Abstract
A finger prosthesis consisting of a Cobalt-chromium (CoCr) proximal component and an Ultra-high-molecular-weight-polyethylene (UHMWPE) medial component (both mounted on hydroxyapatite coated stems) was evaluated to 5,000,000 cycles in an in vitro finger simulator. One “test” prosthesis was cycled through flexion-extension (90°–30°) with a dynamic load of 10 N, whilst immersed in a lubricant of dilute bovine serum. Additionally, a static load of 100 N was applied for 45 s every 3000 cycles to simulate a static gripping force. A second “control” prosthesis was immersed in the same lubricant to account for absorption. Gravimetric and Sa (3D roughness) measurements were taken at 1,000,000 cycle intervals. Micrographs and Sa values revealed negligible change to the CoCr surfaces after 5,000,000 cycles. The UHMWPE also exhibited no distinctive Sa trend, however the micrographs indicate that polishing occurred. Both the CoCr and UHMWPE test components progressively decreased in weight. The CoCr control component did not change in weight, whilst the UHMWPE component gained weight through absorption. To account for the disparity between surface and gravimetric results, the hydroxyapatite coatings were examined. Micrographs of the test stems revealed that the hydroxyapatite coating was partially removed, whilst the micrographs of the control stems exhibited a uniform coating.
1. Introduction
The proximal interphalangeal (PIP) is the joint that connects the first and second phalanges of the finger. The two main indications for surgical replacement of the joint (arthroplasty) are osteoarthritis and rheumatoid-arthritis, both of which affect mainly women over the age of 50 [1,2]. The Swanson single piece silicone prosthesis is considered to be the “replacement of choice” for finger joint arthroplasty [3], and has been used extensively [4,5,6,7,8]. It has, however, been argued that surface replacements (SR) with a bicondylar design have the potential to restore greater post-operative function and range of motion [3], since they replicate the natural anatomy of the PIP joint. The prosthesis used in this present study is such a SR design.
The MatOrtho proximal interphalangeal replacement (PIPR), formerly the Finsbury PIPR (Figure 1a), is an anatomical SR prosthesis used for arthroplasty of the PIP joint. The articulating surface materials are Cobalt-Chromium (CoCr) for the proximal component; and Ultra High Molecular Weight Polyethylene (UHMWPE) for the medial component; both of which are mounted on CoCr stems with an external hydroxyapatite coating. A short-term study [9], with a minimum follow up of 12 months, evaluated 43 implanted prostheses and. reported improvements in pain, function and range of motion. However it has been acknowledged that longer-term study is required to assess the longevity of the prosthesis [10].
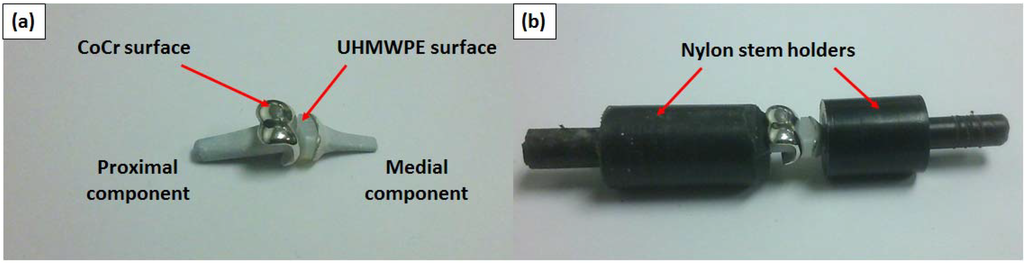
Figure 1.
(a) The unconstrained prosthesis pair with articulating surfaces labelled and (b) the prosthesis pair constrained in the nylon stem holders.
The Small Bone Innovations (formerly Avanta Orthopaedics) SR PIP (surface replacement proximal interphalangeal prosthesis) has yielded mixed results clinically [11,12,13,14]. This prosthesis has the same articulating surface materials (CoCr and UHMWPE), but is mounted on porous titanium stems rather than hydroxyapatite coated CoCr stems. Two clinical studies with mean follow-up periods of 15 [12] and 27 months [14] evaluated 67 and 20 PIP prostheses respectively. Both studies found significantly reduced pain and negligible loss in the preoperative range of motion. However, high rates of loosening have also been reported for the SR PIP, with one study reporting a 33% rate of loosening [11] and another reporting a 68% rate [13]. This loosening indicates that the titanium stems have not integrated well with the cancellous bone.
A different type of CoCr-UHMWPE prosthesis (the Kls-Martin CapFlex PIP) was recently evaluated [15], with 10 prostheses observed over 12 months. This study reported no cases of loosening with overall improvements to strength, range of motion and pain relief. The key difference between this prosthesis and the two other mentioned anatomical prostheses (SR PIP and MatOrtho PIPR) is a shorter central stem, with additional short stems either side to improve lateral joint stability.
To date, no in vitro wear testing of any of the above mentioned CoCr on UHMWPE prosthesis designs has been reported in the scientific literature, despite being implanted into hundreds of people. The advantage of in vitro testing is that throughout the duration of testing, test conditions can be carefully controlled. Testing can also be paused at regular intervals to allow for precise measurements to be taken. With a clinical study, the prostheses are in situ limiting the analysis that can be performed. The aim of this present study was to determine the wear characteristics of the CoCr and UHMWPE surfaces through such a program of in vitro wear testing.
2. Methods
A MatOrtho PIPR size 8 proximal (CoCr) component with a nominal weight of 1.55 g was tested against a size 7 medial (UHMWPE) component with a nominal weight of 0.45 g (Figure 1a). This combination was tested to five million cycles of flexion-extension, with a 60° arc of motion used to represent the functional “day to day” range of motion in the hand [16]. Other recommended test conditions [17] were followed, including: Limiting the test frequency to 1.5 Hz; and use of dilute bovine serum (maintained at a temperature of 37 °C) as a lubricant. The protein concentration used was 20 g/L, which met the requirements for testing joint prostheses in vitro [18,19]. It has been demonstrated in a previous study [20] that CoCr-UHMWPE prostheses adhere to a boundary lubrication regime when tested in dilute bovine serum at low frequency. This was the case for prostheses with equivalent spherical radii ranging from 3 to 10 mm. It is evident that boundary lubrication would not be desirable for prosthesis pairs of the same material (metal-on-metal, ceramic-on-ceramic); however, a previous in vitro experiment [21] found that a metal-on-polymer prosthesis had relatively low wear after five million cycles of flexion-extension, despite adhering to an apparent boundary lubrication regime.
The same combination of sizes was used as a “control” prosthesis pair, which did not undergo any flexion-extension cycles but was however immersed in the same lubricant in the test chamber to account for any lubricant uptake. The test regime was identical to that used by Naylor et al. for the evaluation of the Ascension Pyrocarbon PIPJ [22,23], and the in vitro finger simulator (Figure 2) has been described in much greater detail elsewhere [6,24,25]. A brief description of the test procedure and simulator is provided below.
The flexion-extension mechanism was driven by two small (10 mm diameter) pneumatic cylinders, the pistons of which were connected to braided polyethylene “flexor” and “extensor” tendons which translated through a pulley system to connect to the nylon medial component holder. The proximal component remained stationary throughout testing, with the medial component holder oscillating about the axis of the proximal component. Regulators were used to ensure that the pressure entering each of the dynamic cylinders was 1.3 bar, which in turn was measured using pressure gauges. This resulted in dynamic loads of approximately 10 N in both flexion and extension, which is consistent with dynamic finger activity such as operating a keyboard [26].
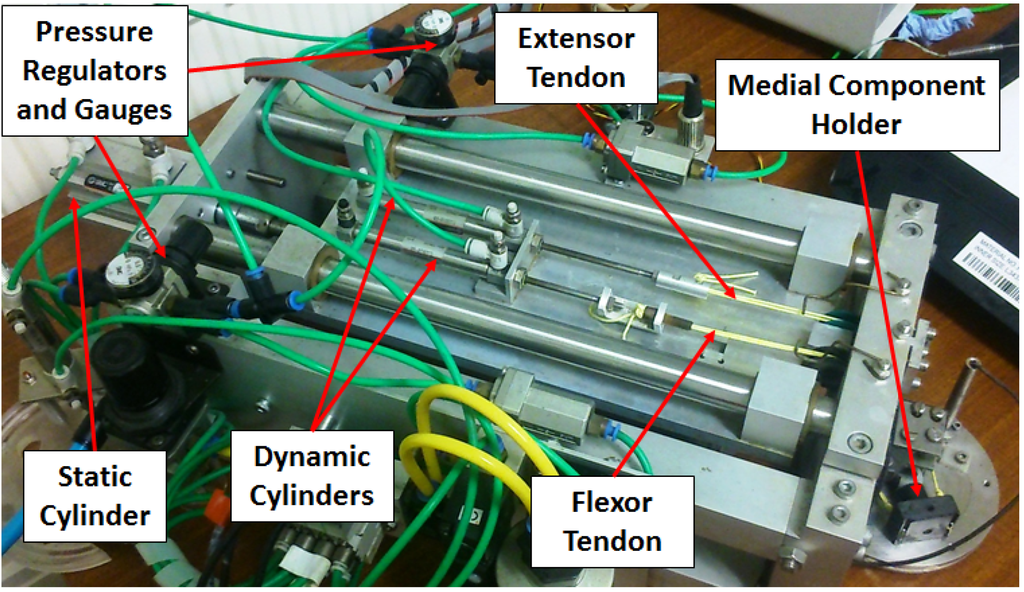
Figure 2.
The finger simulator used to conduct the experimentation.
To provide a biomechanical simulation of gripping and pinching, the dynamic cylinders momentarily paused at 3000 cycles to allow for a larger static cylinder (32 mm diameter) to actuate. This cylinder moved the entire platform on which the dynamic cylinders were fixed, subsequently pulling the flexor tendon in tension (air was dumped from the extensor cylinder). As with the dynamic cylinders, a regulator was used to ensure that the pressure entering the “static” cylinder was 1.3 bar, verified by an inline pressure gage. This resulted in a static load of approximately 100 N, which conforms to the strength of the arthritic finger [27,28].
Nylon has been commonly used as an orthopaedic bone substitute [29,30], with a yield stress similar to cortical bone ranging from approximately 50–100 MPa and hence was selected to be an appropriate “bone substitute” material for the stem holders (Figure 1b). A light push fit was incorporated into the design of the stem holders to prevent displacement during flexion-extension. Such a holder and fit allowed the test components to be removed for topographic and gravimetric measurements.
Gravimetric measurements were taken at one million cycle intervals, for a total of five million cycles. First the prosthetic components were removed and sterilized using Virkon disinfectant. The articulating surfaces were then subject to further cleaning using isopropanol and lint free cloths. After allowing approximately half an hour for any residual moisture to evaporate, the prosthetic components were then weighed using a Denver Instruments TB215D balance to a sensitivity of 0.1 mg. The CoCr proximal and UHMWPE medial components were weighed separately to the nearest 0.1 mg to determine any gravimetric change. 3D roughness (Sa) values of the articulating surfaces were obtained using a ZYGO NewView 5000 optical surface profiler with a resolution of 1 nm [31]. For the proximal component five Sa measurements were taken at and around the apex of each of the two CoCr condyles. The geometric constraints of the UHMWPE medial component limited the surface roughness measurements to one at each of the “poles” of the UHMWPE convex surfaces. This resulted in ten Sa measurements for each CoCr surface and a further two values for the UHMWPE surfaces. As with the gravimetric measurements, this process was conducted prior to testing, then at one million cycle intervals during testing. Micrographic \analysis of the stems was also conducted using the optical surface profiler to provide a visual comparison of the test and control stems at the end of testing.
3. Results
3.1. Surface Analysis
Prior to testing, the measured Sa values for the CoCr test surfaces ranged from 28 to 57 nm, compared with 34 to 81 nm for the control. At five million test cycles the Sa values for the test surfaces ranged from 35 to 68 nm, compared with 26 to 53 nm for the control. Sa values for the UHMWPE surfaces taken across five million cycles ranged from 1100 to 2900 nm, which although two orders of magnitude higher than the CoCr components, is still representative of roughness values for UHMWPE finger components [20,32]. No distinctive trends emerged from the Sa measurements for either the CoCr or the UHMWPE test components over the five million test cycles (Figure 3). Correspondingly, micrographic images of the CoCr surfaces exhibited little or no change after five million cycles (Figure 4). Micro-machining marks were visible on the surface of the UHMWPE test surfaces prior to testing, but had been removed by five million cycles (Figure 5).
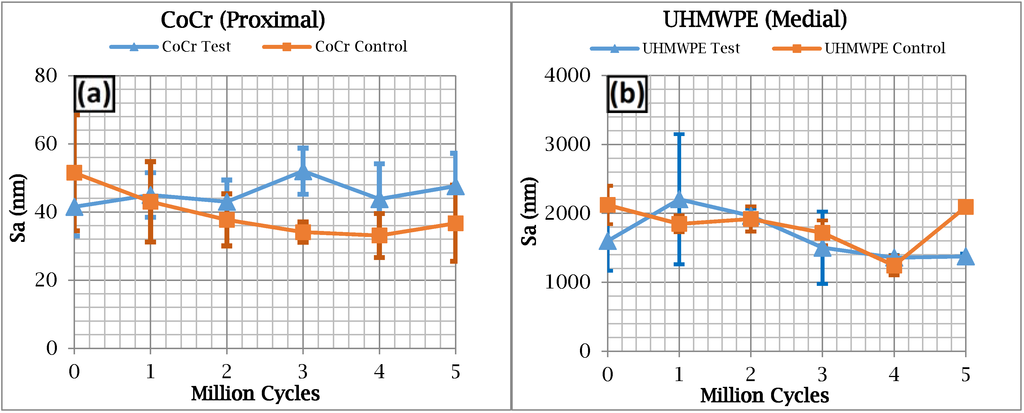
Figure 3.
3D surface roughness (Sa) results for (a) the cobalt-chromium (CoCr) proximal surfaces and (b) the ultra-high-molecular-weight-polyethylene (UHMWPE) surfaces.
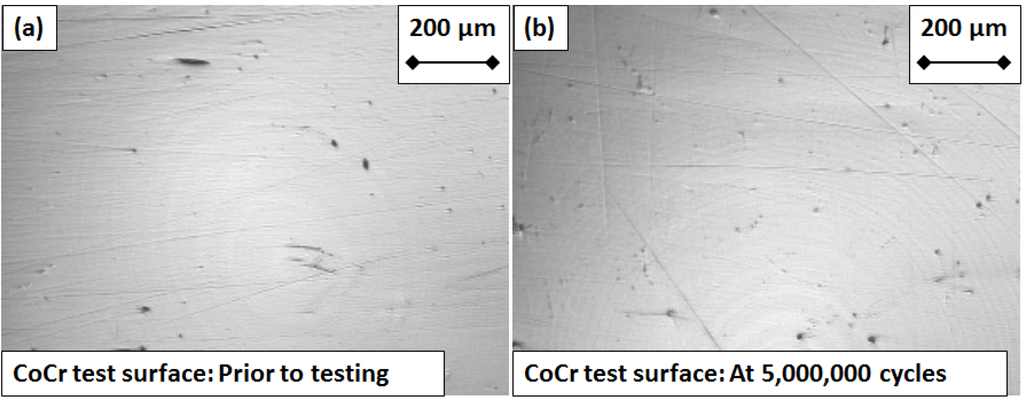
Figure 4.
Micrograph images of the CoCr articulating “test” surface (a) prior to testing and (b) after five million cycles of flexion and extension.
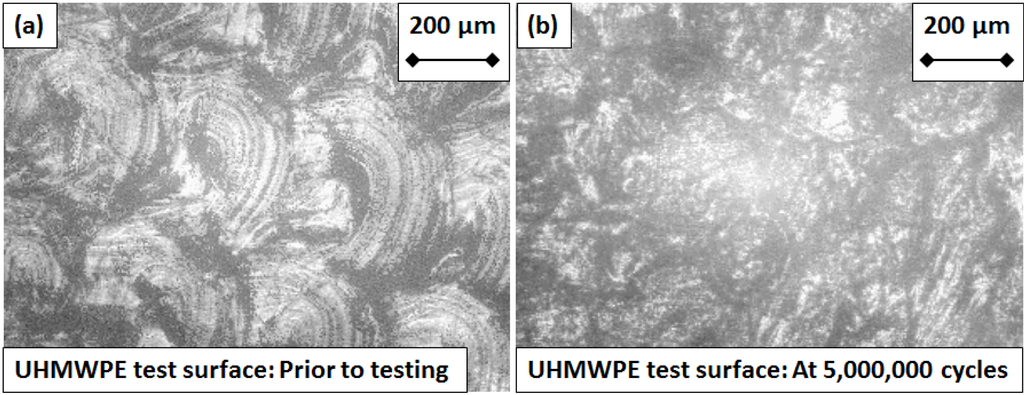
Figure 5.
Micrograph images of the UHMWPE articulating “test” surface (a) prior to testing and (b) after five million cycles of flexion and extension.
3.2. Gravimetric Analysis
In contrast to the surface roughness evaluation, a distinctive trend was exhibited for change in mass (Δm) over the five million cycles for both CoCr proximal and the UHMWPE medial test components (Figure 6a). The apparent rate of gravimetric loss for the proximal CoCr test component was approximately 0.7 mg per million cycles, and 0.3 mg per million cycles for the medial UHMWPE test component. The control CoCr proximal component exhibited a negligible change in mass over the five million test cycles. In comparison the control medial UHMWPE component gained mass through the absorption of bovine serum at a substantial rate of 0.1 mg per million test cycles. The gain in mass of the control components was subtracted from the absolute gravimetric values (Figure 6a) to provide the more realistic relative gravimetric values (Figure 6b). These compensated rates of gravimetric loss were 0.7 mg and 0.4 mg per million cycles for the proximal CoCr and medial UHMWPE test components respectively.
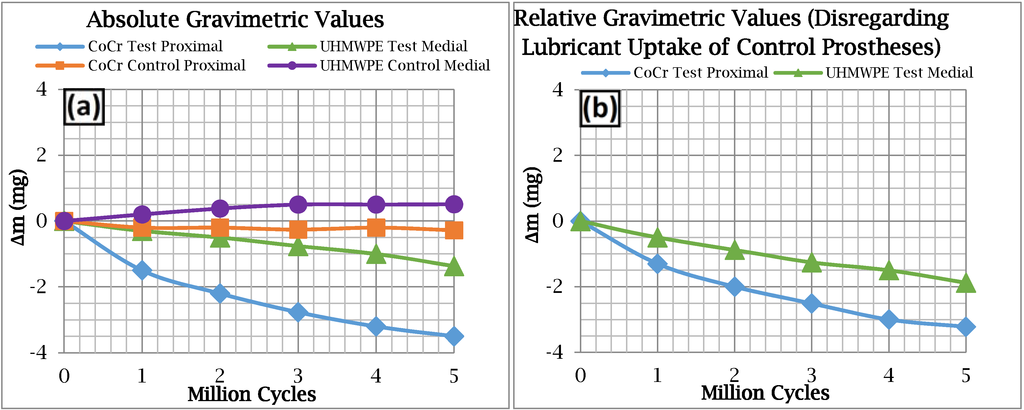
Figure 6.
Gravimetric results for (a) all of the test and control components; and (b) The CoCr and UHMWPE test components minus the “soak” acquired by the control components.
3.3. Micrographic Analysis of the Stems
For the control prosthesis, the stems of both the proximal and medial components exhibited a uniform coating of hydroxyapatite at five million cycles of testing (Figure 7). In contrast, for the test prosthesis, the stems of the proximal and medial components exhibited a less uniform coating of hydroxyapatite (Figure 8), showing areas where the coating has been partially removed.
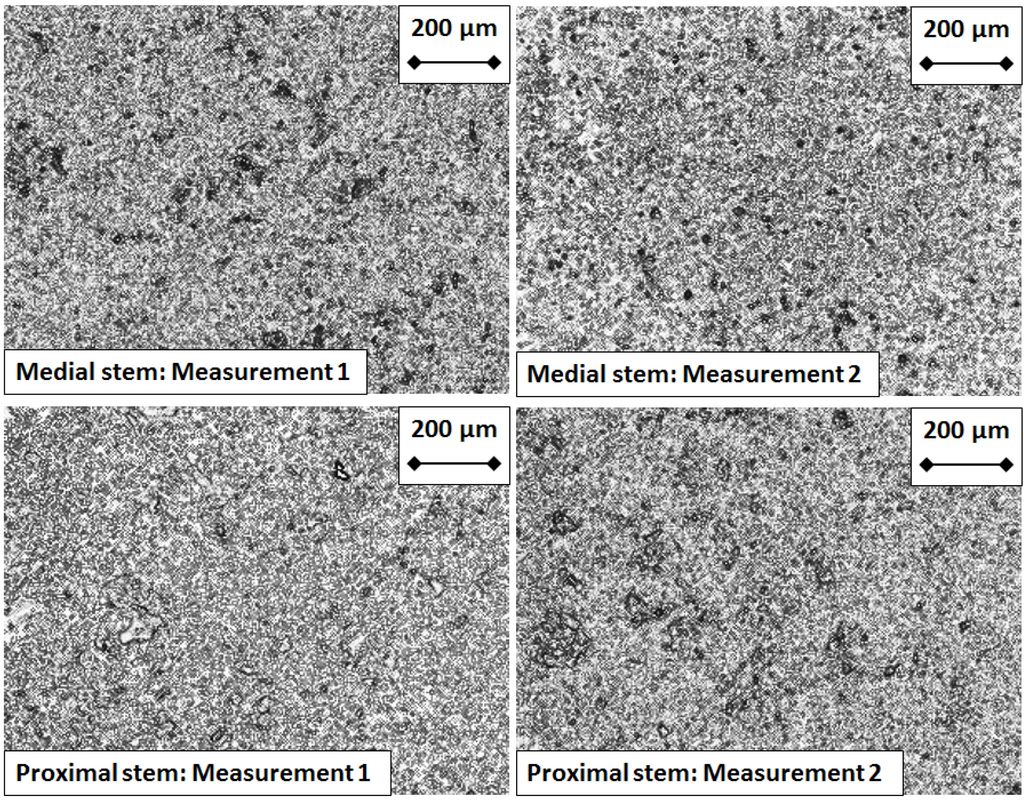
Figure 7.
Micrographs of the Hydroxyapatite coating taken from the control prosthesis proximal and medial stems, at the end of experimentation.
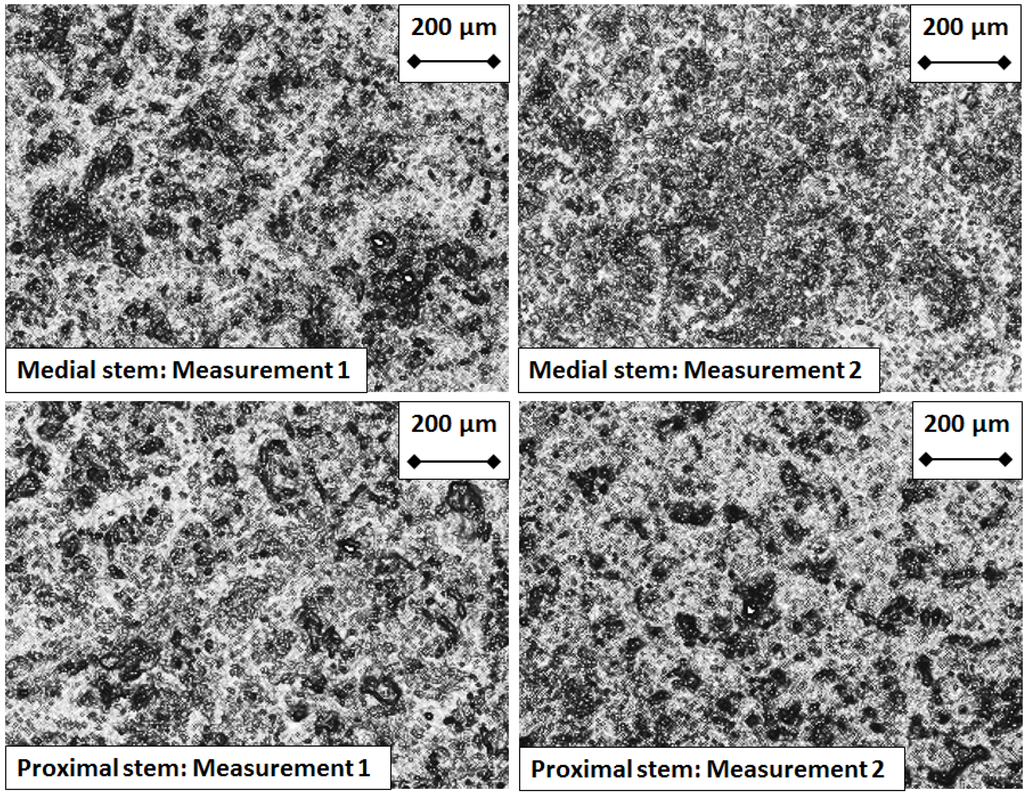
Figure 8.
Micrographs of the Hydroxyapatite coating taken from the test prosthesis proximal and medial stems, after five million cycles of flexion and extension.
4. Discussion
No relationship was found between the roughness of the articulating surfaces and the progressive number of test cycles (Figure 3). This was supported by the fact that, visually, the CoCr test surface did not exhibit any signs of wear at five million test cycles (Figure 4). The micro-machining marks visible on the UHMWPE test surface prior to testing gradually faded with each million cycles of testing, yet as with the CoCr surface, neither an increase nor decrease in Sa values were observed over the duration of the experiment. The gradual fading of the micro-machining marks was consistent with a polishing effect.
From a previous in vitro study [32], comparative roughness values, taken from the articulating surfaces of a stainless steel (SS) versus UHMWPE two-part finger prosthesis (Digital Joint Operative Arthroplasty (DJOA); Landos, Chaumont, France) provided evidence of a polishing effect on the UHMWPE. Initially the UHMWPE surface had a roughness of 1300 nm, which decreased to 270 nm after seven million cycles of flexion-extension testing. Measurements were made with a Mitutoyo Form-tracer 4100, providing roughness in 2D rather than the 3D offered by the ZYGO NewView 5000 optical profiler. The SS surface had an initial roughness of 147 nm, which increased to 209 nm after seven million test cycles. When comparing these results with this present study it must be stated that the CoCr surface was lower in roughness than the SS surface, and further it did not exhibit any change in roughness over the five million test cycles. In part these differences could be explained by the higher hardness of the CoCr compared with the SS, which meant it was more scratch resistant. A potential explanation for the differences in initial roughness values is that the polishing process employed by the manufacturer of the contemporary MatOrtho prosthesis may have been superior to that of the DJOA which dates from the 1980s.
Changes in weight due to lubricant uptake can be an important factor. Previous in vitro studies evaluating the polymeric components of two-part finger prostheses [21,32,33], tested over a comparable range of cycles to this study, have reported an increase in weight due to lubricant uptake. This uptake has often been for both the test and the control polymeric components [21,32,33]. UHMWPE [21,32] test components increased in weight, but the gravimetric measurements for the control components showed a similar increase to that of the test components. Interestingly, cross linked polyethylene (XLPE) [33] test components increased in weight, but no more than those of the control. For both materials, UHMWPE and XLPE, the results indicated that no measurable wear occurred. In contrast to this, a poly ether ether ketone (PEEK) [21] test component from a finger prosthesis increased in weight, but less than the PEEK control component. This difference indicated that wear of the PEEK component occurred. The UHMWPE test component evaluated in this present study decreased in weight, whilst the UHMWPE control component increased. This is not consistent with the previous in vitro studies and indicates that a different “wear” mechanism occurred for the UHMWPE medial component. Gravimetric results for a Stainless Steel (SS) [32] proximal component tested against an UHMWPE component showed no observable change for either the SS test or SS control components. Although this is comparable to the “control” CoCr component from this present study, which also exhibited no gravimetric change, it is not comparable with the “test” CoCr component, which exhibited gravimetric loss over five million test cycles. Once again, this indicated that a different “wear” mechanism occurred for the CoCr proximal component when compared to SS component.
It was surprising to find that the hard CoCr test component exhibited a weight loss, and particularly that it showed a greater weight loss than the much softer UHMWPE test component. From a tribological point of view this is an unexpected result as it is the softer component which is expected to wear. Moreover the roughness results showed that the CoCr articulating surfaces were relatively unchanged in terms of their Sa values during and at the end of five million cycles of testing. What could explain this inconsistency between gravimetric results indicating wear and roughness results indicating no wear? To try and find an answer, the surfaces of the hydroxyapatite covered stems were investigated, with the test stems compared with control stems. The control stems exhibited a uniform coating of hydroxyapatite, whilst the test stems did not. Instead the micrograph images of the test stems revealed distinct areas where the hydroxyapatite had been removed (Figure 7 and Figure 8). Therefore, an explanation for the decrease in weight of the test components over five million cycles of testing is that this decrease was linked to removal of hydroxyapatite. That this decrease only affected the test components and not the control components indicates that hydroxyapatite removal did not occur due to the cleaning and weighing protocol, but was instead associated with testing itself. This information could be vital to other researchers who undertake in vitro wear tests of artificial joints with hydroxyapatite covered stems.
One limitation of this study is the small sample size. One MatOrtho PIPR prosthesis pair was tested in vitro, with a further pair used to account for lubricant uptake. However, this matches the quantity used for in vitro testing of: Single piece silicone prostheses [6]; and for SS-UHMWPE prostheses [32], both of which were evaluated using the simulator featured in this present study. Five million test cycles were undertaken, which conforms to the recommended standards for testing hip [18] and knee [19] prostheses in vitro. This paper offers the first ever reported in vitro wear tests of a metal-on-polymeric “proximal interphalangeal” joint in the scientific literature. This is important as clinical failures of finger prostheses continue to occur [34,35,36,37,38]. It has been argued previously that appropriate in vitro testing of finger prostheses can help to improve the performance of these implants [39] and this paper offers further evidence in support of this ideal.
5. Conclusions
A commercially available design of PIP prosthesis was tested to five million cycles of flexion-extension in a clinically validated simulator. Surface roughness and visual results indicated that minimal wear of the articulating surfaces occurred. However, weight loss of the test components was found. This has been explained by the loss of material from the hydroxyapatite coated stems, rather than loss of material from the articulating surfaces.
Acknowledgments
The authors would like to thank Anthony Unsworth of Durham University for allowing the finger simulator to be used at Newcastle University for this study and for his comments on a draft of the manuscript. The prostheses evaluated in this study were supplied free of charge by the Upper Limb Unit, Wrightington hospital, Wigan, UK.
Author Contributions
Andrew Naylor conducted the experimental work and wrote the initial manuscript. Ian A. Trail and Sumedh C. Talwalkar provided the prostheses and reviewed the study from a clinical perspective. Thomas J. Joyce was the principal investigator, reviewed the manuscript and was one of the original designers of the finger simulator used for this study.
Conflicts of Interest
The MatOrtho PIPR was designed in collaboration with one of the authors of this study, Ian A. Trail.
References
- Lawrence, J.S.; Bremner, J.M.; Bier, F. Osteo-arthrosis. Prevalence in the population and relationship between symptoms and x-ray changes. Ann. Rheum. Dis. 1966, 25, 1–24. [Google Scholar] [PubMed]
- Tsai, C.-L.; Liu, T.-K. Osteoarthritis in women: Its relationship to estrogen and current trends. Life Sci. 1992, 50, 1739–1744. [Google Scholar] [CrossRef]
- Linscheid, R.L. Implant arthroplasty of the hand: Retrospective and prospective considerations. J. Hand Surg. Am. 2000, 25, 796–816. [Google Scholar] [PubMed]
- Ashworth, C.R.; Hansraj, K.K.; Todd, A.O.; Dukhram, K.M.; Ebramzadeh, E.; Boucree, J.B.; Hansraj, M.S. Swanson proximal interphalangeal joint arthroplasty in patients with rheumatoid arthritis. Clin. Orthop. Relat. Res. 1997, 34–37. [Google Scholar] [CrossRef]
- Bales, J.G.; Wall, L.B.; Stern, P.J. Long-term results of swanson silicone arthroplasty for proximal interphalangeal joint osteoarthritis. J. Hand Surg. Am. 2014, 39, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Joyce, T.J.; Unsworth, A. The design of a finger wear simulator and preliminary results. Proc. Inst. Mech. Eng. H. 2000, 214, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Joyce, T.J.; Unsworth, A. A literature review of “failures” of the swanson finger prosthesis in the metacarpophalangeal joint. Hand Surg. 2002, 7, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Takigawa, S.; Meletiou, S.; Sauerbier, M.; Cooney, W.P. Long-term assessment of swanson implant arthroplasty in the proximal interphalangeal joint of the hand. J. Hand Surg. Am. 2004, 29, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, M.; Birch, A.; Trail, I. A short-term review of the finsbury proximal interphalangeal joint replacement. In Proceedings of the British Society for Surgery of the Hand, Autumn Scientific Meeting, Nottingham, UK, 12 November 2009.
- Watts, A.; Trail, I. Anatomical small joint replacement of the hand. Available online: http://www.boneandjoint.org.uk/content/focus/anatomical-small-joint-replacement-hand (accessed on 9 February 2015).
- Daecke, W.; Kaszap, B.; Martini, A.K.; Hagena, F.W.; Rieck, B.; Jung, M. A prospective, randomized comparison of 3 types of proximal interphalangeal joint arthroplasty. J. Hand Surg. Am. 2012, 37, 1770–1779. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, B.R. Proximal interphalangeal joint surface replacement arthroplasty. Hand Surg. 2001, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, B.R.; Fitzgerald, M.; Smith, K.R.; Currie, L.J. Cemented versus uncemented surface replacement arthroplasty of the proximal interphalangeal joint with a mean 5-year follow-up. J. Hand Surg. Am. 2008, 33, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.M.; Linscheid, R.L.; Cooney, W.P.; Baker, V.; Heckman, M.G. Long-term outcomes of proximal interphalangeal joint surface replacement arthroplasty. J. Bone Jt. Surg. Am. 2012, 94, 1120–1128. [Google Scholar] [CrossRef]
- Schindele, S.F.; Hensler, S.; Audigé, L.; Marks, M.; Herren, D.B. A modular surface gliding implant (capflex-pip) for proximal interphalangeal joint osteoarthritis: A prospective case series. J. Hand Surg. Am. 2015, 40, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Selier, J.G. American Society for Surgery of the Hand: Essentials of Hand Surger; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2002. [Google Scholar]
- Joyce, T.J.; Unsworth, A. A test procedure for artificial finger joints. Proc. Inst. Mech. Eng. H. 2002, 216, 105–110. [Google Scholar] [CrossRef] [PubMed]
- British Standards Institute. BS ISO 14242-3: Implants for Surgery. Wear of Total Hip-Joint Prostheses; British Standards Institute: London, UK, 2009. [Google Scholar]
- British Standards Institute. BS ISO 14243-3: Implants for Surgery. Wear of Total Knee-Joint Prostheses; British Standards Institute: London, UK, 2014. [Google Scholar]
- Joyce, T.J. Prediction of lubrication regimes in two-piece metacarpophalangeal prostheses. Med. Eng. Phys. 2007, 29, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Joyce, T.J.; Rieker, C.; Unsworth, A. Comparative in vitro wear testing of peek and uhmwpe capped metacarpophalangeal prostheses. Biomed. Mater. Eng. 2006, 16, 1–10. [Google Scholar] [PubMed]
- Naylor, A.; Bone, M.C.; Unsworth, A.; Talwalkar, S.C.; Trail, I.A.; Joyce, T.J. Evaluating the wear and surface roughness of pyrocarbon finger prostheses tested in vitro. In Proceedings of the 10th Anniversary Bath Biomechanics Symposium: Current Issues and Future Opportunities in Orthopaedic Research, Bath, UK, 15 September 2014.
- Naylor, A.; Bone, M.C.; Unsworth, A.; Trail, I.A.; Talwalkar, S.C.; Joyce, T.J. In vitro testing of pyrolytic carbon proximal interphalangeal prostheses. In Proceedings of the British Society for Surgery of the Hand, Spring Scientific Meeting, Gateshead, UK, 1–2 May 2014.
- Joyce, T.J.; Unsworth, A. Neuflex metacarpophalangeal prostheses tested in vitro. Proc. Inst. Mech. Eng. H. 2005, 219, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Joyce, T.J.; Unsworth, A. The wear of artificial finger joints using different lubricants in a new finger wear simulator. Wear 2001, 250, 199–205. [Google Scholar] [CrossRef]
- Rempel, D.; Dennerlein, J.; Mote, C.D., Jr.; Armstrong, T. A method of measuring fingertip loading during keyboard use. J. Biomech. 1994, 27, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.R.; Unsworth, A.; Haslock, I. A microcomputer controlled hand assessment system used for clinical measurement. Eng. Med. 1985, 14, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Weightman, B.; Amis, A.A. Finger joint force predictions related to design of joint replacements. J. Biomed. Eng. 1982, 4, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Katti, K.S. Biomaterials in total joint replacement. Colloids Surf. B Biointerfaces 2004, 39, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Szycher, M. Biocompatible Polymers, Metals, and Composites; Technomic Pub. Co.: Lancaster, OA, USA, 1983. [Google Scholar]
- Joyce, T.J.; Langton, D.J.; Jameson, S.S.; Nargol, A.V.F. Tribological analysis of failed resurfacing hip prostheses and comparison with clinical data. Proc. Inst. Mech. Eng. J. 2009, 223, 317–323. [Google Scholar] [CrossRef]
- Joyce, T.J. Wear testing of a djoa finger prosthesis in vitro. J. Mater. Sci. Mater. Med. 2010, 21, 2337–2343. [Google Scholar] [CrossRef] [PubMed]
- Joyce, T.J.; Unsworth, A. The influence of bovine serum lubricant on the wear of cross-linked polyethylene finger prostheses. J. Appl. Biomater. Biomech. JABB 2004, 2, 136–142. [Google Scholar]
- Bone, M.C.; Cunningham, J.L.; Lord, J.; Giddins, G.; Field, J.; Joyce, T.J. Analysis of failed van straten lpm proximal interphalangeal prostheses. J. Hand Surg. Eur. Vol. 2013, 38, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Cortés, P.; Pajares-López, M.; Robles-Molina, M.J.; Gómez-Sánchez, R.; Toledo-Romero, M.A.; de Torres-Urrea, J. Two-year outcomes of elektra prosthesis for trapeziometacarpal osteoarthritis: A longitudinal cohort study. J. Hand Surg. Eur. Vol. 2012, 37, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Hobby, J.L.; Edwards, S.; Field, J.; Giddins, G. A report on the early failure of the LPM proximal interphalangeal joint replacement. J. Hand Surg. Eur. Vol. 2008, 33, 526–527. [Google Scholar] [CrossRef] [PubMed]
- Kaszap, B.; Daecke, W.; Jung, M. High frequency failure of the moje thumb carpometacarpal joint arthroplasty. J. Hand Surg. Eur. Vol. 2012, 37, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Middleton, A.; Lakshmipathy, R.; Irwin, L.R. Failures of the rm finger prosthesis joint replacement system. J. Hand Surg. Eur. Vol. 2011, 36, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Joyce, T.J. Snapping the fingers. J. Hand Surg. Br. 2003, 28, 566–567. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).