Characterization of Thermal Stability of Synthetic and Semi-Synthetic Engine Oils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Engine Oil Samples
2.2. Engine Oil Degradation Test
2.3. Thermogravimetric Analysis
2.4. Viscosity
2.5. FluidScan® Lubricant Analysis
2.6. Gas Chromatography/Mass Spectrometry (GC/MS)
2.7. Evaluation of Apparent Activation Energy
3. Results and Discussion
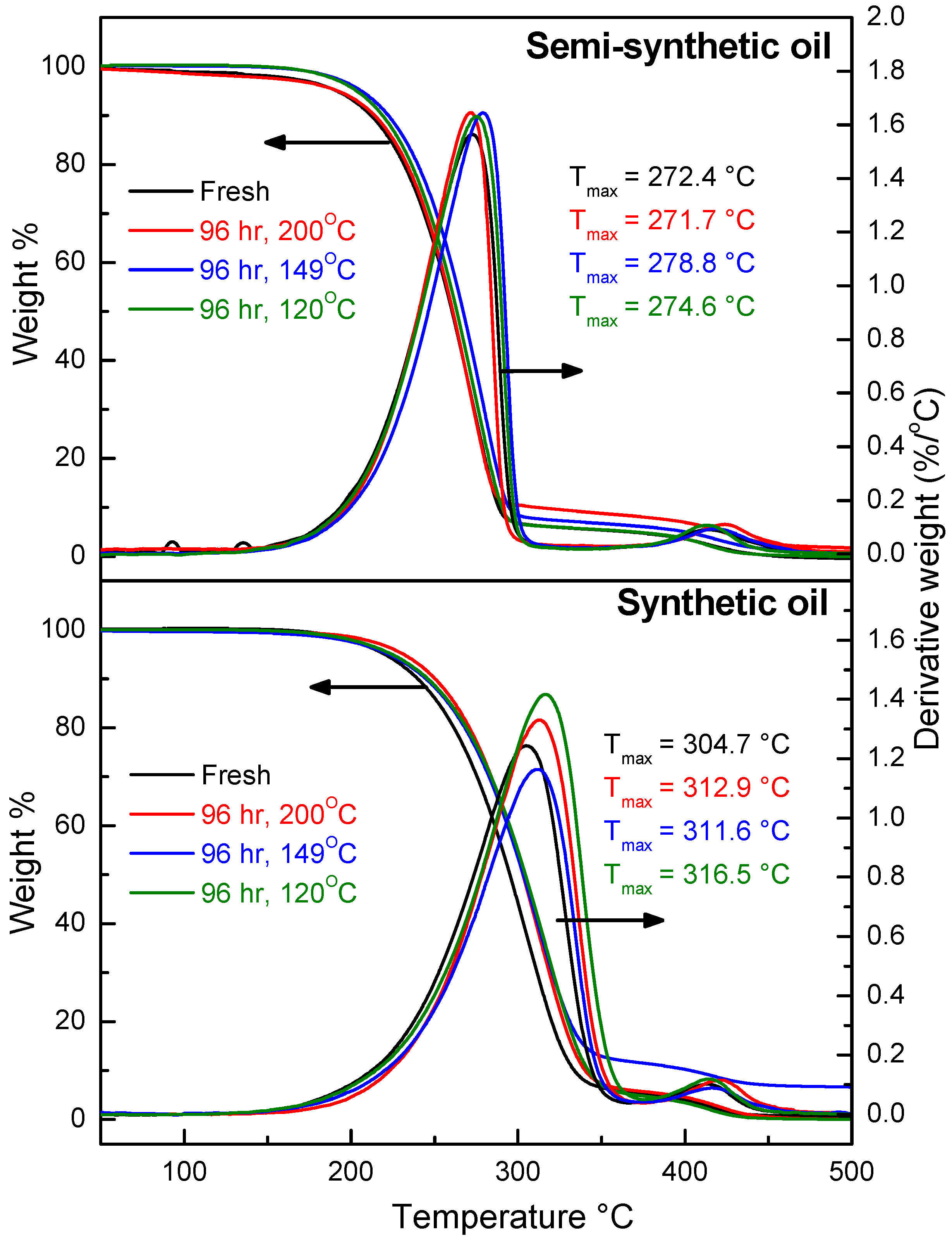
3.1. Thermal Stability of Engine Oils during Ageing
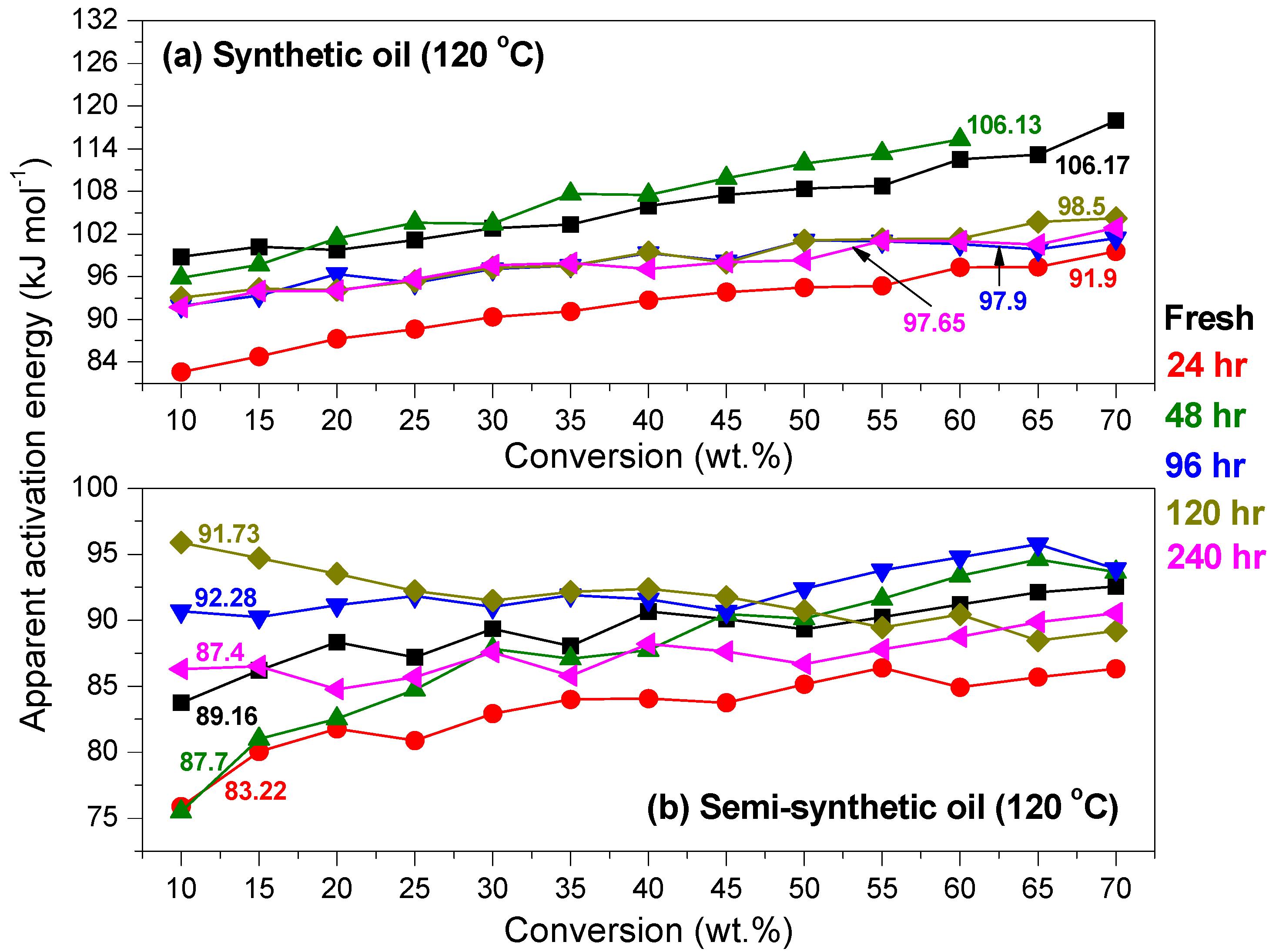
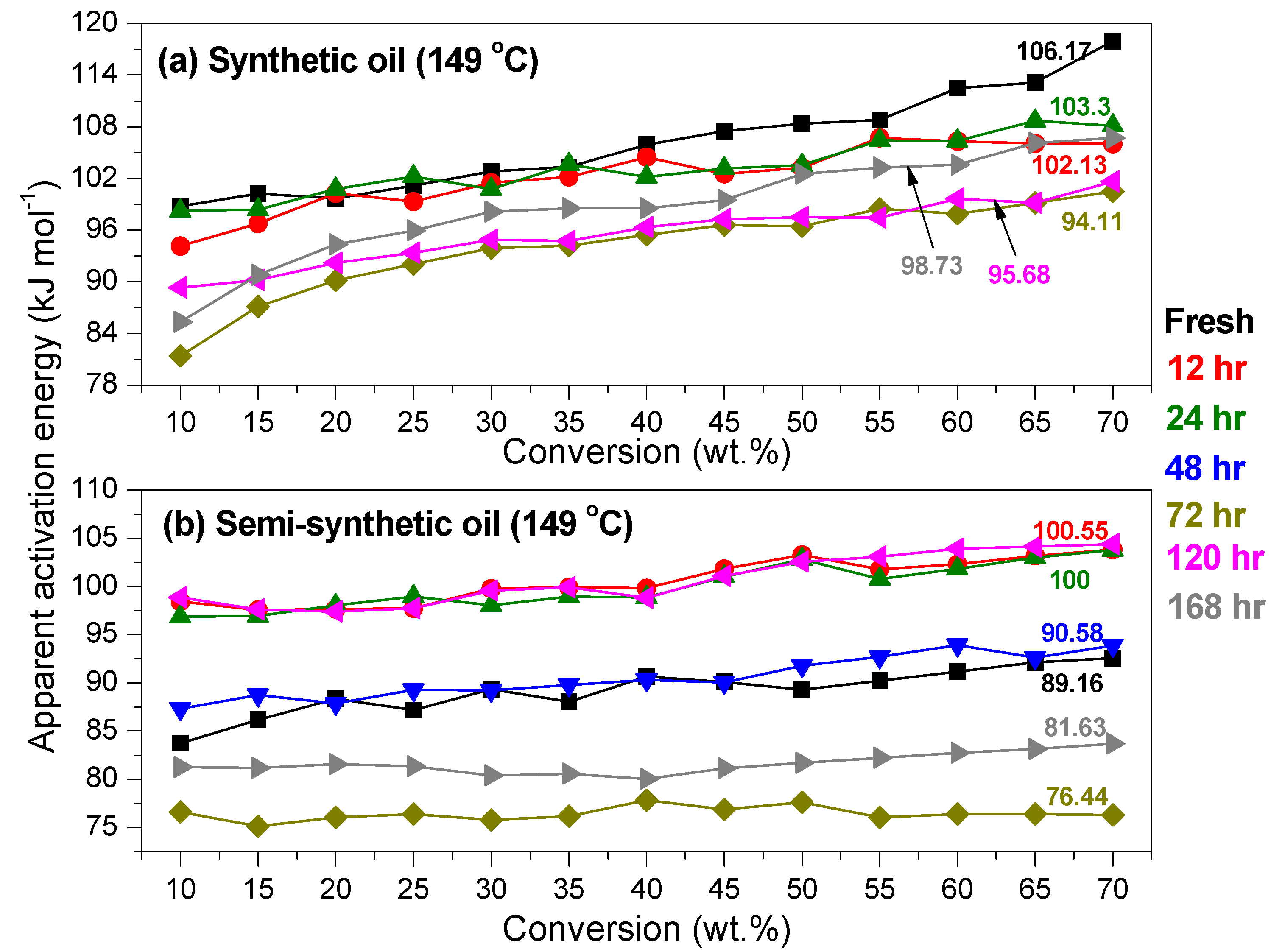
Kinetics of Ageing of Engine Oils
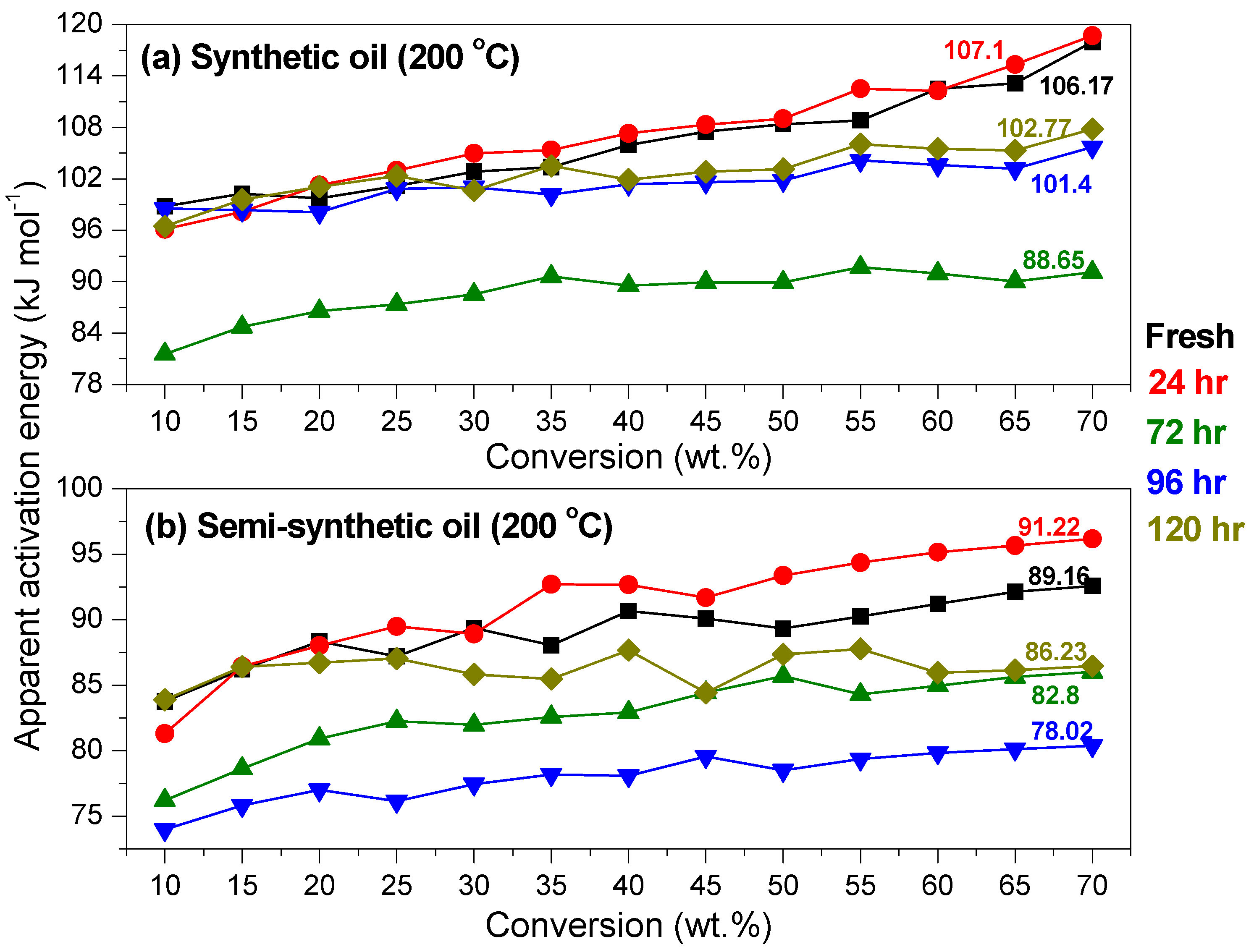
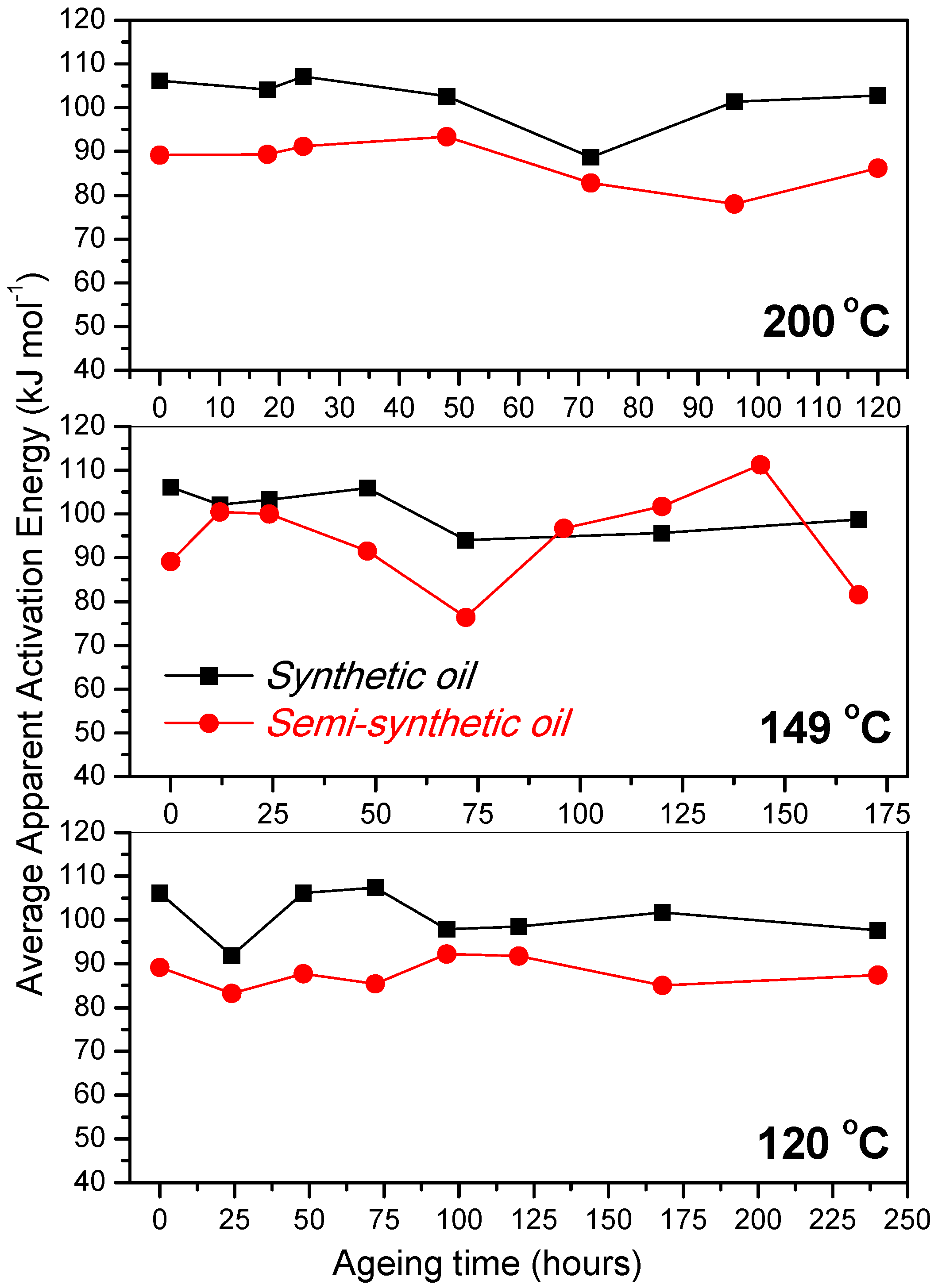
3.2. Variation of Physicochemical Properties of Oils with Ageing
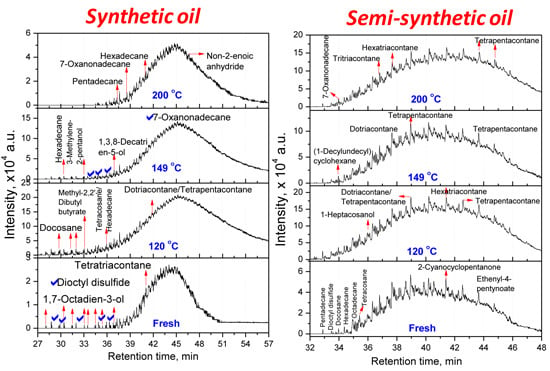
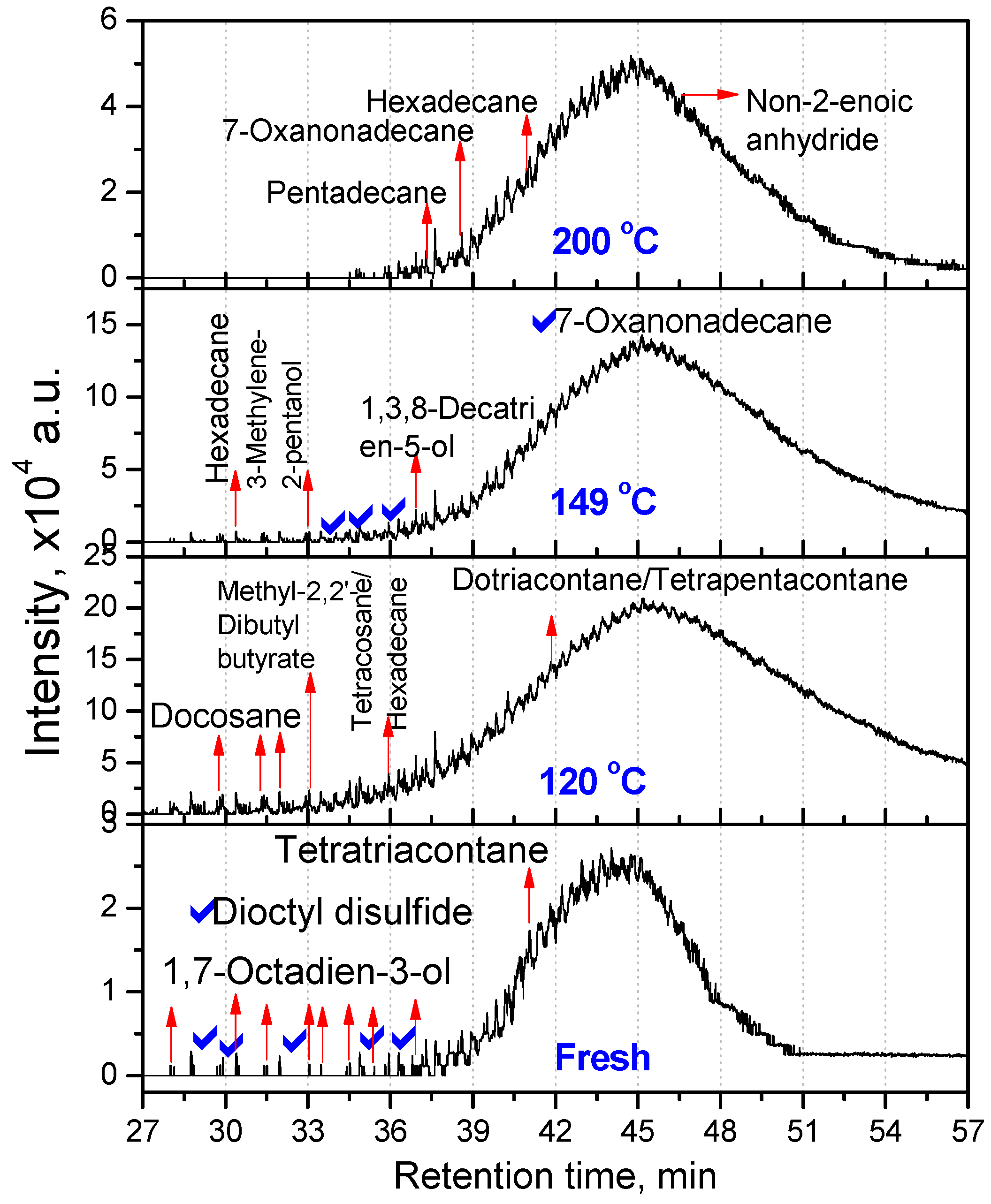
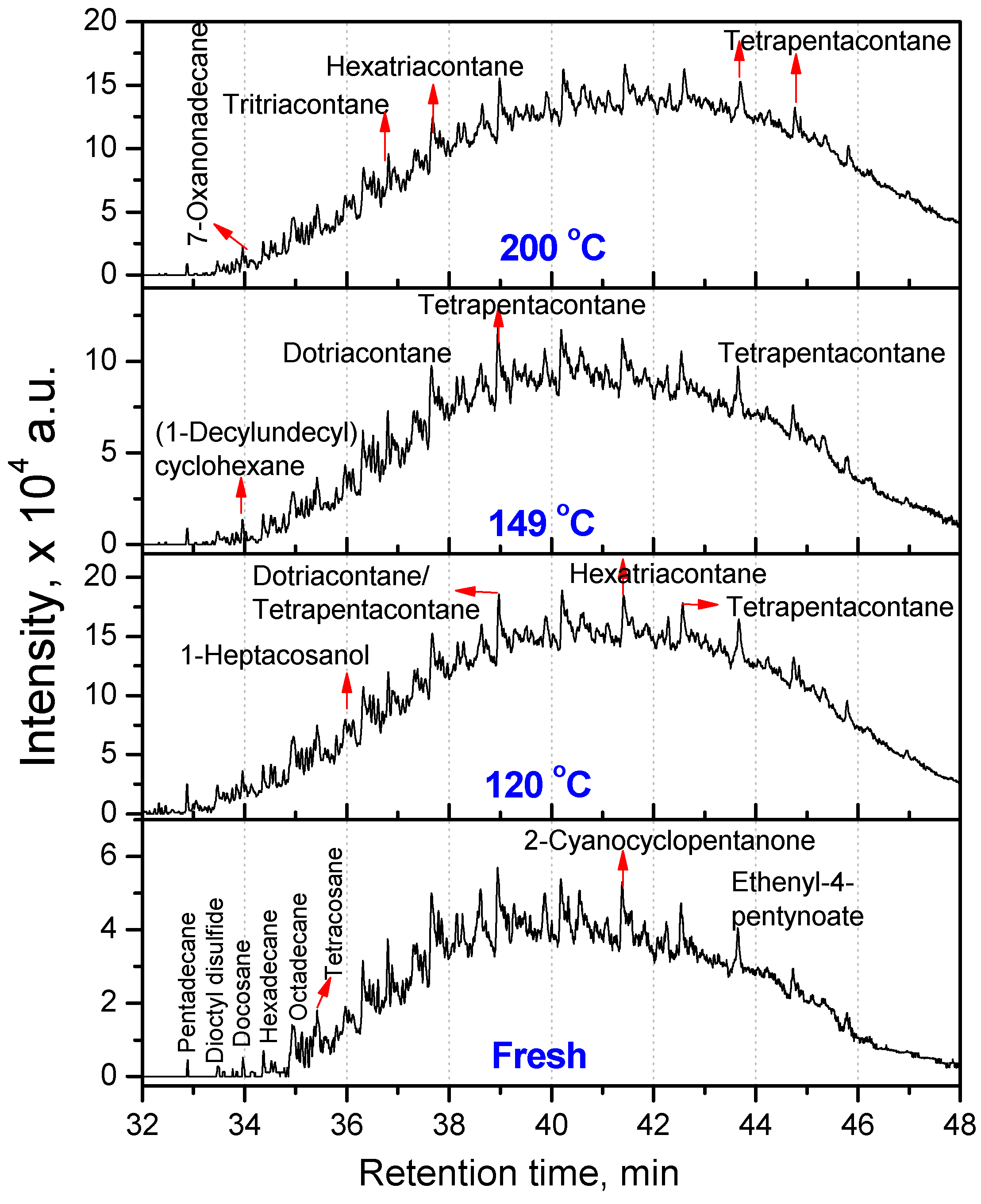
3.2.1. Composition of Aged Engine Oils by GC/MS
| Fresh oil | 120 °C | 149 °C | 200 °C |
|---|---|---|---|
| Synthetic Oil—Hydrocarbons | |||
| Tetratriacontane (C34) | Docosane (C22, 20.66) Tetracosane (C24, 16.08) Dotriacontane (C32, 20.81) Tetrapentacontane (C54, 35.55) Hexacontane (C60, 6.90) | Hexadecane (C16, 25.41) Tetrapentacontane (C54, 74.59) | Pentadecane (C15, 61.90) Hexadecane (C16, 38.10) |
| Semi-synthetic Oil—Hydrocarbons * | |||
| 3,3,4,4-Tetraethyl hexane (C14,22.58) Pentadecane (C15, 2.9) Hexadecane (C14, 6.45) Octadecane (C18, 7.66) 2,6,10,14-Tetramethyl Hexadecane (C20, 25.18) Docosane (C22, 11) Tetracosane (C24, 24.2) | Hexatriacontane (C36, 48.85) Tetrapentacontane (C54, 51.15) | 3-Ethyl-3-methyl decane (C13, 2.95) 3,9-Dimethyl undecane (C13, 2.75) 3,3,8-Trimethyl decane (C13, 2.57) 6-Methyl pentadecane (C16, 4.92) 1,1ʹ-(1,3-propanedi yl)bis-cyclohexane (C16, 5.27) Nonadecane (C19, 3.27) 2,6,10,14-Tetramethyl hexadecane (C20, 3.25) Tetracosane (C24, 2.01) 1-Decylundecyl cyclohexane (C27, 2.76) 2,6,10,15,19,23-Hexamethyl tetracosane (C30, 2.43) Dotriacontane (C32, 11.23) Hexatriacontane (C36, 8.05) Tetratetracontane (C44, 3.3) Tetrapentacontane (C54, 26) | Dotriacontane (C32, 45.21) Tritriacontane (C33, 10.87) Hexatriacontane (C36, 9.46) Tetrapentacontane (C54, 34.46) |
3.2.2. Kinematic Viscosity
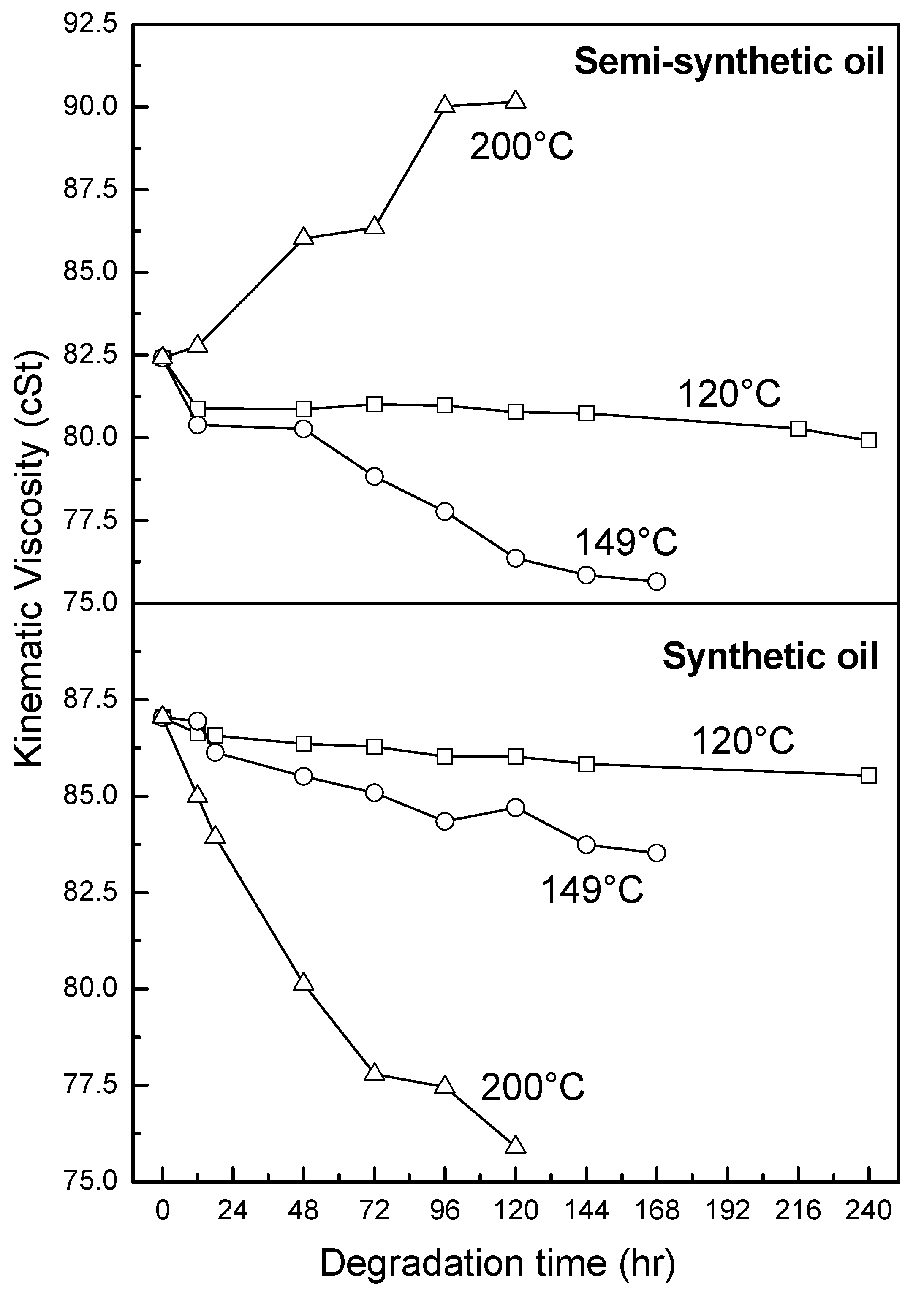
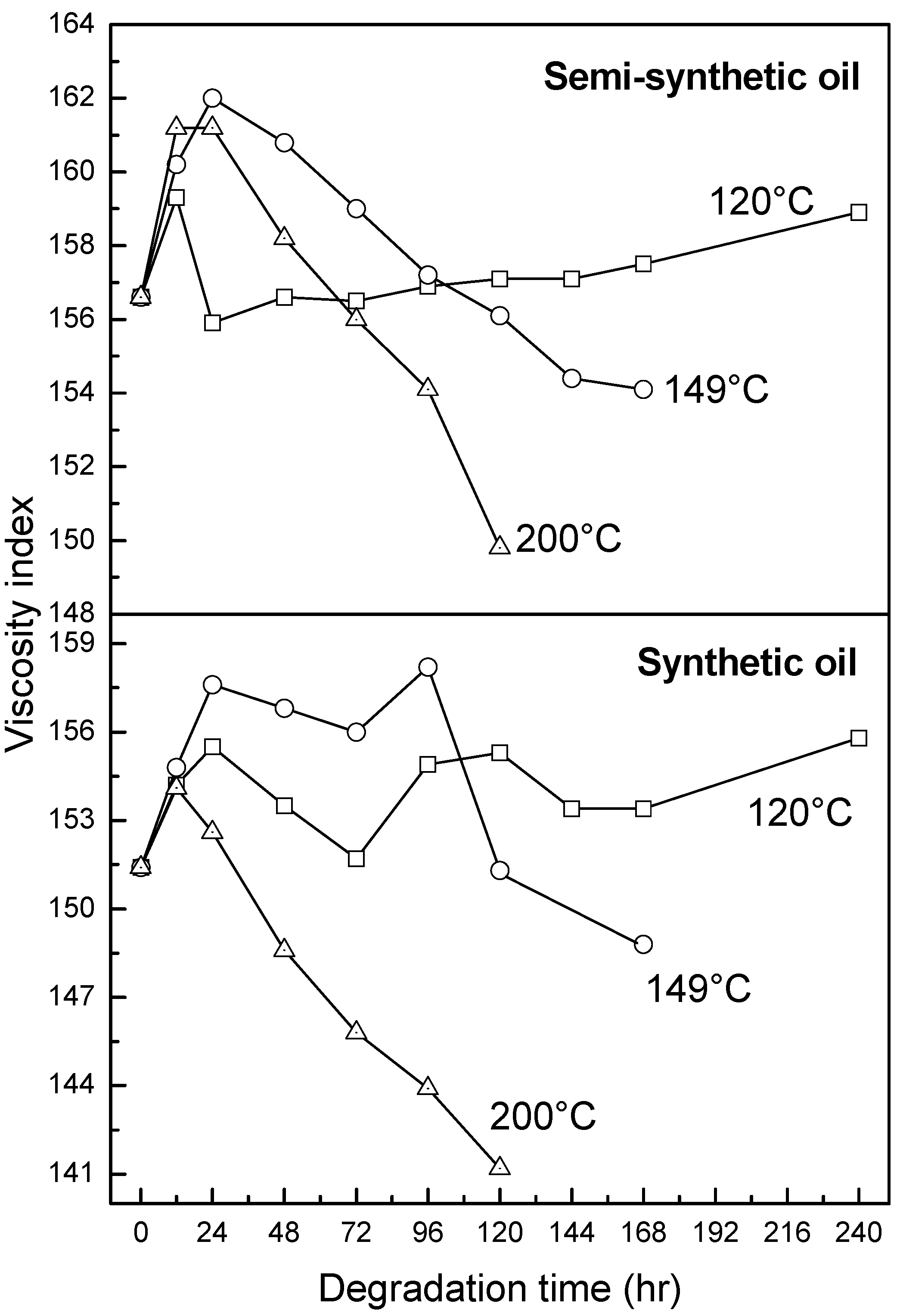
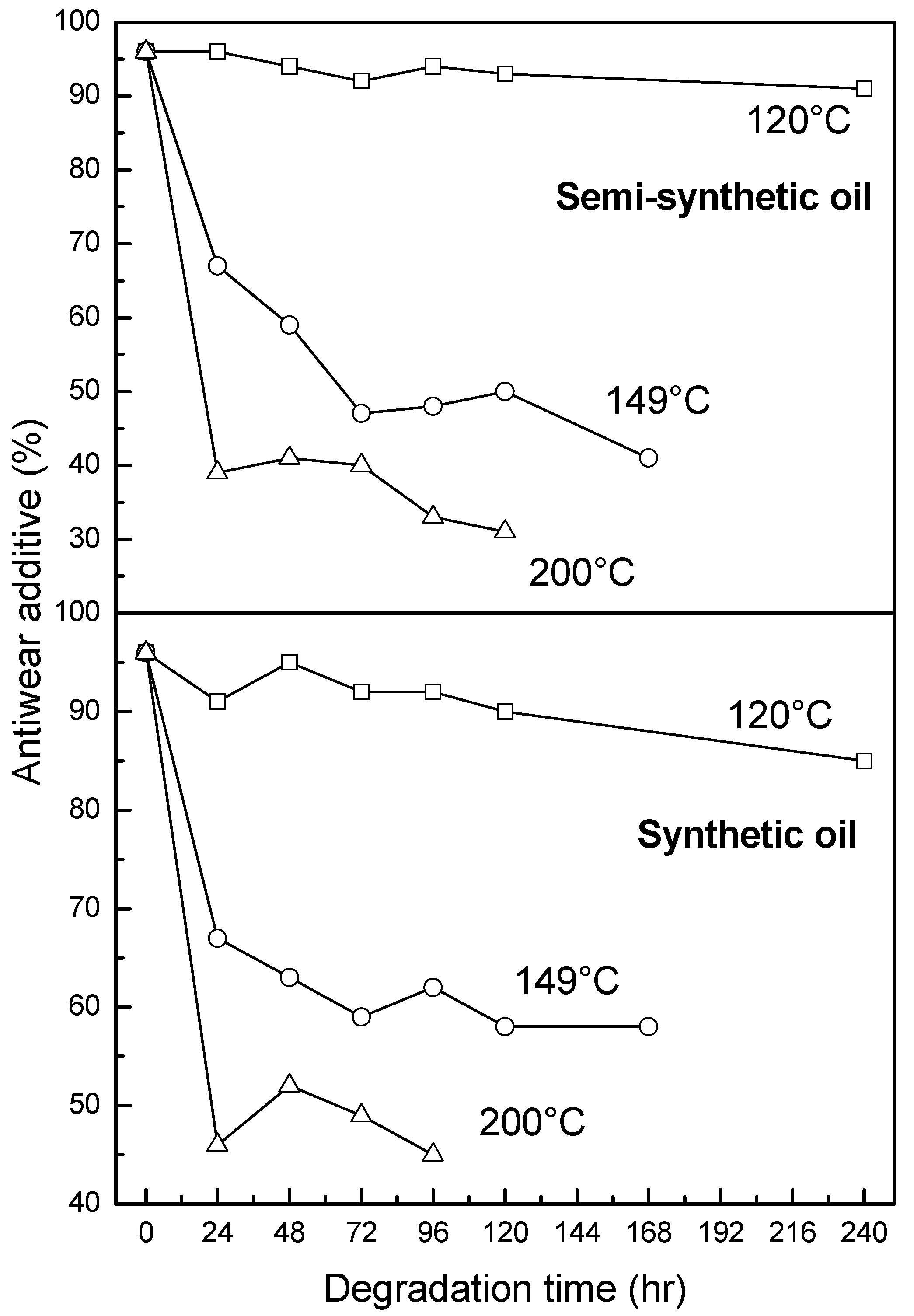
3.2.3. Antiwear Additive
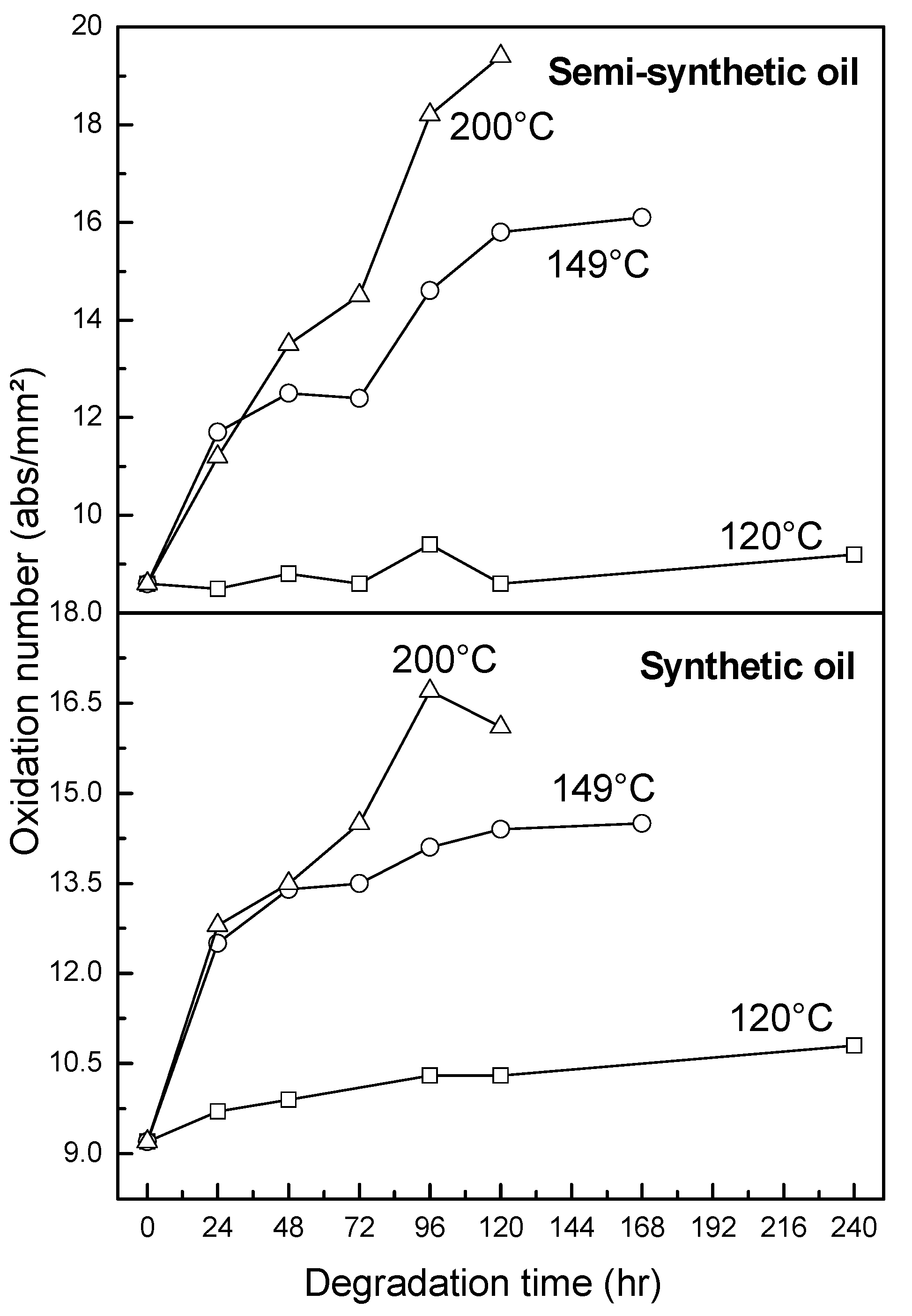
3.2.4. Oxidation, Sulfation and Nitration of Oils
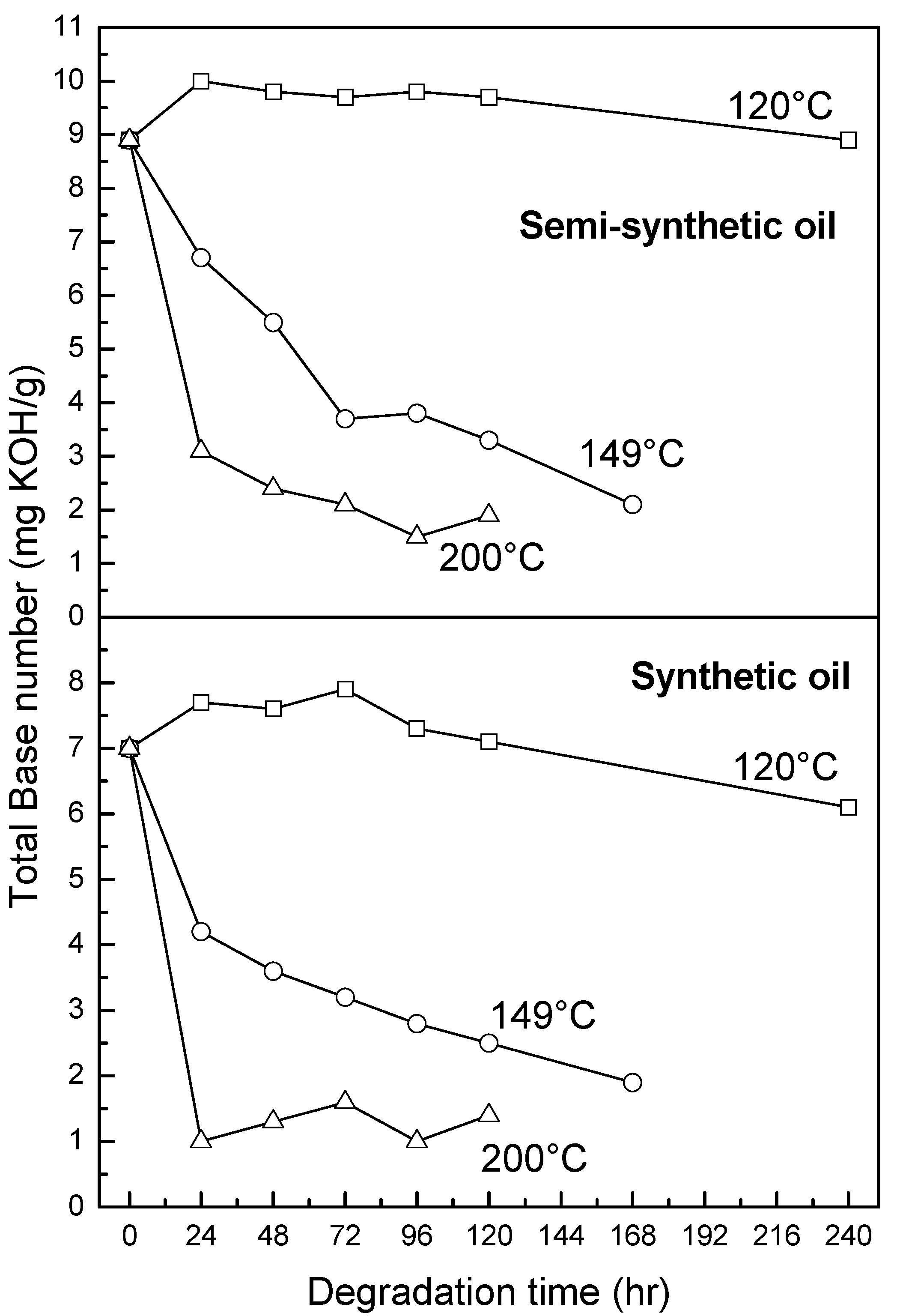
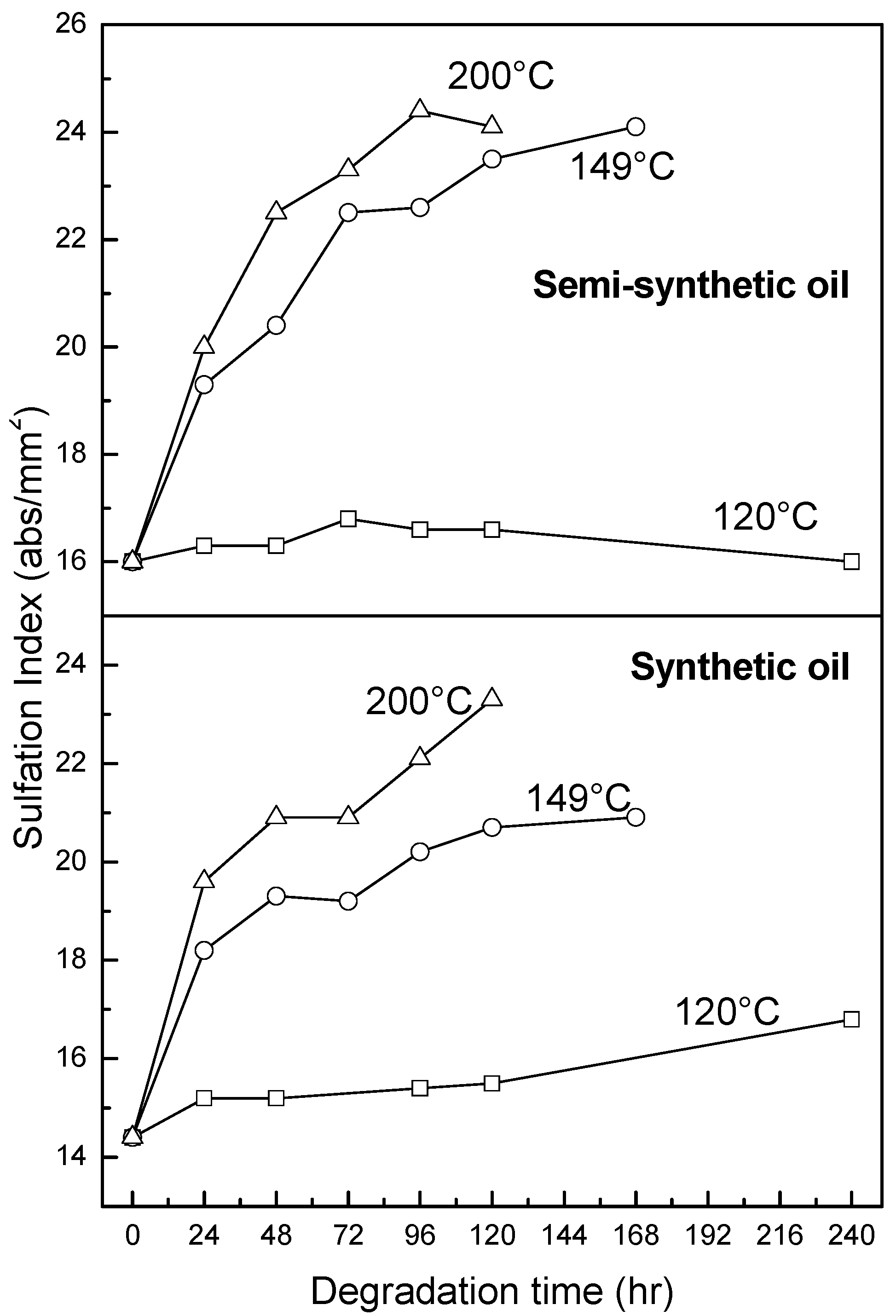
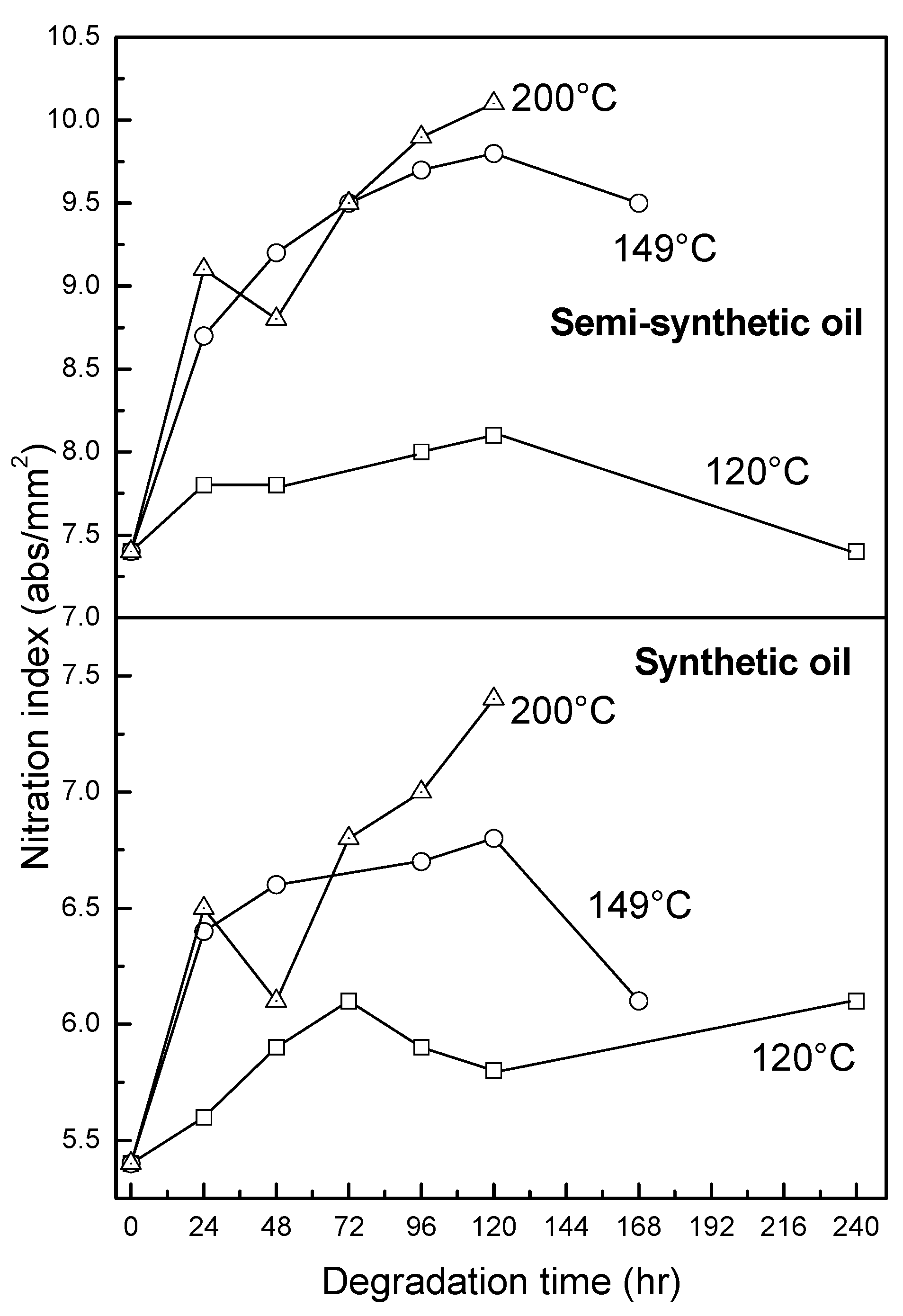
4. Conclusions
- (i)
- At an oxidation temperature of 120 °C, synthetic oil loses stability in the initial time period, while it gains stability at longer ageing periods. Contrastingly, semi-synthetic oil exhibits stability throughout the 240 h of ageing period at 120 °C. This can be correlated with the increase in oxidation number, sulfation index and decrease in antiwear additive content in the case of synthetic oil, while the variation of these quantities is insignificant in the case of semi-synthetic oil. This demonstrates that even though synthetic oil ( = 106.17 kJ mol−1) is intrinsically more stable than semi-synthetic oil ( = 89.16 kJ mol−1), the former loses stability at 120 °C owing to a relatively higher extent of oxidation. The constant value of and the formation of long chain hydrocarbons corroborates that semi-synthetic oil is oxidized to a relatively lesser extent than synthetic oil.
- (ii)
- At intermediate degradation temperatures of 149 °C, a number of linear, branched and cyclic hydrocarbons are formed from semi-synthetic oil. Moreover, the apparent activation energies at different conversions are nearly the same. The above observations show that the decomposition of semi-synthetic oil predominantly involves chain fission and hydrogen abstraction, and mid-chain β-scission as the rate determining pathways. The loss of antiwear additive was more pronounced in the case of semi-synthetic oil than synthetic oil. However, shorter chain saturated and unsaturated alcohols are formed from synthetic oils. The oxidation and sulfation indices increased in the initial 96 h of ageing and reached saturation at longer ageing periods.
- (iii)
- The average apparent activation energies of synthetic oil in the initial 24 h of degradation at 149 and 200 °C were found to be the same, which correlates well with a similar variation of oxidation index.
- (iv)
- At an ageing temperature of 200 °C, both synthetic and semi-synthetic oils exhibit similar trends in variation of . Around 72–96 h, the oils lose their thermal stability, while the stability is regained at long ageing periods. The gain in stability may be correlated with the decrease in oxidation index of synthetic oil after 96 h of ageing and increase in kinematic viscosity of semi-synthetic oil with ageing time. The increase in viscosity is due to the formation of long chain hydrocarbons. Both the oils rapidly lose their lubricity, as evidenced by the high rate of decrease of viscosity index.
- (v)
- The activation energy analysis and physicochemical characterization of fresh and aged engine oils discussed in this work will be useful to develop distributed activation energy models with pseudo components taking part in a multistep mechanism. The apparent activation energies evaluated in this work via KAS method can be used as initial estimates for a more robust semi-mechanistic model of engine oil oxidation.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liston, T.V. Engine lubricant additives. What they are and how they function? Lubr. Eng. 1992, 48, 389–397. [Google Scholar]
- Mortier, R.M.; Orszulik, S.T. Chemistry and Technology of Lubricants; VCH Publishers: New York, NY, USA, 1992. [Google Scholar]
- Syed, R. Lubricant additives. In A Comprehensive Review of Lubricants Chemistry, Technology, Selection, and Design; ASTM International: West Conshohocken, PA, USA, 2009. [Google Scholar] [CrossRef]
- Adhvaryu, A.; Perez, J.M.; Singh, I.D.; Tyagi, O.S. Spectroscopic studies of oxidative degradation of base oils. Energy Fuels 1998, 2, 1369–1374. [Google Scholar]
- Rose, H.R.; Smith, D.R.; Vassallo, A.M. Study of the oxidation of oil shale and kerogen by Fourier transform infrared emission spectrometry. Energy Fuels 1998, 12, 682–688. [Google Scholar]
- Dong, J.; van De Voort, F.R.; Ismail, A.A.; Akochi-Koble, E.; Pinchuk, D. Rapid determination of the carboxylic acid contribution to the total acid number of lubricants by Fourier transform infrared spectroscopy. Lubr. Eng. 2000, 56, 12–20. [Google Scholar]
- Adams, M.J.; Romeo, M.J.; Rawson, P. FTIR analysis and monitoring of synthetic aviation engine oils. Talanta 2007, 73, 629–634. [Google Scholar]
- Al-Ghouti, M.A.; Al-Atoum, L. Virgin and recycled engine oil differentiation: A spectroscopic study. J. Environ. Manag. 2009, 90, 187–195. [Google Scholar]
- Dominguez-Rosado, E.; Pichtel, J. Chemical characterization of fresh, used and weathered motor oil via GC/MS, NMR and FTIR techniques. Proc. Indiana Acad. Sci. 2003, 112, 109–116. [Google Scholar]
- Adhvaryu, A.; Perez, J.M.; Singh, I.D. Application of quantitative NMR spectroscopy to oxidation kinetics of base oils using a pressurized differential scanning calorimetry technique. Energy Fuels 1999, 13, 493–498. [Google Scholar]
- Owrang, F.; Mattsson, H.; Olsson, J.; Pedersen, J. Investigation of oxidation of a mineral and a synthetic engine oil. Thermochim. Acta 2004, 413, 241–248. [Google Scholar]
- Bowman, W.F.; Stachowiak, G.W. Application of sealed capsule differential scanning calorimetry. Part II: Assessing the performance of antioxidants and base oils. Lubr. Eng. 1999, 55, 22–29. [Google Scholar]
- Santos, J.C.O.; dos Santos, I.M.G.; Souza, A.G.; Sobrinho, E.V.; Fernandes, V.J.; Silva, A.J.N. Thermoanalytical and rheological characterization of automotive mineral lubricants after thermal degradation. Fuel 2004, 83, 2393–2399. [Google Scholar]
- Gamlin, C.D.; Dutta, N.K.; Choudhury, N.R.; Kehoe, D.; Matisons, J. Evaluation of kinetic parameters of thermal and oxidative decomposition of base oils by conventional, isothermal and modulated TGA, and pressure DSC. Thermochim. Acta 2002, 393, 357–369. [Google Scholar]
- Crnkovic, P.M.; Leiva, C.R.M.; dos Santos, A.M.; Milioli, F.E. Kinetic study of the oxidative degradation of Brazilian fuel oils. Energy Fuels 2007, 21, 3415–3419. [Google Scholar]
- Barman, B.N. Behavioral differences between group I and group II base oils during thermo-oxidative degradation. Tribol. Int. 2002, 35, 15–26. [Google Scholar]
- Spearot, J.A. Viscosity of severely oxidized engine oil. Ind. Eng. Chem. Prod. Res. Dev. 1974, 13, 259–267. [Google Scholar]
- Cerny, J.; Strnad, Z.; Sebor, G. Composition and oxidation stability of SAE 15W-40 engine oils. Tribol. Int. 2001, 34, 127–134. [Google Scholar]
- Adhvaryu, A.; Sharma, Y.K.; Singh, I.D. Studies on the oxidative behavior of base oils and their chromatographic fractions. Fuel 1999, 78, 1293–1302. [Google Scholar]
- Baker, A.E. Hand Book of Lubrication. Theory and Practice of Tribology; Booser, E.R., Ed.; CRC Press: Boca Raton, FL, USA, 1983; Volume 1, p. 481. [Google Scholar]
- Wooton, D. The lubricant’s nemesis—Oxidation. Practicing Oil Analysis. 2007. Available online: http://www.machinerylubrication.com/Articles/Print/999 (accessed on 20 July 2014).
- Four Stroke Engine Oils. Available online: http://www.shell.com/global/products-services/on-the-road/oils-lubricants/motorcycles/find-right-bike-oils/4-stroke-engine-oils.html (accessed on 30 July 2014).
- Standard test method for evaluation of engine oils for roller follower wear in light-duty diesel engine. ASTM International: West Conshohocken, PA, USA, 2013. [CrossRef]
- Standard test method for evaluation of automotive engine oils in the sequence IIIE, spark-ignition engine. ASTM International: West Conshohocken, PA, USA, 1998. [CrossRef]
- Overview of FluidScan® handheld infrared oil analyzer. Spectro Inc. Available online: http://www.spectrosci.com/products/product/q1100/ (accessed on 15 July 2014).
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar]
- Lehrle, R.S.; Duncan, R.; Liu, Y.; Parsons, I.W.; Rollinson, M.; Lamb, G.; Barr, D. Mass spectrometric methods for assessing the thermal stability of liquid polymers and oils: study of some liquid polyisobutylenes used in the production of crankcase oil additives. J. Anal. Appl. Pyrol. 2002, 64, 207–227. [Google Scholar]
- Vinu, R.; Broadbelt, L.J. Unraveling reaction pathways and specifying reaction kinetics for complex systems. Annu. Rev. Chem. Biomol. Eng. 2012, 3, 29–54. [Google Scholar]
- Roegiers, M.; Zhmud, B. Tribological performance of ionized vegetable oils as lubricity and fatty oiliness additives in lubricants and fuels. Lube 2008, 19–26. [Google Scholar]
- Maleville, X.; Faure, D.; Legros, A.; Hipeaux, J.C. Oxidation of mineral base oils of petroleum origin: The relationship between chemical composition, thickening, and composition of degradation products. Lubr. Sci. 1996, 9, 1–60. [Google Scholar]
- Pfaendtner, J.; Broadbelt, L.J. Mechanistic modelling of lubricant degradation. 1. Structure-reactivity relationships for free-radical oxidation. Ind. Eng. Chem. Res. 2008, 47, 2886–2896. [Google Scholar]
- Pfaendtner, J.; Broadbelt, L.J. Mechanistic modelling of lubricant degradation. 2. The autoxidation of decane and octane. Ind. Eng. Chem. Res. 2008, 47, 2897–2904. [Google Scholar]
- Blaine, S.; Savage, P.E. Reaction pathways in lubricant degradation. 3. Reaction model for n-hexadecane autoxidation. Ind. Eng. Chem. Res. 1992, 31, 69–75. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tripathi, A.K.; Vinu, R. Characterization of Thermal Stability of Synthetic and Semi-Synthetic Engine Oils. Lubricants 2015, 3, 54-79. https://doi.org/10.3390/lubricants3010054
Tripathi AK, Vinu R. Characterization of Thermal Stability of Synthetic and Semi-Synthetic Engine Oils. Lubricants. 2015; 3(1):54-79. https://doi.org/10.3390/lubricants3010054
Chicago/Turabian StyleTripathi, Anand Kumar, and Ravikrishnan Vinu. 2015. "Characterization of Thermal Stability of Synthetic and Semi-Synthetic Engine Oils" Lubricants 3, no. 1: 54-79. https://doi.org/10.3390/lubricants3010054
APA StyleTripathi, A. K., & Vinu, R. (2015). Characterization of Thermal Stability of Synthetic and Semi-Synthetic Engine Oils. Lubricants, 3(1), 54-79. https://doi.org/10.3390/lubricants3010054