The Evolving Landscape of Novel and Old Biomarkers in Localized High-Risk Prostate Cancer: State of the Art, Clinical Utility, and Limitations Toward Precision Oncology
Abstract
1. Introduction
2. Materials and Methods
3. Biomolecular Pitfalls of Prostate Tumorigenesis
4. Genomic Profiling in Localized Prostate Cancer
5. Diagnostic Biomarkers in Localized Prostate Cancer: The Post-PSA Era
6. Prognostic Biomarkers in Localized Prostate Cancer
7. Predictive Biomarkers in Localized Prostate Cancer: An Open Issue
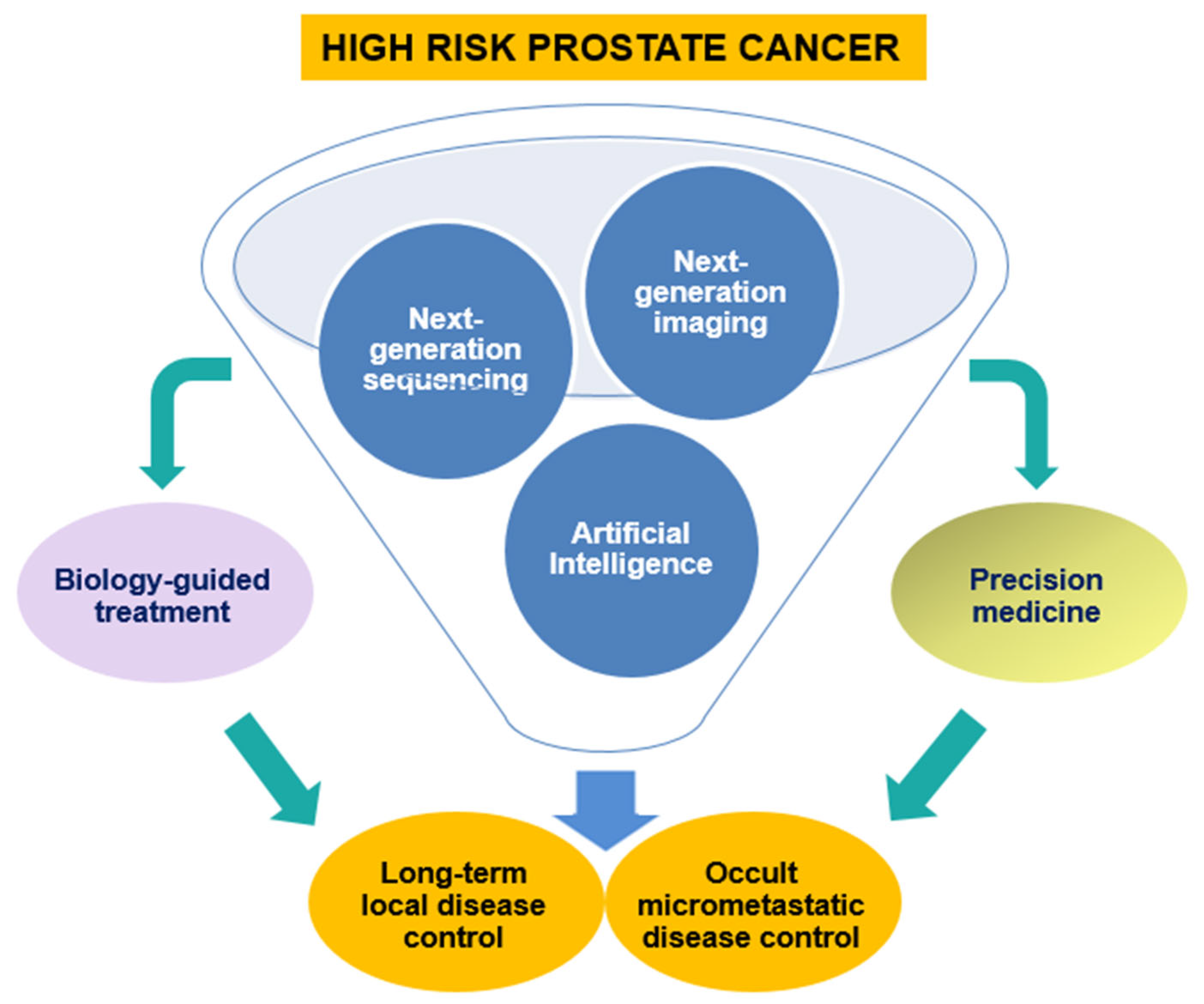
8. Future Perspectives
8.1. Liquid Biopsy in Localized Prostate Cancer
8.1.1. Exosomes and miRNAs
8.1.2. Epigenetic Mechanisms
8.2. The Impact of Artificial Intelligence
9. Discussion
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| %fPSA | Percent free PSA |
| ADT | Androgen deprivation therapy |
| AI | Artificial intelligence |
| APC | Adenomatous polyposis coli |
| AR | Androgen receptor |
| AS | Active surveillance |
| ASCO | American Society of Clinical Oncology |
| ATM | Ataxia-telangiectasia mutated |
| AUA | American Urological Association |
| BCR | Biochemical relapse |
| BRCA | Breast cancer gene |
| CAPRA | Cancer of the Prostate Risk Assessment |
| CCP | Cell-cycle progression |
| CCR | Clinical cell-cycle risk |
| cfDNA | Cell-free DNA |
| CHEK2 | Checkpoint kinase 2 |
| cPSA | Complexed PSA |
| csPC | Clinically significant prostate cancer |
| CSS | Cancer-specific survival |
| CTCs | Circulating tumor cells |
| ctDNA | Circulating tumor DNA |
| DAPK | Death-associated protein kinase 1 |
| DLX1 | Distal-less homeobox 1 |
| DRE | Digital rectal examination |
| EAU | European Association of Urology |
| EBRT | External beam RT |
| ERSPC | European Randomised Study for Screening of Prostate Cancer |
| FDA | Food and Drug Administration |
| FOXA1 | Forkhead box protein A1 |
| fPSA | Free PSA |
| GADD45 | Growth arrest and DNA-damage-inducible α |
| GPS | Genomic Prostate Score |
| GS | Gleason score |
| GSTP1 | Glutathione-S-transferase P1 / Glutathione-S-transferase π |
| HER2 | Human epidermal growth factor receptor 2 |
| HOXB13 | Omeobox B13 |
| HRR | Homologous recombination repair |
| iPARP | Poly-ADP ribose polymerase RASSF inhibitor |
| ISUP | International Society of Urological Pathology |
| mCRPC | Metastatic castration-resistant prostate cancer |
| MFS | Metastasis-free survival |
| MiPS | Mi Prostate Score |
| MLH1 | MutL protein homolog 1 |
| MMR | Mismatch repair |
| MpMRI | Multiparametric magnetic resonance imaging |
| NCCN | National Comprehensive Cancer Network |
| NGI | Next-generation imaging |
| NICE | National Institute for Health and Care Excellence |
| NPV | Negative predictive value |
| OS | Overall survival |
| PALB2 | Partner and localizer of BRCA2 |
| PC | Prostate cancer |
| PCA 3 | Prostate cancer antigen 3 |
| PHI | Prostate Health Index |
| PI-RADS | Prostate Imaging Reporting & Data System |
| PMS2 | Postmeiotic segregation increased 2 |
| PSA | Prostate-specific antigen |
| PSA-D | PSA density |
| PSMA-PET/CT | Prostate-specific membrane antigen–positron emission tomography/computed tomography |
| PTEN | Phosphatase and tensin homolog |
| QoL | Quality of life |
| RASSF1 | Ras association domain family member 1 / Ras association domain-containing protein 1 |
| RB1 | Retinoblastoma transcriptional corepressor |
| RP | Radical prostatectomy |
| RT | Radiotherapy |
| RTOG | Radiation Therapy Oncology Group |
| RUNX3 | Runt-related transcription factor 3 |
| SEER | Surveillance, Epidemiology, and End Results |
| SNPs | Single-nucleotide polymorphisms |
| SNVs | Single-nucleotide variants |
| SPOP | Speckle-type POZ protein |
| TGFβ | Transforming growth factor β |
| TME | Tumor microenvironment |
| TMPRSS2:ERG | The fusion of the transmembrane protease serine 2 gene with the erythroblast transformation-specific-related gene |
| TNFRSF10c | Tumor necrosis factor receptor superfamily member 10c |
| tPSA | Total PSA |
| US | United States |
References
- Culp, M.B.; Soerjomataram, I.; Efstathiou, J.A.; Bray, F.; Jemal, A. Recent Global Patterns in Prostate Cancer Incidence and Mortality Rates. Eur. Urol. 2020, 77, 38–52. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Hussain, M.; Lin, D.; Saad, F.; Vapiwala, N.; Chapin, B.F.; Sandler, H.; Evans, C.P.; Carducci, M.A.; Sachdev, S. Newly Diagnosed High-Risk Prostate Cancer in an Era of Rapidly Evolving New Imaging: How Do We Treat? J. Clin. Oncol. 2021, 39, 13–16. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017; ISBN 9781119263579. [Google Scholar]
- D’Amico, A.V.; Whittington, R.; Malkowicz, S.B.; Schultz, D.; Blank, K.; Broderick, G.A.; Tomaszewski, J.E.; Renshaw, A.A.; Kaplan, I.; Beard, C.J.; et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998, 280, 969–974. [Google Scholar] [CrossRef]
- Eastham, J.A.; Auffenberg, G.B.; Barocas, D.A.; Chou, R.; Crispino, T.; Davis, J.W.; Eggener, S.; Horwitz, E.M.; Kane, C.J.; Kirkby, E.; et al. Clinically Localized Prostate Cancer: AUA/ASTRO Guideline, Part I: Introduction, Risk Assessment, Staging, and Risk-Based Management. J. Urol. 2022, 208, 10–18. [Google Scholar] [CrossRef]
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Brunckhorst, O.; Darraugh, J.; Eberli, D.; De Meerleer, G.; De Santis, M.; Farolfi, A.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer-2024 Update. Part I: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2024, 86, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, P.; Davis, J.; Hughes, S. NICE guidelines on prostate cancer 2019. BJU Int. 2019, 124, 1. [Google Scholar] [CrossRef] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Prostate Cancer Version24.2025—April 16, 2025. Available online: https://www.nccn.org/patients/guidelines/content/PDF/prostate-early-patient.pdf (accessed on 4 July 2025).
- Roach, M.; Lu, J.; Pilepich, M.V.; Asbell, S.O.; Mohiuddin, M.; Terry, R.; Grignon, D. Four prognostic groups predict long-term survival from prostate cancer following radiotherapy alone on Radiation Therapy Oncology Group clinical trials. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 609–615, Erratum in Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 313. Mohuidden M [corrected to Mohiuddin M]. [Google Scholar] [CrossRef]
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. proPSMA Study Group Collaborators. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomized, multicentre study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Downes, M.R.; Xu, B.; van der Kwast, T.H. Cribriform architecture prostatic adenocarcinoma in needle biopsies is a strong independent predictor for lymph node metastases in radical prostatectomy. Eur. J. Cancer 2021, 148, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Lozano, R.; Salles, D.C.; Sandhu, S.; Aragón, I.M.; Thorne, H.; López-Campos, F.; Rubio-Briones, J.; Gutierrez-Pecharroman, A.M.; Maldonado, L.; di Domenico, T.; et al. Association between BRCA2 alterations and intraductal and cribriform histologies in prostate cancer. Eur. J. Cancer 2021, 147, 74–83. [Google Scholar] [CrossRef]
- Fraser, M.; Sabelnykova, V.Y.; Yamaguchi, T.N.; Heisler, L.E.; Livingstone, J.; Huang, V.; Shiah, Y.J.; Yousif, F.; Lin, X.; Masella, A.P.; et al. Genomic hallmarks of localized, non-indolent prostate cancer. Nature 2017, 541, 359–364. [Google Scholar] [CrossRef]
- Marino, F.; Totaro, A.; Gandi, C.; Bientinesi, R.; Moretto, S.; Gavi, F.; Pierconti, F.; Iacovelli, R.; Bassi, P.; Sacco, E. Germline mutations in prostate cancer: A systematic review of the evidence for personalized medicine. Prostate Cancer Prostatic Dis. 2023, 26, 655–664. [Google Scholar] [CrossRef]
- Haffner, M.C.; Zwart, W.; Roudier, M.P.; True, L.D.; Nelson, W.G.; Epstein, J.I.; De Marzo, A.M.; Nelson, P.S.; Yegnasubramanian, S. Genomic and phenotypic heterogeneity in prostate cancer. Nat. Rev. Urol. 2021, 18, 79–92. [Google Scholar] [CrossRef]
- Hamdy, F.C.; Donovan, J.L.; Lane, J.A.; Metcalfe, C.; Davis, M.; Turner, E.L.; Martin, R.M.; Young, G.J.; Walsh, E.I.; Bryant, R.J.; et al. ProtecTStudy Group Fifteen-Year Outcomes after Monitoring Surgery or Radiotherapy for Prostate Cancer. N. Engl. J. Med. 2023, 388, 1547–1558. [Google Scholar] [CrossRef]
- Lam, T.B.L.; MacLennan, S.; Willemse, P.M.; Mason, M.D.; Plass, K.; Shepherd, R.; Baanders, R.; Bangma, C.H.; Bjartell, A.; Bossi, A.; et al. EAU-EANM-ESTRO-ESUR-SIOG Prostate Cancer Guideline Panel Consensus Statements for Deferred Treatment with Curative Intent for Localised Prostate Cancer from an International Collaborative Study (DETECTIVE Study). Eur. Urol. 2019, 76, 790–813. [Google Scholar] [CrossRef]
- Espiritu, S.M.G.; Liu, L.Y.; Rubanova, Y.; Bhandari, V.; Holgersen, E.M.; Szyca, L.M.; Fox, N.S.; Chua, M.L.K.; Yamaguchi, T.N.; Heisler, L.E.; et al. The Evolutionary Landscape of Localized Prostate Cancers Drives Clinical Aggression. Cell 2018, 173, 1003–1013.e15. [Google Scholar] [CrossRef]
- Das, C.J.; Razik, A.; Netaji, A.; Verma, S. Prostate MRI-TRUS fusion biopsy: A review of the state of the art procedure. Abdom. Radiol. 2020, 45, 2176–2183. [Google Scholar] [CrossRef] [PubMed]
- Thorwarth, D. Biologically adapted radiation therapy. Z. Med. Phys. 2018, 28, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. The Molecular Taxonomy of Primary Prostate Cancer. Cell 2015, 163, 1011–1025. [Google Scholar] [CrossRef] [PubMed]
- Brady, L.; Carlsson, J.; Baird, A.M.; Casey, O.; Vlajnic, T.; Murchan, P.; Cormican, D.; Costigan, D.; Gray, S.; Sheils, O.; et al. Correlation of integrated ERG/PTEN assessment with biochemical recurrence in prostate cancer. Cancer Treat. Res. Commun. 2021, 29, 100451. [Google Scholar] [CrossRef]
- Leinonen, K.A.; Saramäki, O.R.; Furusato, B.; Kimura, T.; Takahashi, H.; Egawa, S.; Suzuki, H.; Keiger, K.; Ho Hahm, S.; Isaacs, W.B.; et al. Loss of PTEN is associated with aggressive behavior in ERG-positive prostate cancer. Cancer Epidemiol. Biomarkers Prev. 2013, 22, 2333–2344. [Google Scholar] [CrossRef]
- Kobelyatskaya, A.A.; Pudova, E.A.; Katunina, I.V.; Snezhkina, A.V.; Fedorova, M.S.; Pavlov, V.S.; Kotelnikova, A.O.; Nyushko, K.M.; Alekseev, B.Y.; Krasnov, G.S.; et al. Transcriptome Profiling of Prostate Cancer, Considering Risk Groups and the TMPRSS2-ERG Molecular Subtype. Int. J. Mol. Sci. 2023, 24, 9282. [Google Scholar] [CrossRef]
- Mavingire, N.; Moore, J.C.; Johnson, J.R.; Dwead, A.M.; Cropp, C.D.; Mechref, Y.; Kobeissy, F.; Rais-Bahrami, S.; Woods-Burnham, L. Revisiting HER2 in Prostate Cancer from an Inclusive Perspective: From Biomarkers to Omics. Cancers 2024, 16, 3262. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Li, K.; Zhang, H.; Lai, G.; Li, D.; Zhong, X. Identification and validation of an immune-related gene pairs signature for three urologic cancers. Aging 2022, 14, 1429–1447. [Google Scholar] [CrossRef] [PubMed]
- Yanai, Y.; Kosaka, T.; Mikami, S.; Hongo, H.; Yasumizu, Y.; Takeda, T.; Matsumoto, K.; Miyauchi, J.; Kitano, S.; Oya, M. CD8-positive T cells and CD204-positive M2-like macrophages predict postoperative prognosis of very high-risk prostate cancer. Sci. Rep. 2021, 11, 22495. [Google Scholar] [CrossRef] [PubMed]
- Magrowski, Ł.; Masri, O.; Ciepał, J.; Depowska, G.; Nowicka, Z.; Stando, R.; Chimiak, K.; Bylica, G.; Czapla, B.; Masri, M.; et al. Pre-Treatment Hemoglobin Concentration and Absolute Monocyte Count as Independent Prognostic Factors for Survival in Localized or Locally Advanced Prostate Cancer Patients Undergoing Radiotherapy. Biomedicines 2022, 10, 2514. [Google Scholar] [CrossRef]
- Olczak, M.; Orzechowska, M.J.; Bednarek, A.K.; Lipiński, M. The Transcriptomic Profiles of ESR1 and MMP3 Stratify the Risk of Biochemical Recurrence in Primary Prostate Cancer beyond Clinical Features. Int. J. Mol. Sci. 2023, 24, 8399. [Google Scholar] [CrossRef]
- Kang, D.W.; Barnes, O.; Vander Heiden, M.G.; Dieli-Conwright, C.M. Effect of exercise on tumor markers—Is exercise anti-tumorigenic in humans?: A scoping review of preliminary clinical investigations. Crit. Rev. Oncol. Hematol. 2022, 178, 103779. [Google Scholar] [CrossRef]
- Wei, J.T.; Barocas, D.; Carlsson, S.; Coakley, F.; Eggener, S.; Etzioni, R.; Fine, S.W.; Han, M.; Kim, S.K.; Kirkby, E.; et al. Early Detection of Prostate Cancer: AUA/SUO Guideline Part I: Prostate Cancer Screening. J. Urol. 2023, 210, 46–52. [Google Scholar] [CrossRef]
- Schröder, F.H.; Roobol, M.J.; van der Kwast, T.H.; Kranse, R.; Bangma, C.H. Does PSA velocity predict prostate cancer in pre-screened populations? Eur. Urol. 2006, 49, 460–465; discussion 465. [Google Scholar] [CrossRef]
- Wolters, T.; Roobol, M.J.; Bangma, C.H.; Schröder, F.H. Is prostate-specific antigen velocity selective for clinically significant prostate cancer in screening? European Randomized Study of Screening for Prostate Cancer (Rotterdam). Eur. Urol. 2009, 55, 385–392. [Google Scholar] [CrossRef]
- Stephan, C.; Stroebel, G.; Heinau, M.; Lenz, A.; Roemer, A.; Lein, M.; Schnorr, D.; Loening, S.A.; Jung, K. The ratio of prostate-specific antigen (PSA) to prostate volume (PSA density) as a parameter to improve the detection of prostate carcinoma in PSA values in the range of <4 ng/mL. Cancer 2005, 104, 993–1003. [Google Scholar] [CrossRef]
- Petrelli, F.; Vavassori, I.; Cabiddu, M.; Coinu, A.; Ghilardi, M.; Borgonovo, K.; Lonati, V.; Barni, S. Predictive Factors for Reclassification and Relapse in Prostate Cancer Eligible for Active Surveillance: A Systematic Review and Meta-analysis. Urology 2016, 91, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Maggi, M.; Panebianco, V.; Mosca, A.; Salciccia, S.; Gentilucci, A.; Di Pierro, G.; Busetto, G.M.; Barchetti, G.; Campa, R.; Sperduti, I.; et al. Prostate Imaging Reporting and Data System 3 Category Cases at Multiparametric Magnetic Resonance for Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. Focus. 2020, 6, 463–478. [Google Scholar] [CrossRef] [PubMed]
- Pagniez, M.A.; Kasivisvanathan, V.; Puech, P.; Drumez, E.; Villers, A.; Olivier, J. Predictive Factors of Missed Clinically Significant Prostate Cancers in Men with Negative Magnetic Resonance Imaging: A Systematic Review and Meta-Analysis. J. Urol. 2020, 204, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Garrido, M.M.; Bernardino, R.M.; Marta, J.C.; Holdenrieder, S.; Guimarães, J.T. Tumour markers in prostate cancer: The post-prostate-specific antigen era. Ann. Clin. Biochem. 2022, 59, 46–58. [Google Scholar] [CrossRef]
- Parekh, D.J.; Punnen, S.; Sjoberg, D.D.; Asroff, S.W.; Bailen, J.L.; Cochran, J.S.; Concepcion, R.; David, R.D.; Deck, K.B.; Dumbadze, I.; et al. A multi-institutional prospective trial in the USA confirms that the 4Kscore accurately identifies men with high-grade prostate cancer. Eur. Urol. 2015, 68, 464–470. [Google Scholar] [CrossRef]
- Braun, K.; Sjoberg, D.D.; Vickers, A.J.; Lilja, H.; Bjartell, A.S. A Four-kallikrein Panel Predicts High-grade Cancer on Biopsy: Independent Validation in a Community Cohort. Eur. Urol. 2016, 69, 505–511. [Google Scholar] [CrossRef]
- Lin, D.W.; Newcomb, L.F.; Brown, M.D.; Sjoberg, D.D.; Dong, Y.; Brooks, J.D.; Carroll, P.R.; Cooperberg, M.; Dash, A.; Ellis, W.J.; et al. Evaluating the Four Kallikrein Panel of the 4Kscore for Prediction of High-grade Prostate Cancer in Men in the Canary Prostate Active Surveillance Study. Eur. Urol. 2017, 72, 448–454. [Google Scholar] [CrossRef]
- Olleik, G.; Kassouf, W.; Aprikian, A.; Hu, J.; Vanhuyse, M.; Cury, F.; Peacock, S.; Bonnevier, E.; Palenius, E.; Dragomir, A. Evaluation of New Tests and Interventions for Prostate Cancer Management: A Systematic Review. J. Natl. Compr. Cancer Netw. 2018, 16, 1340–1351. [Google Scholar] [CrossRef]
- Stovsky, M.; Klein, E.A.; Chait, A.; Manickam, K.; Stephenson, A.J.; Wagner, M.; Dineen, M.; Lotan, Y.; Partin, A.; Baniel, J.; et al. Clinical Validation of IsoPSA™, a Single Parameter, Structure Based Assay for Improved Detection of High Grade Prostate Cancer. J. Urol. 2019, 201, 1115–1120. [Google Scholar] [CrossRef]
- Catalona, W.J.; Partin, A.W.; Sanda, M.G.; Wei, J.T.; Klee, G.G.; Bangma, C.H.; Slawin, K.M.; Marks, L.S.; Loeb, S.; Broyles, D.L.; et al. A multicenter study of [-2]pro-prostate specific antigen combined with prostate specific antigen and free prostate specific antigen for prostate cancer detection in the 2.0 to 10.0 ng/ml prostate specific antigen range. J. Urol. 2011, 185, 1650–1655, Erratum in J. Urol. 2011, 186, 354. [Google Scholar] [CrossRef] [PubMed]
- Guazzoni, G.; Lazzeri, M.; Nava, L.; Lughezzani, G.; Larcher, A.; Scattoni, V.; Gadda, G.M.; Bini, V.; Cestari, A.; Buffi, N.M.; et al. Preoperative prostate-specific antigen isoform p2PSA and its derivatives, %p2PSA and prostate health index, predict pathologic outcomes in patients undergoing radical prostatectomy for prostate cancer. Eur. Urol. 2012, 61, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Tosoian, J.J.; Druskin, S.C.; Andreas, D.; Mullane, P.; Chappidi, M.; Joo, S.; Ghabili, K.; Agostino, J.; Macura, K.J.; Carter, H.B.; et al. Use of the Prostate Health Index for detection of prostate cancer: Results from a large academic practice. Prostate Cancer Prostatic Dis. 2017, 20, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Loeb, S.; Catalona, W.J. The Prostate Health Index: A new test for the detection of prostate cancer. Ther. Adv. Urol. 2014, 6, 74–77. [Google Scholar] [CrossRef]
- de la Calle, C.; Patil, D.; Wei, J.T.; Scherr, D.S.; Sokoll, L.; Chan, D.W.; Siddiqui, J.; Mosquera, J.M.; Rubin, M.A.; Sanda, M.G. Multicenter Evaluation of the Prostate Health Index to Detect Aggressive Prostate Cancer in Biopsy Naïve Men. J. Urol. 2015, 194, 65–72. [Google Scholar] [CrossRef]
- Elyan, A.; Saba, K.; Sigle, A.; Wetterauer, C.; Engesser, C.; Püschel, H.; Attianese, S.; Maurer, P.; Deckart, A.; Cathomas, R.; et al. Prospective Multicenter Validation of the Stockholm3 Test in a Central European Cohort. Eur. Urol. Focus. 2024, 10, 620–626. [Google Scholar] [CrossRef]
- Vigneswaran, H.T.; Eklund, M.; Discacciati, A.; Nordström, T.; Hubbard, R.A.; Perlis, N.; Abern, M.R.; Moreira, D.M.; Eggener, S.; Yonover, P.; et al. SEPTASTHLM3 Study Group Stockholm3 in a Multiethnic Cohort for Prostate Cancer Detection (SEPTA): AProspective Multicentered Trial. J. Clin. Oncol. 2024, 42, 3806–3816. [Google Scholar] [CrossRef]
- Brooks, J.D. Stockholm3 in a Multiethnic Cohort: Optimizing Prostate Cancer Screening to Reduce Harm and Improve Equity. J. Clin. Oncol. 2024, 42, 3768–3772. [Google Scholar] [CrossRef]
- Nordström, T.; Discacciati, A.; Bergman, M.; Clements, M.; Aly, M.; Annerstedt, M.; Glaessgen, A.; Carlsson, S.; Jäderling, F.; Eklund, M.; et al. STHLM3 study group. Prostate cancer screening using a combination of risk-prediction, MRI, and targeted prostate biopsies (STHLM3-MRI): A prospective, population-based, randomised, open-label, non-inferiority trial. Lancet Oncol. 2021, 22, 1240–1249. [Google Scholar] [CrossRef]
- Björnebo, L.; Discacciati, A.; Falagario, U.; Vigneswaran, H.T.; Jäderling, F.; Grönberg, H.; Eklund, M.; Nordström, T.; Lantz, A. Biomarker vs MRI-Enhanced Strategies for Prostate Cancer Screening: The STHLM3-MRI Randomized Clinical Trial. JAMA Netw. Open 2024, 7, e247131. [Google Scholar] [CrossRef]
- Morote, J.; Campistol, M.; Triquell, M.; Celma, A.; Regis, L.; de Torres, I.; Semidey, M.E.; Mast, R.; Santamaria, A.; Planas, J.; et al. Improving the Early Detection of Clinically Significant Prostate Cancer in Men in the Challenging Prostate Imaging-Reporting and Data System 3 Category. Eur. Urol. Open Sci. 2022, 37, 38–44. [Google Scholar] [CrossRef]
- Tosoian, J.J.; Zhang, Y.; Xiao, L.; Xie, C.; Samora, N.L.; Niknafs, Y.S.; Chopra, Z.; Siddiqui, J.; Zheng, H.; Herron, G.; et al. EDRN-PCA3 Study Group. Development and Validation of an 18-Gene Urine Test for High-Grade Prostate Cancer. JAMA Oncol. 2024, 10, 726–736. [Google Scholar] [CrossRef]
- Tomlins, S.A.; Rhodes, D.R.; Perner, S.; Dhanasekaran, S.M.; Mehra, R.; Sun, X.W.; Varambally, S.; Cao, X.; Tchinda, J.; Kuefer, R.; et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 2005, 310, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Van Neste, L.; Hendriks, R.J.; Dijkstra, S.; Trooskens, G.; Cornel, E.B.; Jannink, S.A.; de Jong, H.; Hessels, D.; Smit, F.P.; Melchers, W.J.; et al. Detection of High-grade Prostate Cancer Using a Urinary Molecular Biomarker-Based Risk Score. Eur. Urol. 2016, 70, 740–748. [Google Scholar] [CrossRef]
- Hendriks, R.J.; van der Leest, M.M.G.; Israël, B.; Hannink, G.; YantiSetiasti, A.; Cornel, E.B.; Hulsbergen-van de Kaa, C.A.; Klaver, O.S.; Sedelaar, J.P.M.; Van Criekinge, W.; et al. Clinical use of the SelectMDx urinary-biomarker test with or without mpMRI in prostate cancer diagnosis: A prospective, multicenter study in biopsy-naïve men. Prostate Cancer Prostatic Dis. 2021, 24, 1110–1119. [Google Scholar] [CrossRef]
- Roumiguié, M.; Ploussard, G.; Nogueira, L.; Bruguière, E.; Meyrignac, O.; Lesourd, M.; Péricart, S.; Malavaud, B. Independent Evaluation of the Respective Predictive Values for High-Grade Prostate Cancer of Clinical Information and RNA Biomarkers after Upfront MRI and Image-Guided Biopsies. Cancers 2020, 12, 285. [Google Scholar] [CrossRef]
- Dang, X.T.T.; Kavishka, J.M.; Zhang, D.X.; Pirisinu, M.; Le, M.T.N. Extracellular Vesicles as an Efficient and Versatile System for Drug Delivery. Cells 2020, 9, 2191. [Google Scholar] [CrossRef] [PubMed]
- Donovan, M.J.; Noerholm, M.; Bentink, S.; Belzer, S.; Skog, J.; O’Neill, V.; Cochran, J.S.; Brown, G.A. A molecular signature of PCA3 and ERG exosomal RNA from non-DRE urine is predictive of initial prostate biopsy result. Prostate Cancer Prostatic Dis. 2015, 18, 370–375. [Google Scholar] [CrossRef] [PubMed]
- McKiernan, J.; Donovan, M.J.; Margolis, E.; Partin, A.; Carter, B.; Brown, G.; Torkler, P.; Noerholm, M.; Skog, J.; Shore, N.; et al. A Prospective Adaptive Utility Trial to Validate Performance of a Novel Urine Exosome Gene Expression Assay to Predict High-grade Prostate Cancer in Patients with Prostate-specific Antigen 2-10ng/ml at Initial Biopsy. Eur. Urol. 2018, 74, 731–738. [Google Scholar] [CrossRef]
- Nordström, T.; Engel, J.C.; Bergman, M.; Egevad, L.; Aly, M.; Eklund, M.; Palsdottir, T.; Grönberg, H. Identifying Prostate Cancer Among Men with Lower Urinary Tract Symptoms. Eur. Urol. Open Sci. 2021, 24, 11–16. [Google Scholar] [CrossRef]
- Eggener, S.E.; Rumble, R.B.; Armstrong, A.J.; Morgan, T.M.; Crispino, T.; Cornford, P.; van der Kwast, T.; Grignon, D.J.; Rai, A.J.; Agarwal, N.; et al. Molecular Biomarkers in Localized Prostate Cancer: ASCO Guideline. J. Clin. Oncol. 2020, 38, 1474–1494. [Google Scholar] [CrossRef]
- Mohler, J.L.; Antonarakis, E.S.; Armstrong, A.J.; D’Amico, A.V.; Davis, B.J.; Dorff, T.; Eastham, J.A.; Enke, C.A.; Farrington, T.A.; Higano, C.S.; et al. Prostate cancer, version 2.2019, NCCN Clinical Practice Guideline in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 479–505. [Google Scholar] [CrossRef]
- Olivier, J.; Stavrinides, V.; Kay, J.; Freeman, A.; Pye, H.; Ahmed, Z.; Carmona Echeverria, L.; Heavey, S.; Simmons, L.A.M.; Kanthabalan, A.; et al. Immunohistochemical biomarker validation in highly selective needle biopsy microarrays derived from mpMRI-characterized prostates. Prostate 2018, 78, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Salmasi, A.; Said, J.; Shindel, A.W.; Khoshnoodi, P.; Felker, E.R.; Sisk, A.E., Jr.; Grogan, T.; McCullough, D.; Bennett, J.; Bailey, H.; et al. A 17-Gene Genomic Prostate Score Assay Provides Independent Information on Adverse Pathology in the Setting of Combined Multiparametric Magnetic Resonance Imaging Fusion Targeted and Systematic Prostate Biopsy. J. Urol. 2018, 200, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Leapman, M.S.; Westphalen, A.C.; Ameli, N.; Lawrence, H.J.; Febbo, P.G.; Cooperberg, M.R.; Carroll, P.R. Association between a 17-gene genomic prostate score and multi-parametric prostate MRI in men with low and intermediate risk prostate cancer (PCa). PLoS ONE 2017, 12, e0185535. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Associazione Italiana Oncologia Medica (AIOM). Linee Guida CARCINOMA DELLA PROSTATA Edizione 2024 Aggiornamento. 2 Ottobre 2024. Available online: https://www.aiom.it/linee-guida-aiom-2024-carcinoma-della-prostata/ (accessed on 4 July 2025).[Green Version]
- Jairath, N.K.; Dal Pra, A.; Vince, R., Jr.; Dess, R.T.; Jackson, W.C.; Tosoian, J.J.; McBride, S.M.; Zhao, S.G.; Berlin, A.; Mahal, B.A.; et al. A Systematic Review of the Evidence for the Decipher Genomic Classifier in Prostate Cancer. Eur. Urol. 2021, 79, 374–383. [Google Scholar] [CrossRef]
- Erho, N.; Crisan, A.; Vergara, I.A.; Mitra, A.P.; Ghadessi, M.; Buerki, C.; Bergstralh, E.J.; Kollmeyer, T.; Fink, S.; Haddad, Z.; et al. Discovery and validation of a prostate cancer genomic classifier that predicts early metastasis following radical prostatectomy. PLoS ONE 2013, 8, e66855. [Google Scholar] [CrossRef] [PubMed]
- Karnes, R.J.; Choeurng, V.; Ross, A.E.; Schaeffer, E.M.; Klein, E.A.; Freedland, S.J.; Erho, N.; Yousefi, K.; Takhar, M.; Davicioni, E.; et al. Validation of a Genomic Risk Classifier to Predict Prostate Cancer-specific Mortality in Men with Adverse Pathologic Features. Eur. Urol. 2018, 73, 168–175. [Google Scholar] [CrossRef]
- Spratt, D.E.; Dai, D.L.Y.; Den, R.B.; Troncoso, P.; Yousefi, K.; Ross, A.E.; Schaeffer, E.M.; Haddad, Z.; Davicioni, E.; Mehra, R.; et al. Performance of a Prostate Cancer Genomic Classifier in Predicting Metastasis in Men with Prostate-specific Antigen Persistence Postprostatectomy. Eur. Urol. 2018, 74, 107–114. [Google Scholar] [CrossRef]
- Zhao, S.G.; Chang, S.L.; Spratt, D.E.; Erho, N.; Yu, M.; Ashab, H.A.; Alshalalfa, M.; Speers, C.; Tomlins, S.A.; Davicioni, E.; et al. Development and validation of a 24-gene predictor of response to postoperative radiotherapy in prostate cancer: A matched, retrospective analysis. Lancet Oncol. 2016, 17, 1612–1620. [Google Scholar] [CrossRef]
- Berlin, A.; Murgic, J.; Hosni, A.; Pintilie, M.; Salcedo, A.; Fraser, M.; Kamel-Reid, S.; Zhang, J.; Wang, Q.; Ch’ng, C.; et al. Genomic Classifier for Guiding Treatment of Intermediate-Risk Prostate Cancers to Dose-Escalated Image Guided Radiation Therapy Without Hormone Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 84–91. [Google Scholar] [CrossRef]
- Nguyen, P.L.; Huang, H.R.; Spratt, D.E.; Davicioni, E.; Sandler, H.M.; Shipley, W.U.; Efstathiou, J.A.; Simko, J.P.; Pollack, A.; Dicker, A.P.; et al. Analysis of a Biopsy-Based Genomic Classifier in High-Risk Prostate Cancer: Meta-Analysis of the NRG Oncology/Radiation Therapy Oncology Group 9202, 9413, and 9902 Phase 3 Randomized Trials. Int. J. Radiat. Oncol. Biol. Phys. 2023, 116, 521–529. [Google Scholar] [CrossRef]
- Bitting, R.L.; Wu, Y.; Somarelli, J.A.; Proudfoot, J.A.; Liu, Y.; Davicioni, E.; George, D.J.; Armstrong, A.J. Transcriptomic Signatures Associated With Outcomes in Recurrent Prostate Cancer Treated With Salvage Radiation, Androgen-Deprivation Therapy, and Enzalutamide: Correlative Analysis of the STREAM Trial. JCO Precis. Oncol. 2023, 7, e2300214. [Google Scholar] [CrossRef] [PubMed]
- McKay, R.R.; Feng, F.Y.; Wang, A.Y.; Wallis, C.J.D.; Moses, K.A. Recent Advances in the Management of High-Risk Localized Prostate Cancer: Local Therapy, Systemic Therapy, and Biomarkers to Guide Treatment Decisions. Am. Soc. Clin. Oncol. Educ. Book. 2020, 40, e241–e252. [Google Scholar] [CrossRef] [PubMed]
- Cullen, J.; Kuo, H.C.; Shan, J.; Lu, R.; Aboushwareb, T.; Van Den Eeden, S.K. The 17-Gene Genomic Prostate Score Test as a Predictor of Outcomes in Men with Unfavorable Intermediate Risk Prostate Cancer. Urology 2020, 143, 103–111. [Google Scholar] [CrossRef]
- Helfand, B.T.; Paterakos, M.; Wang, C.H.; Talaty, P.; Abran, J.; Bennett, J.; Hall, D.W.; Lehman, A.; Aboushwareb, T. The 17-gene Genomic Prostate Score assay as a predictor of biochemical recurrence in men with intermediate and high-risk prostate cancer. PLoS ONE 2022, 17, e0273782. [Google Scholar] [CrossRef]
- Tward, J.D.; Schlomm, T.; Bardot, S.; Freedland, S.J.; Lenz, L.; Cohen, T.; Stone, S.; Bishoff, J. Ability of the combined clinical cell-cycle risk score to identify patients that benefit from multi versus single modality therapy in NCCN intermediate and high-risk prostate cancer. J. Clin. Oncol. 2020, 38, 346. [Google Scholar] [CrossRef]
- Freedland, S.J.; Gerber, L.; Reid, J.; Welbourn, W.; Tikishvili, E.; Park, J.; Younus, A.; Gutin, A.; Sangale, Z.; Lanchbury, J.S.; et al. Prognostic utility of cell cycle progression score in men with prostate cancer after primary external beam radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 848–853. [Google Scholar] [CrossRef]
- Tward, J.D.; Schlomm, T.; Bardot, S.; Canter, D.J.; Scroggins, T.; Freedland, S.J.; Lenz, L.; Flake DD2nd Cohen, T.; Brawer, M.K.; Stone, S.; et al. Personalizing Localized Prostate Cancer: Validation of a Combined Clinical Cell-cycle Risk (CCR) Score Threshold for Prognosticating Benefit From Multimodality Therapy. Clin. Genitourin. Cancer 2021, 19, 296–304.e3. [Google Scholar] [CrossRef]
- Tward, J.; Lenz, L.; Flake, D.D.I.I.; Rajamani, S.; Yonover, P.; Olsson, C.; Kapoor, D.A.; Mantz, C.; Liauw, S.L.; Antic, T.; et al. The Clinical Cell-Cycle Risk (CCR) Score Is Associated With Metastasis After Radiation Therapy and Provides Guidance on When to Forgo Combined Androgen Deprivation Therapy With Dose-Escalated Radiation. Int. J. Radiat. Oncol. Biol. Phys. 2022, 113, 66–76, Erratum in Int. J. Radiat. Oncol. Biol. Phys. 2023, 115, 543. [Google Scholar] [CrossRef]
- Lenz, L.; Clegg, W.; Iliev, D.; Kasten, C.R.; Korman, H.; Morgan, T.M.; Hafron, J.; DeHaan, A.; Olsson, C.; Tutrone, R.F., Jr.; et al. Active surveillance selection and 3-year durability in intermediate-risk prostate cancer following genomic testing. Prostate Cancer Prostatic Dis. 2024, 28, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.A.; Ramsey, S.D.; Carlson, J.J. Cost-Effectiveness of a Biopsy-Based 8-Protein Prostate Cancer Prognostic Assay to Optimize Treatment Decision Making in Gleason 3 + 3 and 3 + 4 Early Stage Prostate Cancer. Oncologist 2015, 20, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Tuffaha, H.; Edmunds, K.; Fairbairn, D.; Roberts, M.J.; Chambers, S.; Smith, D.P.; Horvath, L.; Arora, S.; Scuffham, P. Guidelines for genetic testing in prostate cancer: A scoping review. Prostate Cancer Prostatic Dis. 2024, 27, 594–603. [Google Scholar] [CrossRef]
- Smith, C.P.; Proudfoot, J.A.; Boutros, P.C.; Reiter, R.E.; Valle, L.; Rettig, M.B.; Nickols, N.G.; Feng, F.Y.; Nguyen, P.L.; Nagar, H.; et al. Transcriptomic Heterogeneity in High-risk Prostate Cancer and Implications for Extraprostatic Disease at Presentation on Prostate-specific Membrane Antigen Positron Emission Tomography. Eur. Urol. Oncol. 2023, 6, 224–227. [Google Scholar] [CrossRef]
- Nikitas, J.; Subramanian, K.; Gozal, N.B.; Ricaurte-Fajardo, A.; Li, E.; Proudfoot, J.A.; Davicioni, E.; Marciscano, A.E.; Osborne, J.R.; Barbieri, C.E.; et al. Transcriptomic Profiling of Primary Prostate Cancers and Nonlocalized Disease on Prostate-Specific Membrane Antigen Positron Emission Tomography/Computed Tomography: A Multicenter Retrospective Study. JCO Precis. Oncol. 2024, 8, e2400161. [Google Scholar] [CrossRef] [PubMed]
- Kensler, K.H.; Baichoo, S.; Pathania, S.; Rebbeck, T.R. The tumor mutational landscape of BRCA2-deficient primary and metastatic prostate cancer. NPJ Precis. Oncol. 2022, 6, 39. [Google Scholar] [CrossRef]
- De Bono, J.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef]
- Smith, M.R.; Scher, H.I.; Sandhu, S.; Efstathiou, E.; Lara, P.N., Jr.; Yu, E.Y.; George, D.J.; Chi, K.N.; Saad, F.; Ståhl, O.; et al. GALAHAD investigators. Niraparib in patients with metastatic castration-resistant prostate cancer and DNA repair gene defects (GALAHAD): A multicentre, open-label, phase 2 trial. Lancet Oncol. 2022, 23, 362–373. [Google Scholar] [CrossRef]
- Abida, W.; Patnaik, A.; Campbell, D.; Shapiro, J.; Bryce, A.H.; McDermott, R.; Sautois, B.; Vogelzang, N.J.; Bambury, R.M.; Voog, E.; et al. TRITON2 investigators Rucaparib in Men With Metastatic Castration-Resistant Prostate Cancer Harboring a BRCA1 or BRCA2 Gene Alteration. J. Clin. Oncol. 2020, 38, 3763–3772. [Google Scholar] [CrossRef]
- Nicolosi, P.; Ledet, E.; Yang, S.; Michalski, S.; Freschi, B.; O’Leary, E.; Esplin, E.D.; Nussbaum, R.L.; Sartor, O. Prevalence of Germline Variants in Prostate Cancer and Implications for Current Genetic Testing Guidelines. JAMA Oncol. 2019, 5, 523–528. [Google Scholar] [CrossRef]
- Abusamra, S.M.; Solorzano, M.A.; Quarles, J.; Luke, M.; Patel, M.; Vince, R., Jr.; Jiang, R.; Volin, J.; Jacobs, M.F.; Kaffenberger, S.; et al. Detection of Germline Variants in Patients With Localized and Metastatic Prostate Cancer Through Guideline-Based Testing. Urol. Pract. 2024, 12, 63–72. [Google Scholar] [CrossRef]
- Carter, H.B.; Helfand, B.; Mamawala, M.; Wu, Y.; Landis, P.; Yu, H.; Wiley, K.; Na, R.; Shi, Z.; Petkewicz, J.; et al. Germline Mutations in ATM and BRCA1/2 Are Associated with Grade Reclassification in Men on Active Surveillance for Prostate Cancer. Eur. Urol. 2019, 75, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.; Kote-Jarai, Z.; Mikropoulos, C.; Eeles, R. Prostate Cancer Germline Variations and Implications for Screening and Treatment. Cold Spring Harb. Perspect. Med. 2018, 8, a030379. [Google Scholar] [CrossRef] [PubMed]
- Leongamornlert, D.; Mahmud, N.; Tymrakiewicz, M.; Saunders, E.; Dadaev, T.; Castro, E.; Goh, C.; Govindasami, K.; Guy, M.; O’Brien, L.; et al. Germline BRCA1 mutations increase prostate cancer risk. Br. J. Cancer 2012, 106, 1697–1701. [Google Scholar] [CrossRef] [PubMed]
- Kote-Jarai, Z.; Leongamornlert, D.; Saunders, E.; Tymrakiewicz, M.; Castro, E.; Mahmud, N.; Guy, M.; Edwards, S.; O’Brien, L.; Sawyer, E.; et al. BRCA2 is a moderate penetrance gene contributing to young-onset prostate cancer: Implications for genetic testing in prostate cancer patients. Br. J. Cancer 2011, 105, 1230–1234. [Google Scholar] [CrossRef]
- Bancroft, E.K.; Page, E.C.; Castro, E.; Lilja, H.; Vickers, A.; Sjoberg, D.; Assel, M.; Foster, C.S.; Mitchell, G.; Drew, K.; et al. Targeted prostate cancer screening in BRCA1 and BRCA2 mutation carriers: Results from the initial screening round of the IMPACT study. Eur. Urol. 2014, 66, 489–499, Erratum in Eur. Urol. 2015, 67, e126. [Google Scholar] [CrossRef]
- Castro, E.; Mikropoulos, C.; Bancroft, E.K.; Dadaev, T.; Goh, C.; Taylor, N.; Saunders, E.; Borley, N.; Keating, D.; Page, E.C.; et al. The PROFILE Feasibility Study: Targeted Screening of Men With a Family History of Prostate Cancer. Oncologist 2016, 21, 716–722. [Google Scholar] [CrossRef]
- Andreassen, C.N.; Rosenstein, B.S.; Kerns, S.L.; Ostrer, H.; De Ruysscher, D.; Cesaretti, J.A.; Barnett, G.C.; Dunning, A.M.; Dorling, L.; West, C.M.L.; et al. Individual patient data meta-analysis shows a significant association between the ATM rs1801516 SNP and toxicity after radiotherapy in 5456 breast and prostate cancer patients. Radiother. Oncol. 2016, 121, 431–439. [Google Scholar] [CrossRef]
- Mandel, P.; Metais, P. Les acides nucléiques du plasma sanguin chez l’homme [Nuclear Acids In Human Blood Plasma]. C R. Seances Soc. Biol. Fil. 1948, 142, 241–243. (In French) [Google Scholar]
- Crowley, E.; Di Nicolantonio, F.; Loupakis, F.; Bardelli, A. Liquid biopsy: Monitoring cancer-genetics in the blood. Nat. Rev. Clin. Oncol. 2013, 10, 472–484. [Google Scholar] [CrossRef]
- Cheng, F.; Su, L.; Qian, C. Circulating tumor DNA: A promising biomarker in the liquid biopsy of cancer. Oncotarget 2016, 7, 48832–48841. [Google Scholar] [CrossRef] [PubMed]
- Mehra, N.; Dolling, D.; Sumanasuriya, S.; Christova, R.; Pope, L.; Carreira, S.; Seed, G.; Yuan, W.; Goodall, J.; Hall, E.; et al. Plasma Cell-free DNA Concentration and Outcomes from Taxane Therapy in Metastatic Castration-resistant Prostate Cancer from Two Phase III Trials (FIRSTANA and PROSELICA). Eur. Urol. 2018, 74, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Hennigan, S.T.; Trostel, S.Y.; Terrigino, N.T.; Voznesensky, O.S.; Schaefer, R.J.; Whitlock, N.C.; Wilkinson, S.; Carrabba, N.V.; Atway, R.; Shema, S.; et al. Low Abundance of Circulating Tumor DNA in Localized Prostate Cancer. JCO Precis. Oncol. 2019, 3, 1–13. [Google Scholar] [CrossRef]
- Bastian, P.J.; Palapattu, G.S.; Yegnasubramanian, S.; Lin, X.; Rogers, C.G.; Mangold, L.A.; Trock, B.; Eisenberger, M.; Partin, A.W.; Nelson, W.G. Prognostic value of preoperative serum cell-free circulating DNA in men with prostate cancer undergoing radical prostatectomy. Clin. Cancer Res. 2007, 13, 5361–5367. [Google Scholar] [CrossRef]
- Chen, E.; Cario, C.L.; Leong, L.; Lopez, K.; Márquez, C.P.; Chu, C.; Li, P.S.; Oropeza, E.; Tenggara, I.; Cowan, J.; et al. Cell-free DNA concentration and fragment size as a biomarker for prostate cancer. Sci. Rep. 2021, 11, 5040. [Google Scholar] [CrossRef]
- Fei, X.; Du, X.; Gong, Y.; Liu, J.; Fan, L.; Wang, J.; Wang, Y.; Zhu, Y.; Pan, J.; Dong, B.; et al. Early Plasma Circulating Tumor DNA as a Potential Biomarker of Disease Recurrence in Non-metastatic Prostate Cancer. Cancer Res. Treat. 2023, 55, 969–977. [Google Scholar] [CrossRef]
- Pope, B.; Park, G.; Lau, E.; Belic, J.; Lach, R.; George, A.; McCoy, P.; Nguyen, A.; Grima, C.; Campbell, B.; et al. Ultrasensitive Detection of Circulating Tumour DNA enriches for Patients with a Greater Risk of Recurrence of Clinically Localised Prostate Cancer. Eur. Urol. 2024, 85, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Mao, X.; Guo, T.; Chan, P.Y.; Shaw, G.; Hines, J.; Stankiewicz, E.; Wang, Y.; Oliver, R.T.D.; Ahmad, A.S.; et al. The Novel Association of Circulating Tumor Cells and Circulating Megakaryocytes with Prostate Cancer Prognosis. Clin. Cancer Res. 2017, 23, 5112–5122. [Google Scholar] [CrossRef]
- Xu, L.; Mao, X.; Grey, A.; Scandura, G.; Guo, T.; Burke, E.; Marzec, J.; Abdu, S.; Stankiewicz, E.; Davies, C.R.; et al. Noninvasive Detection of Clinically Significant Prostate Cancer Using Circulating Tumor Cells. J. Urol. 2020, 203, 73–82. [Google Scholar] [CrossRef]
- Al-Hammouri, T.; Almeida-Magana, R.; Lawrence, R.; Duffy, T.; White, L.; Burke, E.; Kudahetti, S.; Collins, J.; Rajan, P.; Berney, D.; et al. Protocol for a prospective study evaluating circulating tumour cells status to predict radical prostatectomy treatment failure in localised prostate cancer patients (C-ProMeta-1). BMC Cancer 2023, 23, 581. [Google Scholar] [CrossRef]
- Dong, L.; Hu, C.; Ma, Z.; Huang, Y.; Shelley, G.; Kuczler, M.D.; Kim, C.J.; Witwer, K.W.; Keller, E.T.; Amend, S.R.; et al. Urinary extracellular vesicle-derived miR-126-3p predicts lymph node invasion in patients with high-risk prostate cancer. Med. Oncol. 2024, 41, 169. [Google Scholar] [CrossRef] [PubMed]
- McDermott, N.; Meunier, A.; Wong, S.; Buchete, V.; Marignol, L. Profiling of a panel of radioresistant prostate cancer cells identifies deregulation of key miRNAs. Clin. Transl. Radiat. Oncol. 2017, 2, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.; Ahmad, A.; Kong, D.; Ali, S.; Azmi, A.S.; Li, Y.; Banerjee, S.; Padhye, S.; Sarkar, F.H. Hypoxia induced aggressiveness of prostate cancer cells is linked with deregulated expression of VEGF, IL-6 and miRNAs that are attenuated by CDF. PLoS ONE 2012, 7, e43726. [Google Scholar] [CrossRef] [PubMed]
- Kachris, S.; Papadaki, C.; Rounis, K.; Tsitoura, E.; Kokkinaki, C.; Nikolaou, C.; Sourvinos, G.; Mavroudis, D. Circulating miRNAs as Potential Biomarkers in Prostate Cancer Patients Undergoing Radiotherapy. Cancer Manag. Res. 2021, 13, 8257–8271, Erratum in Cancer Manag. Res. 2022, 14, 409–410. https://doi.org/10.2147/CMAR.S360019. [Google Scholar] [CrossRef]
- Melton, C.A.; Freese, P.; Zhou, Y.; Shenoy, A.; Bagaria, S.; Chang, C.; Kuo, C.C.; Scott, E.; Srinivasan, S.; Cann, G.; et al. A Novel Tissue-Free Method to Estimate Tumor-Derived Cell-Free DNA Quantity Using Tumor Methylation Patterns. Cancers 2023, 16, 82. [Google Scholar] [CrossRef] [PubMed]
- Schrag, D.; Beer, T.M.; McDonnell CH3rd Nadauld, L.; Dilaveri, C.A.; Reid, R.; Marinac, C.R.; Chung, K.C.; Lopatin, M.; Fung, E.T.; Klein, E.A. Blood-based tests for multicancer early detection (PATHFINDER): A prospective cohort study. Lancet 2023, 402, 1251–1260. [Google Scholar] [CrossRef]
- Mahal, B.A.; Margolis, M.; Hubbell, E.; Chen, C.; Venstrom, J.M.; Abran, J.; Kartlitz, J.J.; Wyatt, A.W.; Klein, E.A. A Targeted Methylation-Based Multicancer Early Detection Blood Test Preferentially Detects High-Grade Prostate Cancer While Minimizing Overdiagnosis of Indolent Disease. JCO Precis. Oncol. 2024, 8, e2400269, Erratum in JCO Precis. Oncol. 2024, 8, e2400637. https://doi.org/10.1200/PO-24-00637. [Google Scholar] [CrossRef]
- Eismann, L.; von Walter, P.; Jung, A.; Chaloupka, M.; Rodler, S.; Westhofen, T.; Buchner, A.; Stief, C.G.; Stadler, T.; Schlenker, B. Methylation status of various gene loci in localized prostate cancer: Novel biomarkers for diagnostics and biochemical recurrence. Urol. Oncol. 2023, 41, e1–e325. [Google Scholar] [CrossRef]
- Liu, Q.; Reed, M.; Zhu, H.; Cheng, Y.; Almeida, J.; Fruhbeck, G.; Ribeiro, R.; Hu, P. Epigenome-wide DNA methylation and transcriptome profiling of localized and locally advanced prostate cancer: Uncovering new molecular markers. Genomics 2022, 114, 110474. [Google Scholar] [CrossRef]
- Patel, P.; Harmon, S.; Iseman, R.; Ludkowski, O.; Auman, H.; Hawley, S.; Newcomb, L.F.; Lin, D.W.; Nelson, P.S.; Feng, Z.; et al. Artificial Intelligence-Based PTEN Loss Assessment as an Early Predictor of Prostate Cancer Metastasis After Surgery: A Multicenter Retrospective Study. Mod. Pathol. 2023, 36, 100241. [Google Scholar] [CrossRef]
- Shao, Y.; Bazargani, R.; Karimi, D.; Wang, J.; Fazli, L.; Goldenberg, S.L.; Gleave, M.E.; Black, P.C.; Bashashati, A.; Salcudean, S. Prostate Cancer Risk Stratification by Digital Histopathology and Deep Learning. JCO Clin. Cancer Inform. 2024, 8, e2300184. [Google Scholar] [CrossRef]
- Pellegrini, M. Accurate prognosis for localized prostate cancer through coherent voting networks with multi-omic and clinical data. Sci. Rep. 2023, 13, 7875. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, N.M.; Almeida, J.G.; Rodrigues, A.; Vanneschi, L.; Matos, C.; Lisitskaya, M.V.; Uysal, A.; Silva, S.; Papanikolaou, N. Deep Learning Features Can Improve Radiomics-Based Prostate Cancer Aggressiveness Prediction. JCO Clin. Cancer Inform. 2024, 8, e2300180. [Google Scholar] [CrossRef] [PubMed]
- Tward, J.D.; Huang, H.C.; Esteva, A.; Mohamad, O.; van der Wal, D.; Simko, J.P.; DeVries, S.; Zhang, J.; Joun, S.; Showalter, T.N.; et al. Prostate Cancer Risk Stratification in NRG Oncology Phase III Randomized Trials Using Multimodal Deep Learning With Digital Histopathology. JCO Precis. Oncol. 2024, 8, e2400145. [Google Scholar] [CrossRef] [PubMed]
- Fay, M.; Liao, R.S.; Lone, Z.M.; Reddy, C.A.; Muhammad, H.; Xie, C.; Jain, P.; Huang, W.; Basu, H.S.; Nair, S.S.; et al. Artificial Intelligence-Based Digital Histologic Classifier for Prostate Cancer Risk Stratification: Independent Blinded Validation in Patients Treated With Radical Prostatectomy. JCO Clin. Cancer Inform. 2025, 9, e2400292. [Google Scholar] [CrossRef]
- Di Pierro, G.B.; Salciccia, S.; Frisenda, M.; Tufano, A.; Sciarra, A.; Scarrone, E.; Del Giudice, F.; Asero, V.; Bevilacqua, G.; Moriconi, M.; et al. Comparison of Four Validated Nomograms (Memorial Sloan Kettering Cancer Center, Briganti 2012, 2017, and 2019) Predicting Lymph Node Invasion in Patients with High-Risk Prostate Cancer Candidates for Radical Prostatectomy and Extended Pelvic Lymph Node Dissection: Clinical Experience and Review of the Literature. Cancers 2023, 15, 1683. [Google Scholar] [CrossRef]
- Morlacco, A.; Modonutti, D.; Motterle, G.; Martino, F.; Dal Moro, F.; Novara, G. Nomograms in Urologic Oncology: Lights and Shadows. J. Clin. Med. 2021, 10, 980. [Google Scholar] [CrossRef]
- Lorent, M.; Maalmi, H.; Tessier, P.; Supiot, S.; Dantan, E.; Foucher, Y. Meta-analysis of predictive models to assess the clinical validity and utility for patient-centered medical decision making: Application to the CAncer of the Prostate Risk Assessment (CAPRA). BMC Med. Inform. Decis. Mak. 2019, 19, 2. [Google Scholar] [CrossRef]
- Kumar Am, S.; Rajan, P.; Alkhamees, M.; Holley, M.; Lakshmanan, V.K. Prostate cancer theragnostics biomarkers: An update. Investig. Clin. Urol. 2024, 65, 527–539. [Google Scholar] [CrossRef]
- Meijer, D.; van Leeuwen, P.J.; Roberts, M.J.; Siriwardana, A.R.; Morton, A.; Yaxley, J.W.; Samaratunga, H.; Emmett, L.; van de Ven, P.M.; van der Poel, H.G.; et al. External Validation and Addition of Prostate-specific Membrane Antigen Positron Emission Tomography to the Most Frequently Used Nomograms for the Prediction of Pelvic Lymph-node Metastases: An International Multicenter Study. Eur. Urol. 2021, 80, 234–242. [Google Scholar] [CrossRef]
- Virgo, K.S.; Rumble, R.B.; Talcott, J.A. Initial Management of Noncastrate Advanced, Recurrent, or Metastatic Prostate Cancer: ASCO Guideline Update: ASCO Guideline Q and A. JCO Oncol. Pract. 2023, 19, 843–846. [Google Scholar] [CrossRef] [PubMed]
- Eastham, J.A.; Boorjian, S.A.; Kirkby, E. Clinically Localized Prostate Cancer: AUA/ASTRO Guideline. J. Urol. 2022, 208, 505–507. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.T.; Barocas, D.; Carlsson, S.; Coakley, F.; Eggener, S.; Etzioni, R.; Fine, S.W.; Han, M.; Kim, S.K.; Kirkby, E.; et al. Early Detection of Prostate Cancer: AUA/SUO Guideline Part II: Considerations for a Prostate Biopsy. J. Urol. 2023, 210, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Gillessen, S.; Bossi, A.; Davis, I.D.; de Bono, J.; Fizazi, K.; James, N.D.; Mottet, N.; Shore, N.; Small, E.; Smith, M.; et al. Management of Patients with Advanced Prostate Cancer. Part I: Intermediate-/High-risk and Locally Advanced Disease, Biochemical Relapse, and Side Effects of Hormonal Treatment: Report of the Advanced Prostate Cancer Consensus Conference 2022. Eur. Urol. 2023, 83, 267–293. [Google Scholar] [CrossRef]
- Salciccia, S.; Capriotti, A.L.; Laganà, A.; Fais, S.; Logozzi, M.; De Berardinis, E.; Busetto, G.M.; Di Pierro, G.B.; Ricciuti, G.P.; Del Giudice, F.; et al. Biomarkers in Prostate Cancer Diagnosis: From Current Knowledge to the Role of Metabolomics and Exosomes. Int. J. Mol. Sci. 2021, 22, 4367. [Google Scholar] [CrossRef]
- Wilson, T.K.; Zishiri, O.T. Prostate Cancer: A Review of Genetics, Current Biomarkers and Personalised Treatments. Cancer Rep. 2024, 7, e70016. [Google Scholar] [CrossRef]
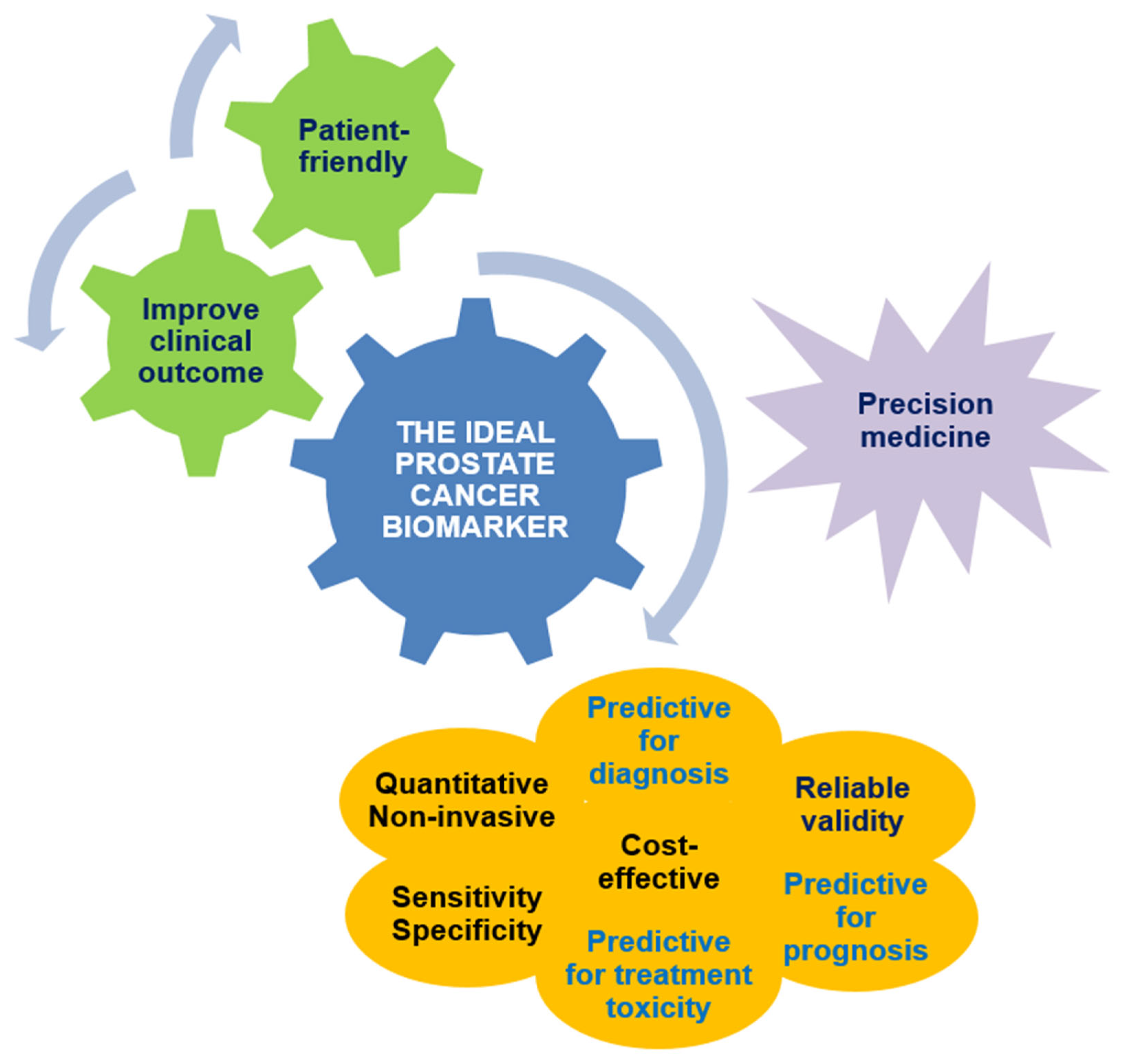
| Marker | Composition | Sample Type | Description | |
|---|---|---|---|---|
| Four-kallikrein score (4K score®) | _tPSA _fPSA _iPSA _human kallikrein 2 combined with clinical data (age, DRE, previous biopsy) | Blood | Available in the US Able to predict high-grade PC and the risk of metastatization | [39,40,41,42,43,44] |
| [-2]proPSA and Prostate Health Index (PHI) | _tPSA _fPSA isoforms from PSA precursor PHI density The ratio between PHI and mpMRI prostate volume | Blood | Available in the US Better diagnostic performance for PSA 2–10 ng/mL than PSA derivatives Uncertain diagnostic accuracy Up to 100% sensitivity for csPC detection with PHI density | [39,45,46,47,48,49] |
| Stockholm-3 test | _age _family history of PC _previous biopsy _tPSA _fPSA/tPSA ratio _human kallikrein 2 _macrophage inhibitory cytokine-1 _microseminoprotein-β [MSMB] _polygenic risk score (SNPs and HOXB13 SNP variant) | Blood | Available in the US and Scandinavian countries Able to predict csPC especially in combination with mpMRI | [39,50,51,52,53,54] |
| Proclarix® | _age _tPSA _fPSA _Thrombospondin 1 _Cathepsin D | Blood | Up to 100% detection of csPC especially for PIRADS 3 mpMRI cases | [55] |
| Progensa® | Hyperexpressed non-coding mRNA sequences (PCA 3) Investigational: Mi Prostate Score (MiPS) combination of tPSA with PCA 3 and the molecular signature TMPRSS2:ERG fusion | Urine | 80% PPV for PCA 3 score ≥ 60 (sensitivity 42%, specificity 91%) 88% NPV for PCA 3 ≤ 20 (sensitivity 75%, specificity 52%) | [39,56,57] |
| SelectMDx® | _HOXC6 mRNA _DLX1 mRNA _KLK3 mRNA combined with clinical information (age, PSA density, DRE, and family history of PC) | Urine | Considered under investigation Unrecognized 6.5% of PIRADS < 4 and 3.2% of PIRADS < 3 csPC Up to 93% NPV in combination with mpMRI | [39,58,59,60] |
| ExoDxTM | _PCA 3 exosomal RNA _TMPRSS2:ERG exosomal RNA _SPDEF exosomal RNA | Urine | Still investigational 15.6 Risk score (range 0–100) associated with increased probability of csPC | [39,61,62,63] |
| ERSPC Risk Calculators | _tPSA _prostate volume _mpMRI features _PHI combined with clinical variables (family history of PC, age, urinary symptoms, DRE, previous negative biopsy) | Mixed | Available online Validated in European and non-European populations Helpful in assessing the risk of csPC | [7] |
| ConfirmMDx® | _ GSTP1 DNA methylation status _APC DNA methylation status _RASSF1 DNA methylation status | Prostate biopsy | Not FDA approved An option for NCCN and European guidelines Able to overcome the challenge of negative biopsy for high probability of csPC | [7,9] |
| Marker | Composition | Sample Type | Description | |
|---|---|---|---|---|
| Decipher GC | 22-gene panel expressed in aggressive PC forms | Prostate tissue (post-prostatectomy and biopsy) | 0.45 and 0.60 cut-off for risk classification Able to predict post-prostatectomy 10y- PC specific mortality and risk for metastasis in case of adverse pathologic features and guide adjuvant treatment decision Prognostic factor for biochemical failure, lethal distant metastases, death for prostate cancer, and OS after primary RT | [71,72,73,74,75,76,77,78,79] |
| OncotypeDx®(GPS™) | 17 relevant genes for PC progression GPS 0–100 | Prostate tissue (post-prostatectomy and biopsy) | Independent predictor of high- and very high-risk PC (GPS 41–100) and time to BCR 21% reduction of interventional treatment for low- and very low-risk disease 35.7% reclassification of low-risk patients into intermediate-risk PC | [39,80,81] |
| Prolaris | 46 genes involved in cell cycle progression combined with clinicopathologic information CCR/CCP score −1.3–4.7 | Prostate tissue (post-prostatectomy and biopsy) | Risk threshold CCR score ≤ 2.112 prognosticates a clinically meaningful different risk of BCR and metastasis Able to guide AS decision | [39,82,83,84,85,86] |
| ProMark® | 8 tissue proteins | Prostate biopsy | 87.2% predictive value for little aggressive tumor in low-risk PC at a score ≤ 0.33 76.9% predictive value for unfavorable pathology at a risk score > 0.8 | [87] |
| Marker | EAU 2025 [7] | NCCN 2025 [9] | ASCO 2020–2023 [65,136] | AUA 2022–2023 [32,137,138] | APCCC 2023 [139] | NICE 2021 [8] |
|---|---|---|---|---|---|---|
| Four-kallikrein score (4K score®) | None | None | None | Not clear utility for reducing unnecessary biopsies | Minimize unnecessary prostate biopsies in men tested with PSA | None |
| [-2]proPSA and Prostate Health Index (PHI) | None | Not clear usefulness | None | Improve the detection of clinically significant prostate cancer | Reduce the number of unnecessary prostate biopsies in PSA-tested men | Not recommended |
| Stockholm-3 test | None | Not clear usefulness | None | Not clear utility for reducing unnecessary biopsies | Reduce the percentage of clinically insignificant cancers when used in combination with MRI in a PSA screening population | None |
| Proclarix® | None | None | None | Not clear utility for reducing unnecessary biopsies | Correlated with the detection of clinically significant PC, notably in the case of equivocal MRI | None |
| Progensa® | None | None | None | Not mentioned | Not clear usefulness | Not recommended |
| SelectMDx® | None | None | None | Not clear utility for reducing unnecessary biopsies | Not clear usefulness | None |
| ExoDxTM | None | None | None | Not clear utility for reducing unnecessary biopsies | Investigational | None |
| ERSPC Risk Calculators | None | None | None | Provide estimates that facilitate clinician/patient discussion of detection risk, but may differ for subgroups | Could help in discriminating between aggressive and non-aggressive tumors | None |
| ConfirmMDx® | None | None | None | Not clear utility for reducing unnecessary biopsies | Not mentioned | None |
| Decipher GC | Predictor of metastasis after RP, but to be used only in clinical trials | Recommended to inform adjuvant treatment post RP Considered as part of counselling for risk stratification in patients with PSA resistance/relapse after RP (category 2B) | Some clinical data Routine use not recommended in the absence of prospective trials | To be validated in prospective clinical trials Routine use not recommended | Prognostic role Routine use not recommended in the absence of prospective trials | None |
| OncotypeDx®(GPS™) | To be used only in clinical trials | Part of counselling for risk stratification | Some clinical data Routine use not recommended in the absence of prospective trials | To be validated in prospective clinical trials Routine use not recommended | Prognostic role Routine use not recommended in the absence of prospective trials | None |
| Prolaris | To be used only in clinical trials | Part of counselling for risk stratification | Some clinical data Routine use not recommended in the absence of prospective trials | To be validated in prospective clinical trials Routine use not recommended | Prognostic role Routine use not recommended in the absence of prospective trials | None |
| ProMark® | None | Not mentioned | Some clinical data Routine use not recommended in the absence of prospective trials | Not mentioned | Prognostic role Routine use not recommended in the absence of prospective trials | None |
| PAM50 | None | None | None | None | None | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bardoscia, L.; Sardaro, A.; Quattrocchi, M.; Cocuzza, P.; Ciurlia, E.; Furfaro, I.; Gilio, M.A.; Mignogna, M.; Detti, B.; Ingrosso, G. The Evolving Landscape of Novel and Old Biomarkers in Localized High-Risk Prostate Cancer: State of the Art, Clinical Utility, and Limitations Toward Precision Oncology. J. Pers. Med. 2025, 15, 367. https://doi.org/10.3390/jpm15080367
Bardoscia L, Sardaro A, Quattrocchi M, Cocuzza P, Ciurlia E, Furfaro I, Gilio MA, Mignogna M, Detti B, Ingrosso G. The Evolving Landscape of Novel and Old Biomarkers in Localized High-Risk Prostate Cancer: State of the Art, Clinical Utility, and Limitations Toward Precision Oncology. Journal of Personalized Medicine. 2025; 15(8):367. https://doi.org/10.3390/jpm15080367
Chicago/Turabian StyleBardoscia, Lilia, Angela Sardaro, Mariagrazia Quattrocchi, Paola Cocuzza, Elisa Ciurlia, Ilaria Furfaro, Maria Antonietta Gilio, Marcello Mignogna, Beatrice Detti, and Gianluca Ingrosso. 2025. "The Evolving Landscape of Novel and Old Biomarkers in Localized High-Risk Prostate Cancer: State of the Art, Clinical Utility, and Limitations Toward Precision Oncology" Journal of Personalized Medicine 15, no. 8: 367. https://doi.org/10.3390/jpm15080367
APA StyleBardoscia, L., Sardaro, A., Quattrocchi, M., Cocuzza, P., Ciurlia, E., Furfaro, I., Gilio, M. A., Mignogna, M., Detti, B., & Ingrosso, G. (2025). The Evolving Landscape of Novel and Old Biomarkers in Localized High-Risk Prostate Cancer: State of the Art, Clinical Utility, and Limitations Toward Precision Oncology. Journal of Personalized Medicine, 15(8), 367. https://doi.org/10.3390/jpm15080367








