Nanocrystallized Surface Effect on the Tribocorrosion Behavior of AISI 420
Abstract
1. Introduction
2. Experimental Procedures
2.1. Nanopeening Treatment
2.2. Samples Characterization
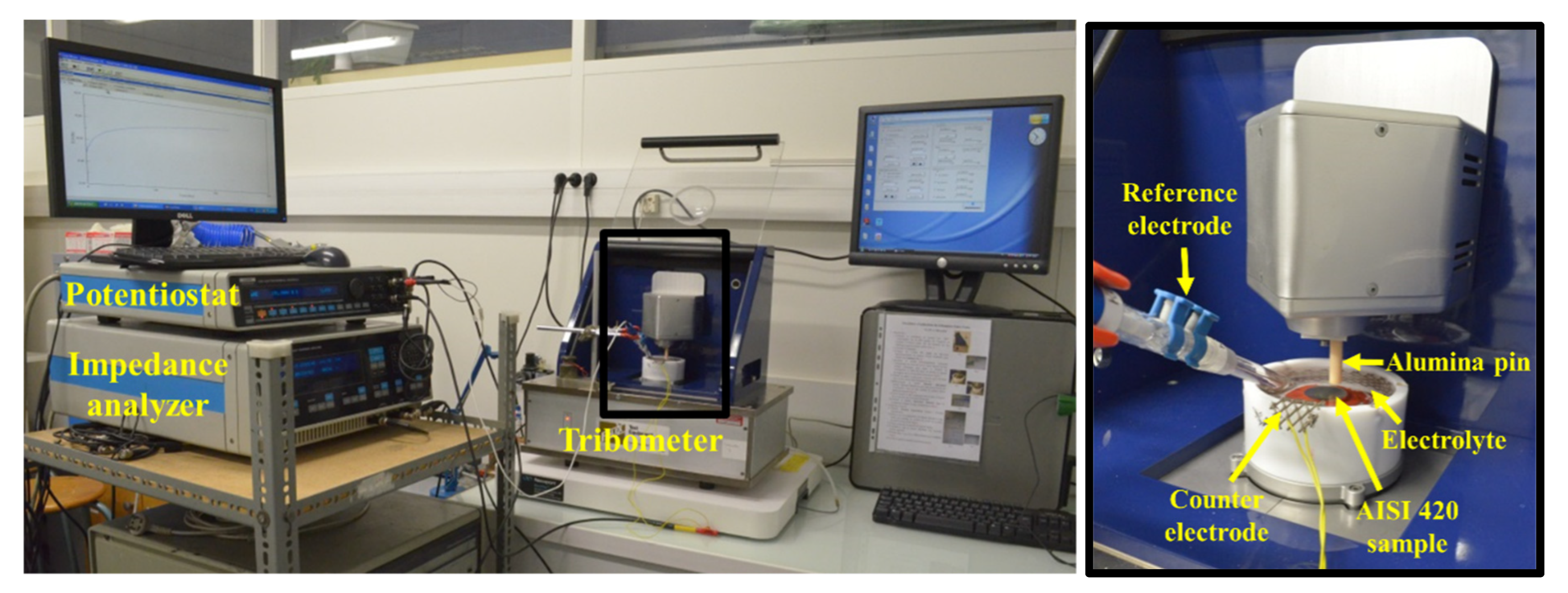
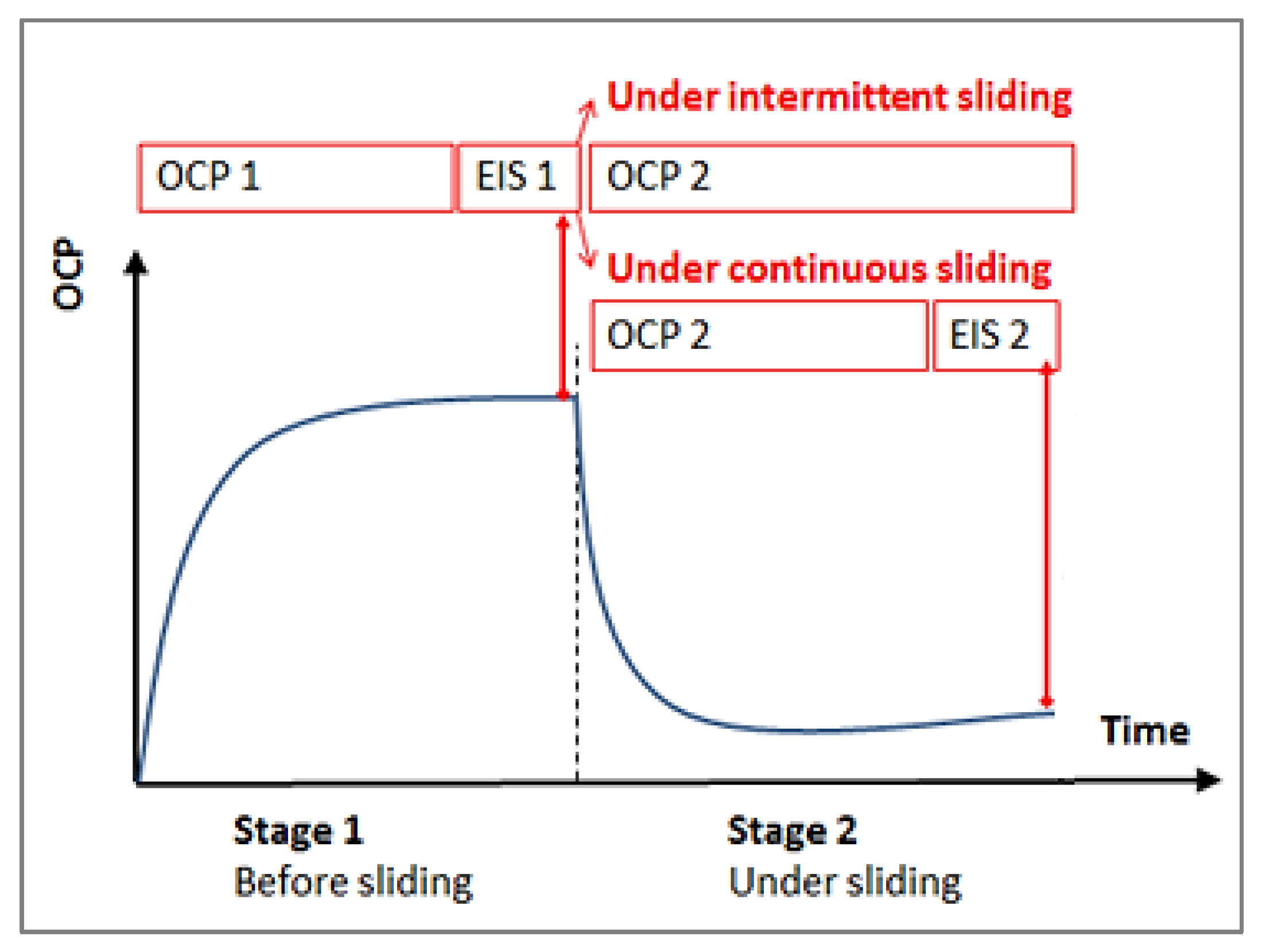
2.3. Tribocorrosion Test
3. Results and Discussions
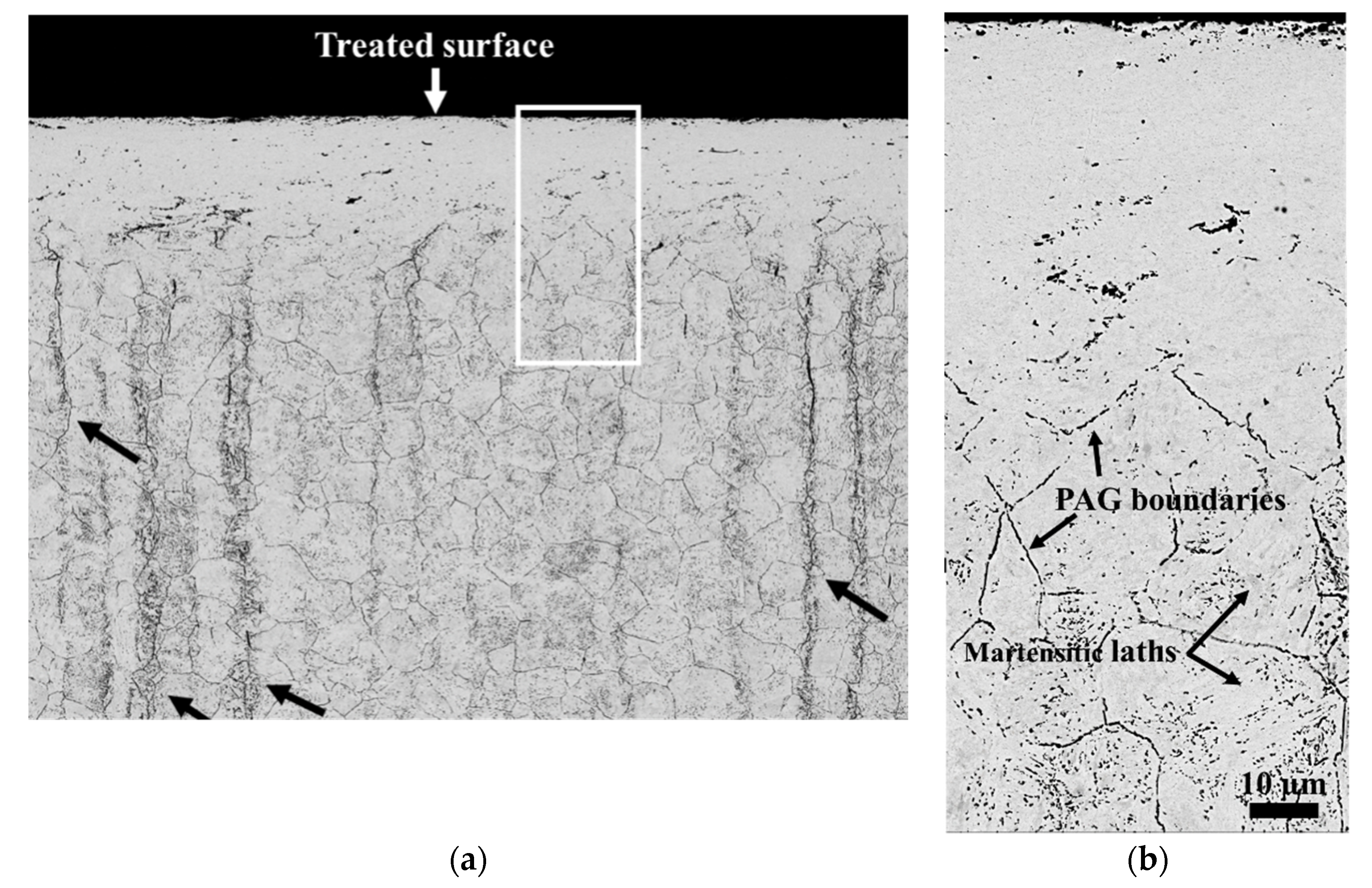
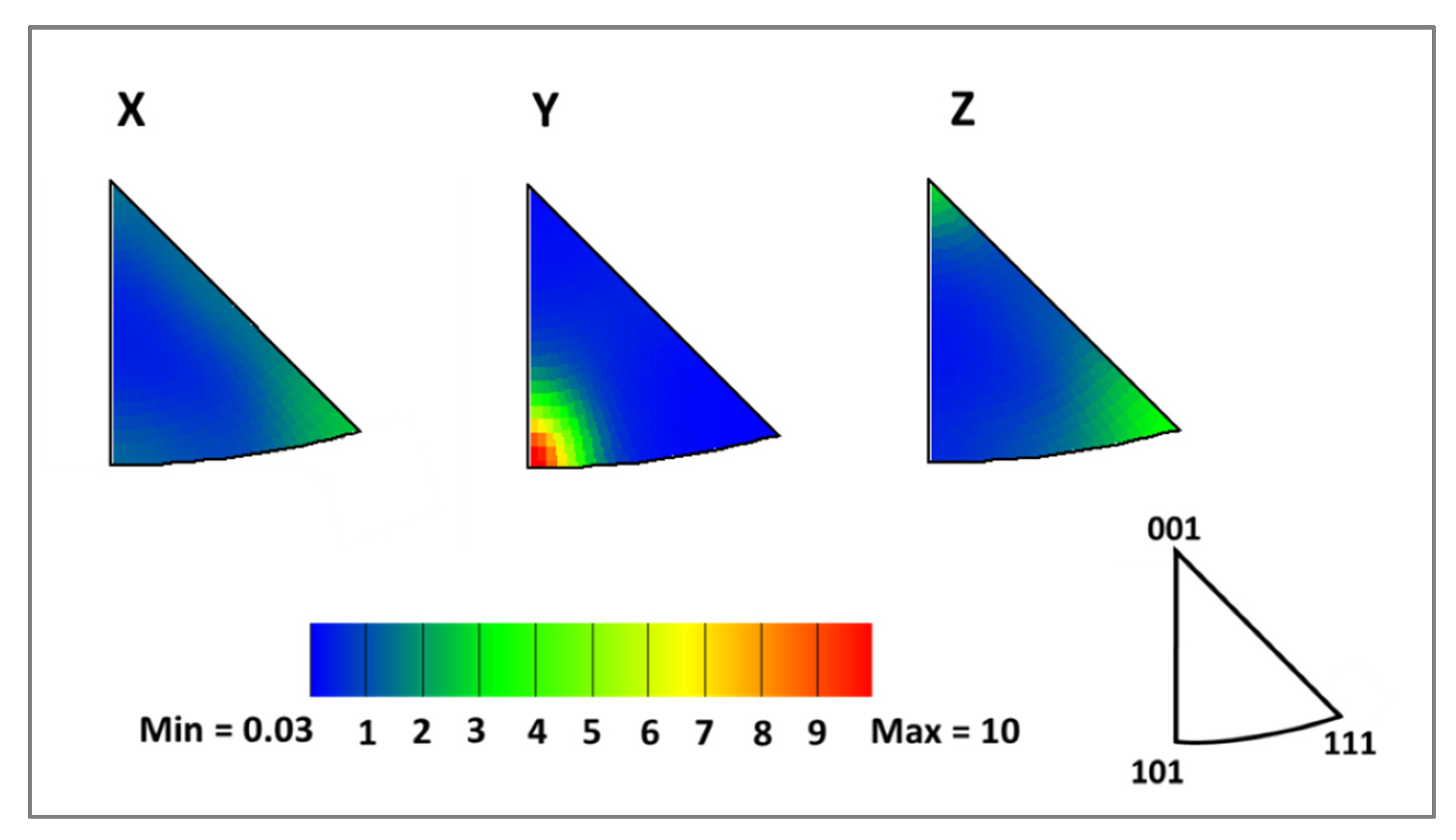
3.1. Microstructure Analyses
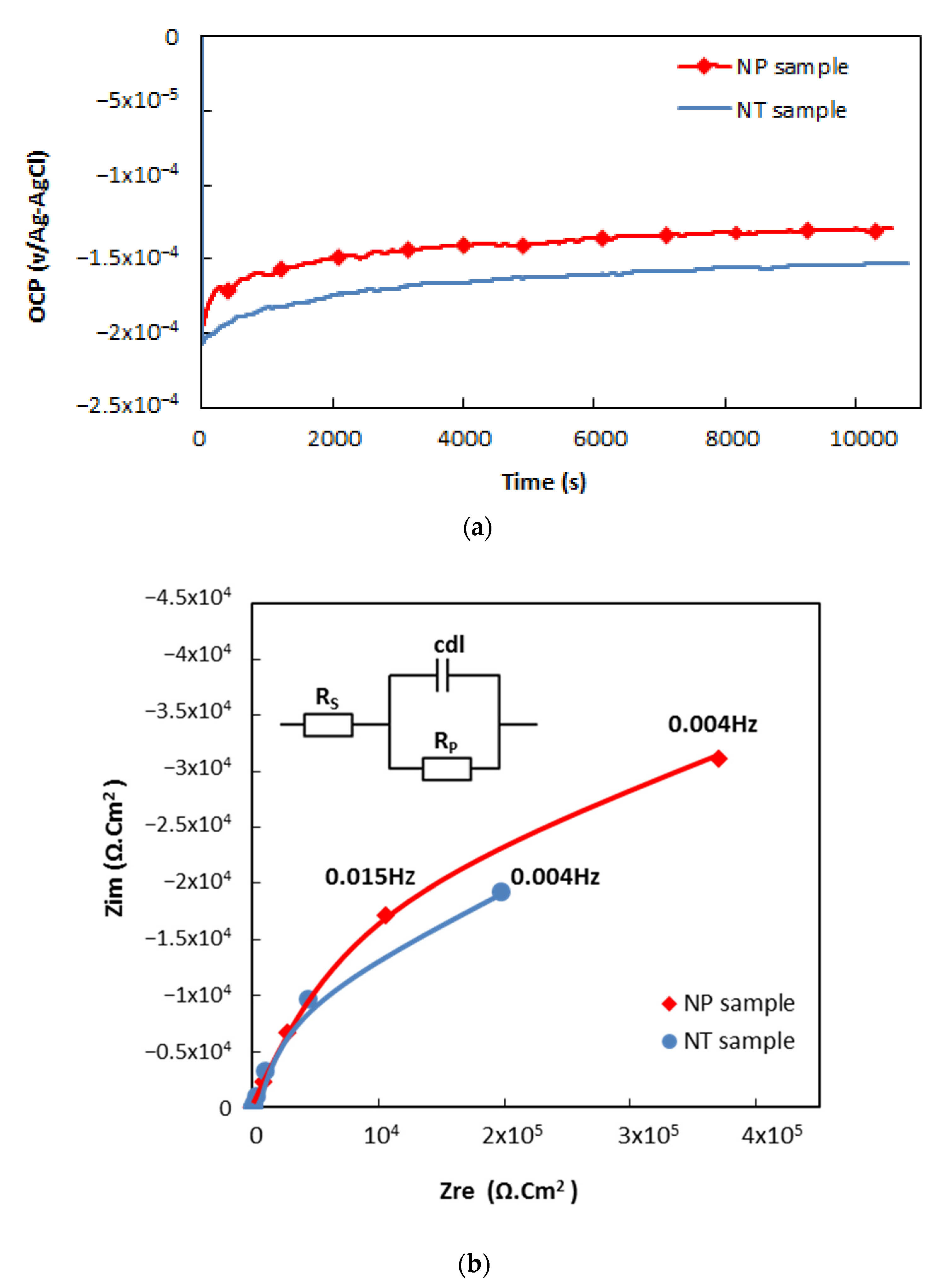
3.2. Corrosion Tests
3.3. Tribocorrosion Tests under Continuous Sliding
3.4. Tribocorrosion Tests under Intermittent Sliding
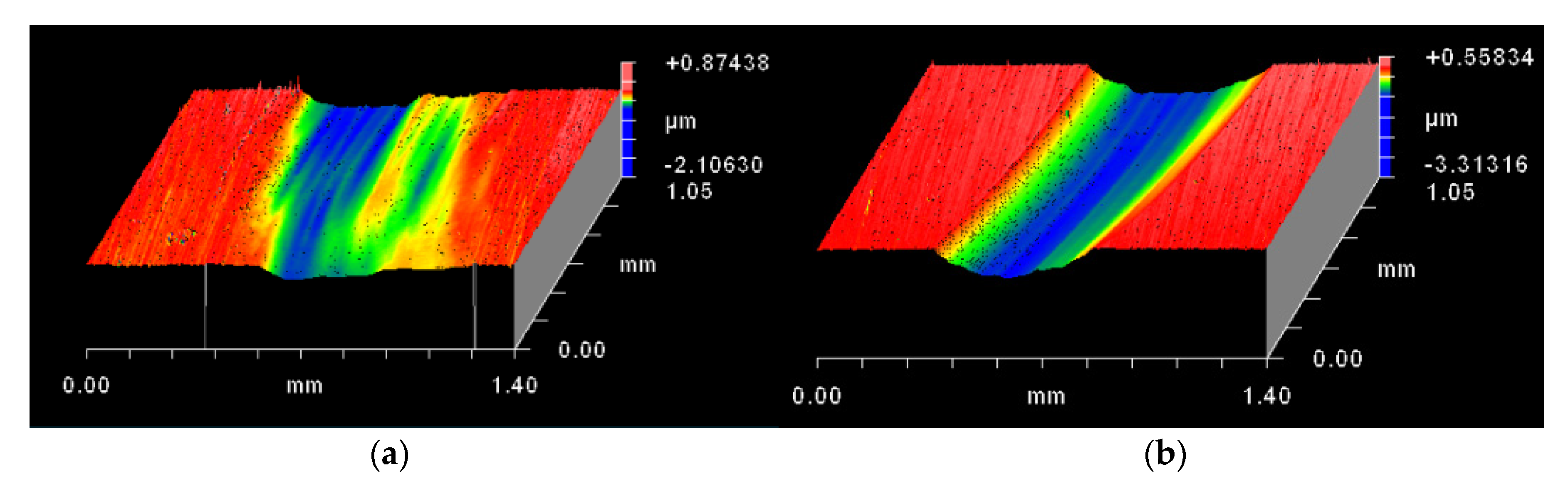
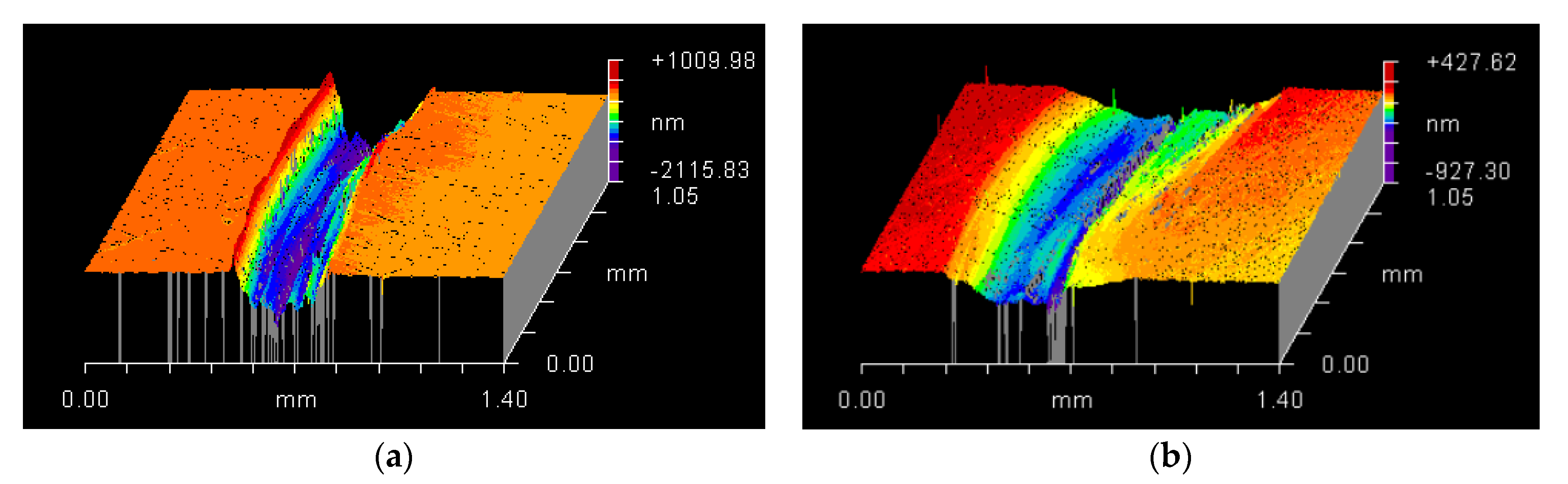
3.5. Surface and Morphological Characterization
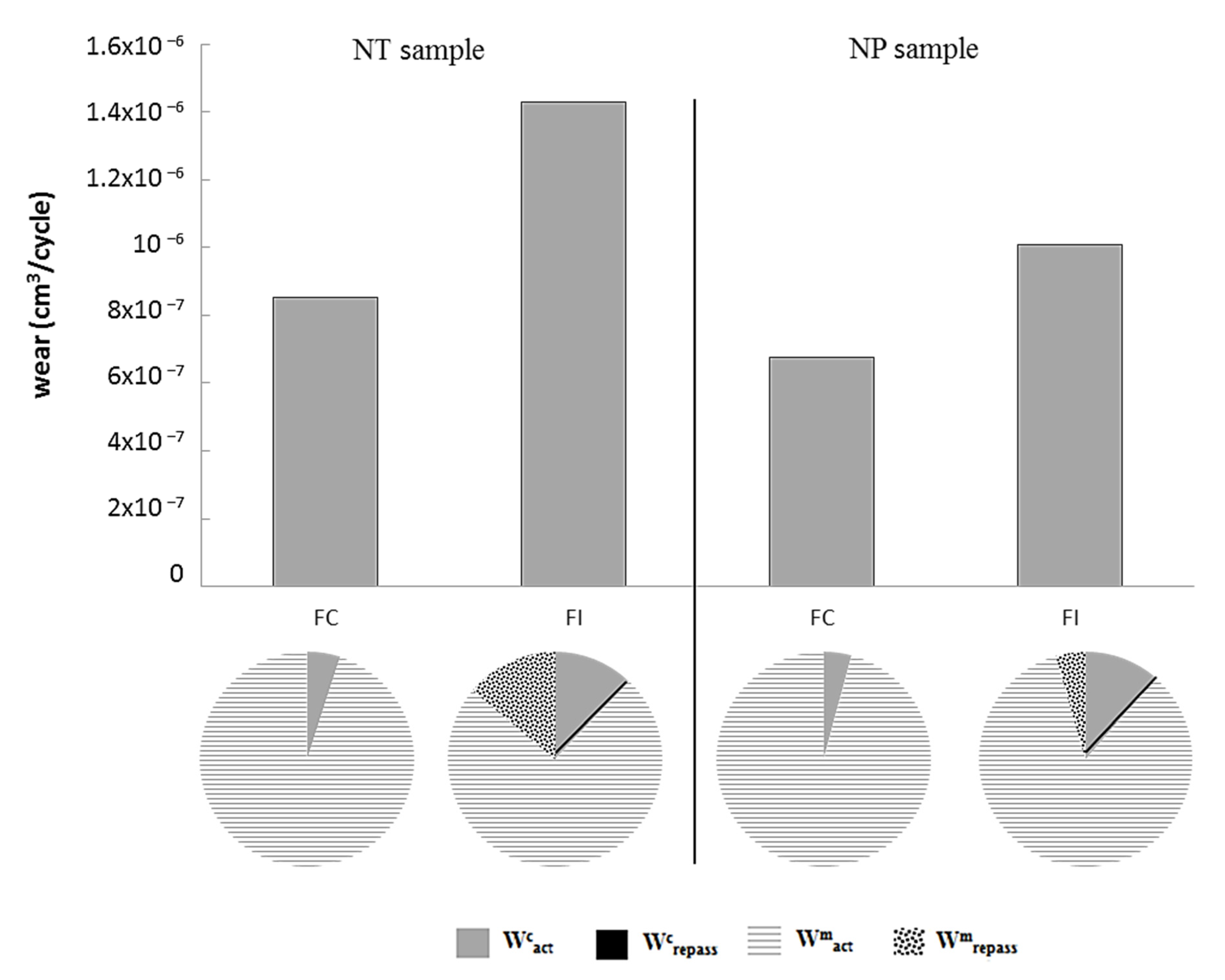
3.6. Wear Analyses
4. Conclusions
- Under both continuous and intermittent sliding, the wear mechanism was dominated by abrasion and adhesion mechanisms. However, the wear of the nanopeened sample was dominated by abrasion, which may give rise to the effect of hard oxide debris.
- The three-dimensional surface profiles of the wear tracks were performed to determine the total wear loss. The tribocorrosion protocol was applied to calculate the different contributions of wear. The tribocorrosion behavior of AISI 420 was improved by applying the nanopeening treatment. Under continuous sliding, both mechanical and corrosion contributions to wear decreased with a similar contribution/total wear ratio. Under intermittent sliding, all wear contributions decreased after the treatment, which greatly ameliorated the mechanical resistance of the repassivated surface.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
List of Symbols
| Cdl | Double layer capacitance |
| Cr | Chromium |
| EBSD | Electron Back Scattered Diffraction |
| EIS | Electrochemical Impedance Measurements |
| ECD | Equivalent Circular Diameter |
| Fe | Iron |
| IPF | Inverse Pole Figure |
| Mo | Molybdenum |
| Ni | Nickel |
| NT | Non-Treated |
| NP | NanoPeened |
| OCP | Open Circuit Potential |
| PAG | Prior Austenite Grain |
| RCCA | Rod Cluster Control Assemblies |
| EC | Equivalent circuit |
| Rp | Polarization Resistance (Ω) |
| Rs | Solution Resistance (Ω) |
| SEM | Scanning Electronic Microscopy |
| tlat | Latency time (s) |
| trot | Rotation time (s) |
| tstop | Stop time (s) |
| Wtr | Total wear (cm3/cycle) |
| Wcact | Material loss due to corrosion of active material in the wear track (cm3/cycle) |
| Wmact | Material loss due to mechanical wear of the active material in the wear track (cm3/cycle) |
| Wcrepass | Material loss due to corrosion of repassivated material in the wear track (cm3/cycle) |
| Wmrepass | Material loss due to the mechanical wear of repassivated material in the wear track (cm3/cycle) |
| Zim | Imaginary part of the impedance (Ω.cm2) |
| Zre | Real part of the impedance (Ω.cm2) |
References
- Lina, A.; Moinereau, D.; Delaune, X.; Phalippou, C.; Reynier, B.; Riberty, P. The influence of water flow on the impact/sliding wear and oxidation of PWR control rods specimens. Wear 2001, 251, 839–852. [Google Scholar] [CrossRef]
- Allen, T.; Busby, J.; Meyer, M.; Petti, D. Materials challenges for nuclear systems. Mater. Today 2010, 13, 14–23. [Google Scholar] [CrossRef]
- Lemaire, E.; Le Calvar, M. Evidence of tribocorrosion wear in pressurized water reactors. Wear 2001, 249, 338–344. [Google Scholar] [CrossRef]
- Benea, L.; Ponthiaux, P.; Wenger, F.; Galland, J.; Hertz, D.; Malo, J. Tribocorrosion of stellite 6 in sulphuric acid medium: Electrochemical behaviour and wear. Wear 2004, 256, 948–953. [Google Scholar] [CrossRef]
- Landolt, D.; Mischler, S. Tribocorrosion of Passive Metals and Coatings, 1st ed.; Elsevier: Amsterdam, The Netherlands; EPFL: Écublens, Switzerland, 2011. [Google Scholar]
- Atamert, S.; Bhadeshia, H.K.D.H. Comparison of the microstructures and abrasive wear properties of stellite hardfacing alloys deposited by arc welding and laser cladding. Met. Mater. Trans. A 1989, 20, 1037–1054. [Google Scholar] [CrossRef]
- Atamert, S.; Stekly, J. Microstructure, wear resistance, and stability of cobalt based and alternative iron based hardfacing alloys. Surf. Eng. 1993, 9, 231–240. [Google Scholar] [CrossRef]
- Dalmau, A.; Rmili, W.; Joly, D.; Richard, C.; Igual-Muñoz, A. Tribological behavior of new martensitic stainless steels using scratch and dry wear test. Tribol. Lett. 2014, 56, 517–529. [Google Scholar] [CrossRef]
- Cozza, R.C.; Schön, C.G. Evidence of superposition between grooving abrasion and rolling abrasion. Tribol. Trans. 2015, 58, 875–881. [Google Scholar] [CrossRef]
- Frenk, A.; Kurtz, W. Microstructural effects on the sliding wear resistance of a cobalt-based alloy. Wear 1994, 174, 81–91. [Google Scholar] [CrossRef]
- Antony, K.C. Wear-resistant cobalt-base alloys. J. Miner. Met. Mater. Soc. TMS 1983, 35, 52–60. [Google Scholar] [CrossRef]
- Agrawal, S.C.; Ocken, H. The microstructure and galling wear of a laser-melted cobalt-base hard facing alloy. Wear 1990, 140, 223–233. [Google Scholar] [CrossRef]
- Shaha, M.; Bakshi, S.D. Three-body abrasive wear of carbide-free bainite, martensite and bainite martensite structure of similar hardness. Wear 2018, 402–403, 207–215. [Google Scholar] [CrossRef]
- Dodds, S.; Jones, A.; Cater, S. Tribological enhancement of AISI 420 martensitic stainless steel through friction-stir processing. Wear 2013, 302, 863–877. [Google Scholar] [CrossRef]
- Trivedi, H.K.; Wedeven, V.; Black, W. Effect of Silicon Nitride Ball on Adhesive Wear of Martensitic Stainless Steel Pyrowear 675 and AISI M-50 Races with Type II Ester Oil. Tribol. Trans. 2016, 59, 363–374. [Google Scholar] [CrossRef]
- Rozing, G.; Alar, V.; Marušić, V. Study of stainless steel resistance in conditions of tribocorrosion wear. Interdiscip. Descr. Complex Syst. 2015, 13, 461–471. [Google Scholar] [CrossRef]
- Scheuer, C.; Possoli, F.; Borges, P.; Cardoso, R.; Brunatto, S. AISI 420 martensitic stainless steel corrosion resistance enhancement by low-temperature plasma carburizing. Electrochim. Acta 2019, 317, 70–82. [Google Scholar] [CrossRef]
- Dalmau, A.; Richard, C.; Igual–Muñoz, A. Degradation mechanisms in martensitic stainless steels: Wear, corrosion and tribocorrosion appraisal. Tribol. Int. 2018, 121, 167–179. [Google Scholar] [CrossRef]
- Prieto, G.; Tuckart, W.R. Influence of cryogenic treatments on the wear behavior of AISI 420 martensitic stainless steel. J. Mater. Eng. Perform. 2017, 26, 5262–5271. [Google Scholar] [CrossRef]
- Li, Y.; He, Y.; Xiu, J.; Wang, W.; Zhu, Y.; Hu, B. Wear and corrosion properties of AISI 420 martensitic stainless steel treated by active screen plasma nitriding. Surf. Coat. Technol. 2017, 329, 184–192. [Google Scholar] [CrossRef]
- Isfahany, A.N.; Saghafian, H.; Borhani, G. The effect of heat treatment on mechanical properties and corrosion behavior of AISI420 martensitic stainless steel. J. Alloys Compd. 2011, 509, 3931–3936. [Google Scholar] [CrossRef]
- Pan, L.; Kwok, C.; Lo, K. Enhancement in hardness and corrosion resistance of AISI 420 martensitic stainless steel via friction stir processing. Surf. Coat. Technol. 2018, 357, 339–347. [Google Scholar] [CrossRef]
- Samih, Y.; Marcos, G.; Stein, N.; Allain, N.; Fleury, E.; Dong, C.; Grosdidier, T. Microstructure modifications and associated hardness and corrosion improvements in the AISI 420 martensitic stainless steel treated by High Current Pulsed Electron Beam (HCPEB). Surf. Coat. Technol. 2014, 259, 737–745. [Google Scholar] [CrossRef]
- Toro, A.; Sinatora, A.; Tanaka, D.; Tschiptschin, A. Corrosion–erosion of nitrogen bearing martensitic stainless steels in seawater–quartz slurry. Wear 2001, 251, 1257–1264. [Google Scholar] [CrossRef]
- López, D.; Congote, J.; Cano, J.; Toro, A.; Tschiptschin, A. Effect of particle velocity and impact angle on the corrosion–erosion of AISI 304 and AISI 420 stainless steels. Wear 2005, 259, 118–124. [Google Scholar] [CrossRef]
- Venske, A.F.; de Castro, V.V.; da Costa, E.M.; dos Santos, C.A. Sliding wear behavior of an AISI 440b martensitic stainless steel lubricated with biodiesel and diesel–biodiesel blends. J. Mater. Eng. Perform. 2018, 27, 5427–5437. [Google Scholar] [CrossRef]
- Mischler, S.; Spiegel, A.; Landolt, D. The role of passive oxide films on the degradation of steel in tribocorrosion systems. Wear 1999, 225–229, 1078–1087. [Google Scholar] [CrossRef]
- Rodríguez-Bravo, G.; Vite-Torres, M.; Godínez-Salcedo, J. Corrosion rate and wear mechanisms comparison for AISI 410 stainles steel exposed to pure corrosion and abrasion-corrosion in a simulated marine environment. Tribol. Ind. 2019, 41, 394–400. [Google Scholar] [CrossRef]
- Aquino, J.M.; Della Rovere, C.A.; Kuri, S.E. Localized corrosion susceptibility of supermartensitic stainless steel in welded joints, Corrosion. Corrosion 2008, 64, 35–39. [Google Scholar] [CrossRef]
- Dalmau, A.; Rmili, W.; Richard, C.; Igual–Muñoz, A. Tribocorrosion behavior of new martensitic stainless steels in sodium chloride solution. Wear 2016, 368–369, 146–155. [Google Scholar] [CrossRef]
- Ben Saada, F.; Elleuch, K.; Ponthiaux, P. On the Tribocorrosion responses of two stainless steels. Tribol. Trans. 2017, 61, 53–60. [Google Scholar] [CrossRef]
- Wu, H.; Li, Y.; Lu, Y.; Li, Z.; Cheng, X.; Hasan, M.; Zhang, H.; Jiang, Z. Influences of load and microstructure on tribocorrosion behaviour of high strength hull steel in saline solution. Tribol. Lett. 2019, 67, 124. [Google Scholar] [CrossRef]
- Stachowiak, A.; Zwierzycki, W. Tribocorrosion modeling of stainless steel in a sliding pair of pin-on-plate type. Tribol. Int. 2011, 44, 1216–1224. [Google Scholar] [CrossRef]
- Esfandiari, M.; Dong, H. The corrosion and corrosion–wear behaviour of plasma nitrided 17-4PH precipitation hardening stainless steel. Surf. Coat. Technol. 2007, 202, 466–478. [Google Scholar] [CrossRef]
- Lin, N.; Xie, F.; Yang, H.; Tian, W.; Wang, H.; Tang, B. Assessments on friction and wear behaviors of P110 steel and chromizing coating sliding against two counterparts under dry and wet conditions. Appl. Surf. Sci. 2012, 258, 4960–4970. [Google Scholar] [CrossRef]
- Ben Saada, F.; Antar, Z.; Elleuch, K.; Ponthiaux, P.; Gey, N. The effect of nanocrystallized surface on the tribocorrosion behavior of 304L stainless steel. Wear 2018, 394–395, 71–79. [Google Scholar] [CrossRef]
- Prezeau, T.; Muller, T.; Dransart, E.; Giraud, Y. Amélioration par un prétraitement mécanique de NanoPeening® des performances des traitements thermochimiques (Improvement by a mechanical pretreatment of NanoPeening® of the performance of thermochemical treatments). Traitements Matér. 2011, 412, 37–43. (In French) [Google Scholar]
- Erdman, N.; Campbell, R.; Asahina, S. Precise SEM cross section polishing via argon beam milling. Microsc. Today 2006, 14, 22–25. [Google Scholar] [CrossRef]
- Ben Saada, M.; Gey, N.; Beausir, B.; Iltis, X.; Mansour, H.; Maloufi, N. Sub-boundaries induced by dislocational creep in uranium dioxide analyzed by advanced diffraction and channeling electron microscopy. Mater. Charact. 2017, 133, 112–121. [Google Scholar] [CrossRef]
- Chui, P.; Sun, K.; Sun, C.; Yang, X.; Shan, T. Effect of surface nanocrystallization induced by fast multiple rotation rolling on hardness and corrosion behavior of 316L stainless steel. Appl. Surf. Sci. 2011, 257, 6787–6791. [Google Scholar] [CrossRef]
- Wang, R.; Zheng, Z.; Zhou, Q.; Gao, Y. Effect of surface nanocrystallization on the sensitization and desensitization behavior of Super304H stainless steel. Corros. Sci. 2016, 111, 728–741. [Google Scholar] [CrossRef]
- Lee, J.B. Effects of alloying elements, Cr, Mo and N on repassivation characteristics of stainless steels using the abrading electrode technique. Mater. Chem. Phys. 2006, 99, 224–234. [Google Scholar] [CrossRef]
- Bastidas, J.M.; Torres, C.L.; Cano, E.; Polo, J.L. Influence of molybdenum on passivation of polarized stainless steels in a chloride environment. Corros. Sci. 2002, 44, 625–633. [Google Scholar] [CrossRef]
- Bagherifard, S.; Fernandez-Pariente, I.; Ghelichi, R.; Guagliano, M. Effect of severe shot peening on microstructure and fatigue strength of cast iron. Int. J. Fatigue 2014, 65, 64–70. [Google Scholar] [CrossRef]
- Wang, T.; Yu, J.; Dong, B. Surface nanocrystallization induced by shot peening and its effect on corrosion resistance of 1Cr18Ni9Ti stainless steel. Surf. Coat. Technol. 2006, 200, 4777–4781. [Google Scholar] [CrossRef]





| Elements | C | Si | Mn | P | S | Cr | Ni | Mo |
|---|---|---|---|---|---|---|---|---|
| NT sample | 0.3 | 0.36 | 0.64 | 0.02 | 0.02 | 13 | 0.22 | 0.04 |
| NP sample | 0.02 | 0.37 | 1.6 | 0.03 | 0.04 | 16.5 | 10.14 | 2.2 |
| NT Samples | NP Samples | |||
|---|---|---|---|---|
| Sliding | Continuous | Intermittent | Continuous | Intermittent |
| Wcact (cm3/cycle) | 4.24 × 10−8 | 1.73 × 10−7 | 2.86 × 10−8 | 1.14 × 10−7 |
| Wcrepass (cm3/cycle) | - | 1.65 × 10−11 | - | 6.89 × 10−12 |
| Wmact (cm3/cycle) | 8.1 × 10−8 | 1.06 × 10−6 | 6.48 × 10−7 | 8.49 × 10−7 |
| Wmrepass (cm3/cycle) | - | 1.99 × 10−7 | - | 4.51 × 10−8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Saada, F.; Ben Saada, M.; Elleuch, K.; Ponthiaux, P. Nanocrystallized Surface Effect on the Tribocorrosion Behavior of AISI 420. Lubricants 2022, 10, 304. https://doi.org/10.3390/lubricants10110304
Ben Saada F, Ben Saada M, Elleuch K, Ponthiaux P. Nanocrystallized Surface Effect on the Tribocorrosion Behavior of AISI 420. Lubricants. 2022; 10(11):304. https://doi.org/10.3390/lubricants10110304
Chicago/Turabian StyleBen Saada, Fatma, Mariem Ben Saada, Khaled Elleuch, and Pierre Ponthiaux. 2022. "Nanocrystallized Surface Effect on the Tribocorrosion Behavior of AISI 420" Lubricants 10, no. 11: 304. https://doi.org/10.3390/lubricants10110304
APA StyleBen Saada, F., Ben Saada, M., Elleuch, K., & Ponthiaux, P. (2022). Nanocrystallized Surface Effect on the Tribocorrosion Behavior of AISI 420. Lubricants, 10(11), 304. https://doi.org/10.3390/lubricants10110304





