3. Experimental Section
The new lubricant consisted of a mixture of sodium alginate and gellan gum, and aimed to match the rheology of synovial fluid. Sodium alginate was used as a synthetic substitute to hyaluronic acid, giving the lubricant non-Newtonian characteristics as seen with synovial fluid, while the gellan gum replaced the lubricin in synovial fluid and aimed to reproduce the viscoelasticity of synovial fluid. Stock solutions of the test lubricants were prepared as previously described [
10]. A 50:50 mix of 2%
w/
w alginate (Protanal LF200) and 0.75%
w/
w gellan gum (kelcogel CG-LA) were subjected to viscosity measurements at 37 °C using a sheer ramp from 0.1–10 s
−1. These parameters were chosen to match similar shear rates the lubricant was subjected to during wear testing. Oscillatory shear measurements of storage modulus (G′) and loss modulus (G″) were taken at a constant strain of 2% (within the linear viscoelastic region) across a frequency range of 0.1–100 rad·s
−1 at 37 °C. Both viscosity measurements and oscillatory measurements were performed on a Bohlin Gemini nano rheometer using a 55 mm parallel plate geometry with a 100 mm gap. All rheological measurements were performed using the set up and parameters as used previously by Smith
et al. [
10].
The four-station wear test rig has been described previously [
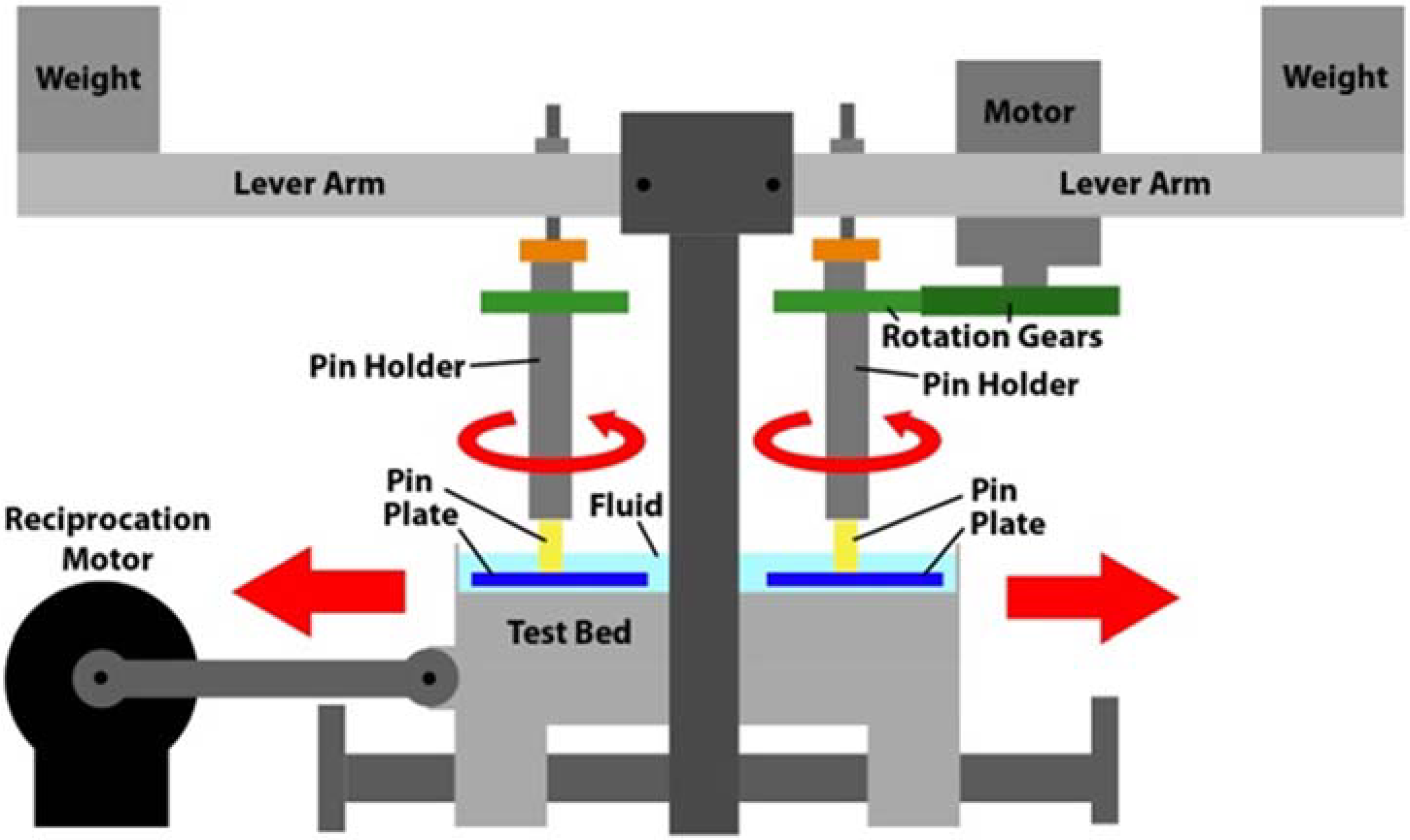
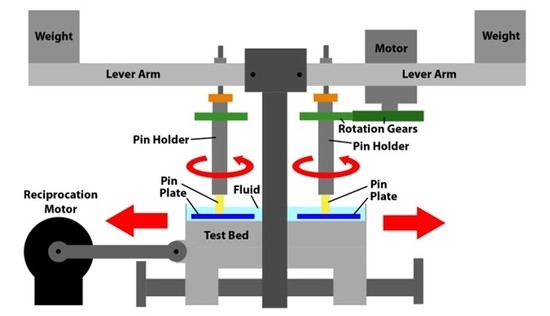
18]. A schematic image of the rig is offered in
Figure 5. As can be seen the key elements are a motor which provided the reciprocating motion to the test bed upon which were held the four test plates. Each test plate sat within an individual bath. Each test pin, which was held within a pin holder, was also subject to a rotational motion, which was provided by a 12 V motor via a pair of spur gears. Each test pin was subject to load which was applied by a weight mounted towards the end of a lever arm.
Figure 5.
Schematic drawing of the pin-on-plate test rig.
Figure 5.
Schematic drawing of the pin-on-plate test rig.
Each of the four test stations applied rotational motion at 1 Hz to 6 mm diameter test pins which were loaded at 40 N against 316 L stainless steel test plates (50 mm × 25 mm × 3 mm) which had been polished to a mean surface finish of 0.015 µm Rq. The 40 N load resulted in a nominal stress of approximately 1.4 MPa. This not only matched that used in previous tests [
18] but also fitted well with research which indicates that the average contact pressure in an artificial hip joint is likely within the range 1–2 MPa [
22]. A reciprocating motion, again at 1 Hz, was applied to the test plates. The stroke length was 30 mm. Pins were manufactured from UHMWPE and the test pins were subject to multi-directional motion through the combination of the rotational and reciprocating motion. Such a combined motion resulted in each point on the wear face of the test pins following elliptical or quasi-elliptical wear tracks [
11], similar to those motions seen on implanted hip prostheses [
33]. The lubricant was not heated as it is recognized that, with higher temperatures, increased protein precipitation occurs during testing of hip implants and that this served to decrease wear, through the formation of an adherent layer on the surfaces of artificial hips [
34]. Similarly other research has shown that not heating the bulk lubricant to 37 °C resulted in less evaporation of lubricant (so that experimental conditions remained largely unchanged); reduced microbial growth (and thus no need for additives which are both toxic and may change the wear mechanisms); reduced protein precipitation; and, most importantly, wear results which were similar to clinical findings [
22].
At regular intervals of approximately 60 h the test was stopped, “test” and “control” lubricant was collected into individual containers, pins and plates were cleaned and weighed three times to a consistent protocol on a balance with a sensitivity of 10 µg. “Control” pins were employed to take account of any lubricant uptake or fluctuations in weight. They were kept in the same test lubricant and subject to cleaning and weighing at the same intervals as the test pins. From such compensated weight changes, a volume change was obtained by using the density of UHMWPE, which was taken to be 949 kg/m
3. Using linear regression and plotting compensated mass loss against sliding distance, the wear rate was computed. Then the wear rate was divided by the density, load and sliding distance to give a wear factor. Thus the wear factor (
k, units mm
3/Nm) for each pin was defined as the volume lost (
V, units mm
3) divided by the product of the load (
L, units N) and the sliding distance (
D, units m):
Prior to, and at the end of testing, fifty readings of the roughness of the wear track on each of the test plates was measured using a ZYGO 5000 non-contacting profilometer, which had a vertical resolution of better than 1 nm [
35,
36].
Accumulation of wear debris in the test lubricants was verified using nanoparticle tracking analysis (Nanosight LM10). In order to analyze the wear debris the polysaccharides were removed from the test lubricant by ethanol extraction. Briefly, at the end point of the wear test the lubricant was collected and a diluted 1:100 with ultrapure water (18.2 MΩ·cm). A threefold volume of cold ethanol (95% v/v) was then added and the precipitated polysaccharides were removed with a spatula. The remaining solution was filtered using a Buchner funnel with a pore size of 11 µm. The filtrate was collected and the ethanol removed using a rotary evaporator. The remaining wear debris was then re-suspended in ultrapure water prior to analysis.