Evaluation of a New Multiparametric Microdot Array-Based Immunoassay Panel for Systemic Autoimmune Disease Diagnosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Methods
2.2.1. ZENIT AMiDot CTD panel
2.2.2. FEIA method (EliA Test) for Anti-ENA specificities
2.2.3. CLIA PRIME ZENIT
2.2.4. LIA Euroline ANA Profile 3 plus DFS70 IgG
3. Statistical Analysis
4. Results
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tozzoli, R. Recent advances in diagnostic technologies and their impact in autoimmune diseases. Autoimmun. Rev. 2007, 6, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Sack, U.; Bossuyt, X.; Andreeva, H.; Antal-Szalmás, P.; Bizzaro, N.; Bogdanos, D.; Borzova, E.; Conrad, K.; Dragon-Durey, M.-A.; Eriksson, C.; et al. European Autoimmunity Standardisation Initiative. Quality and best practice in medical laboratories: Specific requests for autoimmunity testing. Auto. Immun. Highlights 2020, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Sciascia, S.; Bizzaro, N.; Meroni, P.L.; Dimitrios, B.; Borghi, M.O.; Bossuyt, X.; Grossi, C.; Tornai, D.; Papp, M.; Shoenfeld, Y.; et al. Autoantibodies testing in autoimmunity: Diagnostic, prognostic and classification value. Autoimmun. Rev. 2023, 22, 103356. [Google Scholar] [CrossRef] [PubMed]
- Tozzoli, R.; D’Aurizio, F.; Villalta, D.; Bizzaro, N. Automation, consolidation, and integration in autoimmune diagnostics. Auto. Immun. Highlights 2015, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lalvani, A.; Meroni, P.L.; Millington, K.A.; Modolo, M.L.; Plebani, M.; Tincani, A.; Villalta, D.; Doria, A.; Ghirardello, A. Recent advances in diagnostic technology: Applications in autoimmune and infectious diseases. Clin. Exp. Rheumatol. 2008, 26, S62–S66. [Google Scholar] [PubMed]
- Sharp, V.; Utz, P.J. Technology insight: Can autoantibody profiling improve clinical practice? Nat. Clin. Pract. Rheumatol. 2007, 3, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Plebani, M.; Pittoni, M.; Celadin, M.; Bernardi, D.; Mion, M.M. Recent advances in diagnostic technologies for autoimmune diseases. Autoimmun. Rev. 2009, 8, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Cinquanta, L.; Infantino, M.; Bizzaro, N. Detecting Autoantibodies by Multiparametric Assays: Impact on Prevention, Diagnosis, Monitoring, and Personalized Therapy in Autoimmune Diseases. J. Appl. Lab. Med. 2022, 7, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Bizzaro, N. Autoantibody Profiles in Autoimmune Rheumatic Diseases. Mediterr. J. Rheumatol. 2019, 30, 86–89. [Google Scholar]
- Bossuyt, X.; De Langhe, E.; Borghi, M.O.; Meroni, P.L. Understanding and interpreting antinuclear antibody tests in systemic rheumatic diseases. Nat. Rev. Rheumatol. 2020, 16, 715–726. [Google Scholar] [CrossRef]
- Bonroy, C.; Vercammen, M.; Fierz, W.; Andrade, L.E.C.; Van Hoovels, L.; Infantino, M.; Fritzler, M.J.; Bogdanos, D.; Kozmar, A.; Nespola, B.; et al. Detection of antinuclear antibodies: Recommendations from EFLM, EASI and ICAP. Clin. Chem. Lab. Med. 2023, 61, 1167–1198. [Google Scholar] [CrossRef]
- Meroni, P.L.; Schur, P.H. ANA screening: An old test with new recommendations. Ann. Rheum. Dis. 2010, 69, 1420–1422. [Google Scholar] [CrossRef] [PubMed]
- Vulsteke, J.B.; Van Hoovels, L.; Willems, P.; Vander Cruyssen, B.; Vanderschueren, S.; Westhovens, R.; Blockmans, D.; De Langhe, E.; Bossuyt, X. Titre specific positive predictive value of anti-nuclear antibody patterns. Ann. Rheum. Dis. 2021, 80, e128. [Google Scholar] [CrossRef] [PubMed]
- Damoiseaux, J.; Andrade, L.E.C.; Carballo, O.G.; Conrad, K.; Francescantonio, P.L.C.; Fritzler, M.J.; de la Torre, I.G.; Herold, M.; Klotz, W.; Cruvinel, W.d.M.; et al. Clinical relevance of HEp-2 indirect immunofluorescent patterns: The International Consensus on ANA patterns (ICAP) perspective. Ann. Rheum. Dis. 2019, 78, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Tozzoli, R.; Bonaguri, C.; Melegari, A.; Antico, A.; Bassetti, D.; Bizzaro, N. Current state of diagnostic technologies in the autoimmunology laboratory. Clin. Chem. Lab. Med. 2013, 51, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, R.M.; Tan, E.M. Recent progress in the study of autoantibodies to nuclear antigens. Hum. Pathol. 1978, 9, 85–91. [Google Scholar] [CrossRef] [PubMed]
- De Rooij, D.J.; van de Putte, L.B.; Habets, W.J.; Verbeek, A.L.; van Venrooij, W.J. The use of immunoblotting to detect antibodies to nuclear and cytoplasmic antigens. Clinical and serological associations in rheumatic diseases. Scand. J. Rheumatol. 1988, 17, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Tozzoli, R.; Villalta, D.; Bizzaro, N. Challenges in the Standardization of Autoantibody Testing: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2017, 53, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.H.; DiGennaro, C.; Hueber, W.; Haab, B.B.; Kamachi, M.; Dean, E.J.; Fournel, S.; Fong, D.; Genovese, M.C.; De Vegvar, H.E.N.; et al. Autoantigen microarrays for multiplex characterization of autoantibody responses. Nat. Med. 2002, 8, 295–301. [Google Scholar] [CrossRef]
- Carbone, T.; Infantino, M.; Antico, A.; Porcelli, B.; Villalta, D.; Pafundi, V.; Bizzaro, N. An Italian nationwide survey on the evolution of autoantibody diagnostics in autoimmune rheumatic diseases. Clin. Exp. Rheumatol. 2023, 41, 2277–2285. [Google Scholar] [CrossRef]
- González, D.A.; de León, A.C.; Pérez, M.D.C.R.; Díaz, B.B.; Hernández, A.G.; García, D.G.; Moncholi, C.V.; Jaime, A.A. Efficiency of different strategies to detect autoantibodies to extractable nuclear antigens. J. Immunol. Methods 2010, 360, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Kahng, J.; Kim, Y.; Park, Y.J.; Han, K.; Kwok, S.K.; Park, S.H.; Oh, E.J. Comparative study of immunofluorescent antinuclear antibody test and line immunoassay detecting 15 specific autoantibodies in patients with systemic rheumatic disease. J. Clin. Lab. Anal. 2012, 26, 307–314. [Google Scholar] [CrossRef] [PubMed]
- López-Longo, F.J.; Rodríguez-Mahou, M.; Escalona-Monge, M.; González, C.M.; Monteagudo, I.; Carreño-Pérez, L. Simultaneous identification of various antinuclear antibodies using an automated multiparameter line immunoassay system. Lupus 2003, 12, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Mahler, M.; Betteridge, Z.; Bentow, C.; Richards, M.; Seaman, A.; Chinoy, H.; McHugh, N. Comparison of Three Immunoassays for the Detection of Myositis Specific Antibodies. Front. Immunol. 2019, 10, 848. [Google Scholar] [CrossRef] [PubMed]
- Infantino, M.; Carbone, T.; Brusca, I.; Alessio, M.G.; Previtali, G.; Platzgummer, S.; Paura, G.; Castiglione, C.; Fabris, M.; Pesce, G.; et al. Study Group on Autoimmune Diseases of the Italian Society of Clinical Pathology and Laboratory Medicine. Current technologies for anti-ENA antibody detection: State-of-the-art of diagnostic immunoassays. J. Immunol. Methods 2022, 507, 113297. [Google Scholar] [CrossRef] [PubMed]
- Infantino, M.; Bentow, C.; Seaman, A.; Benucci, M.; Atzeni, F.; Sarzi-Puttini, P.; Olivito, B.; Meacci, F.; Manfredi, M.; Mahler, M. Highlights on novel technologies for the detection of antibodies to Ro60, Ro52, and SS-B. Clin. Dev. Immunol. 2013, 2013, 978202. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.K.; Fritzler, M.J.; Wiik, A.; Andrade, L.E.; Reeves, W.H.; Tincani, A.; Meroni, P.L.; IUIS/WHO/AF/CDC Committee for the Standardization of Autoantibodies in Rheumatic and Related Diseases. AutoAbSC.Org—Autoantibody Standardization Committee in 2006. Autoimmun. Rev. 2007, 6, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Dillaerts, D.; De Baere, H.; Bossuyt, X. Clinical autoantibody detection by microarray. Clin. Chem. Lab. Med. 2017, 55, 578–585. [Google Scholar] [CrossRef]
- Norimatsu, Y.; Matsuda, K.M.; Yamaguchi, K.; Ono, C.; Okumura, T.; Kogo, E.; Kotani, H.; Hisamoto, T.; Kuzumi, A.; Fukasawa, T.; et al. The Autoantibody Array Assay: A Novel Autoantibody Detection Method. Diagnostics 2023, 13, 2929. [Google Scholar] [CrossRef]
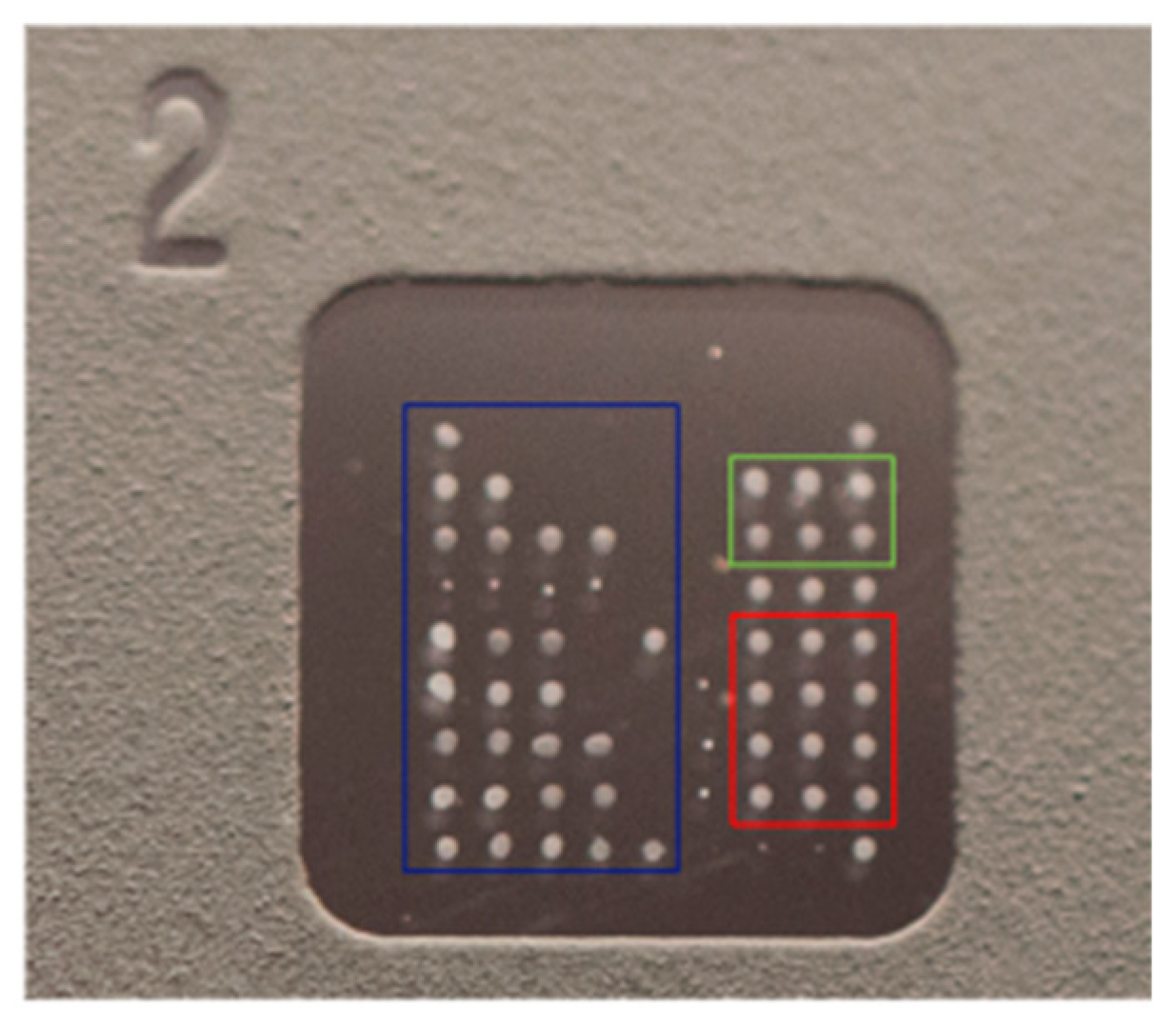
| ARD | Patients (n) | Ratio F/M | Age (Years) Mean ± SD | Disease Duration (Days) Mean ± SD |
|---|---|---|---|---|
| SSc | 24 | 17/7 | 62.0 ± 12.0 | 85.6 ± 73.5 |
| SjS | 21 | 15/6 | 69.2 ± 9.7 | 182.4 ± 13.1 |
| SLE | 16 | 10/6 | 58.4 ± 13.5 | 122.4 ± 61.6 |
| PM/DM | 8 | 7/1 | 60.3 ± 13.7 | 145.4 ± 43.1 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Registration | 0 ug/mL Cal | 0 ug/mL Cal | 0 ug/mL Cal | Positive Control | Positive Control | Positive Control | Registration | |
| 2 | 25 ug/mL Cal | 25 ug/mL Cal | 25 ug/mL Cal | Conjugate Control | Conjugate Control | Conjugate Control | |||
| 3 | Ro60 | Ro52 | SSB/La | Sm | RNP A/C/68kD | Jo-1 | Scl70 | PMScl100 | dsDNA |
| 4 | Ro60 | Ro52 | SSB/La | Sm | RNP A/C/68kD | Jo-1 | Scl70 | PMScl100 | dsDNA |
| 5 | Ro60 | Ro52 | SSB/La | Sm | RNP A/C/68kD | Jo-1 | Scl70 | PMScl100 | dsDNA |
| 6 | Ribosomal P0 | CENP B | DFS70 | PCNA | Ku | Mi-2 | PL7/PL12 | M2 | Nucleosome |
| 7 | Ribosomal P0 | CENP B | DFS70 | PCNA | Ku | Mi-2 | PL7/PL12 | M2 | Nucleosome |
| 8 | Ribosomal P0 | CENP B | DFS70 | PCNA | Ku | Mi-2 | PL7/PL12 | M2 | Nucleosome |
| 9 | Registration | sp100 | sp100 | sp100 | gp210 | gp210 | gp210 | Registration |
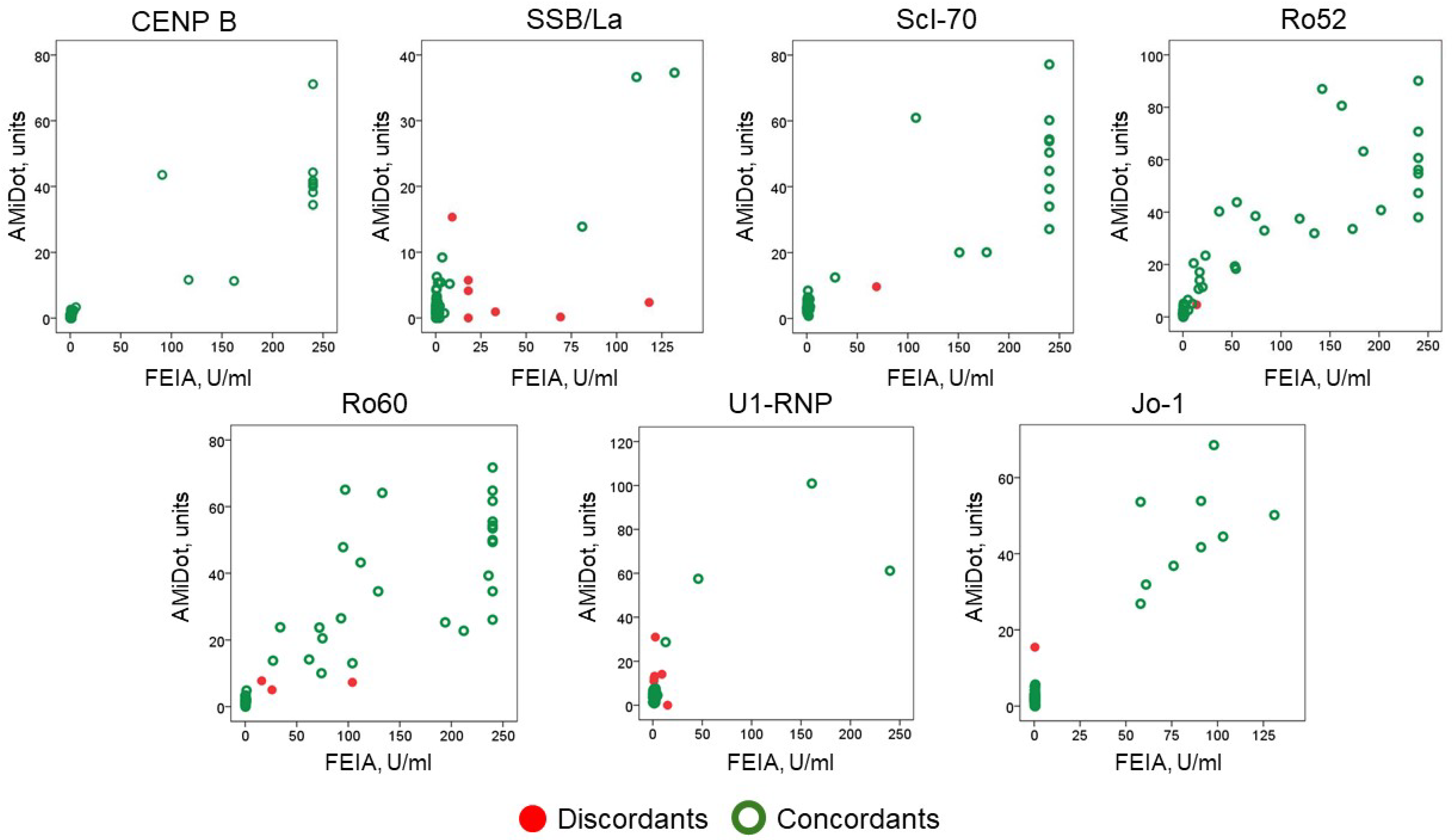
| k | k (95% CI) | p-Value | AMiDot(+) FEIA(+) | AMiDot(−) FEIA(−) | AmiDot(+) FEIA(−) | AmiDot(−) FEIA(+) | Concordance (%) AMiDot-FEIA | |
|---|---|---|---|---|---|---|---|---|
| CENP B | 1.000 | 1.000–1.000 | <0.001 | 10 | 59 | 0 | 0 | 100% |
| SSB/La | 0.415 | 0.070–0.759 | <0.001 | 3 | 59 | 1 | 6 | 90% |
| Scl-70 | 0.954 | 0.865–1.000 | <0.001 | 13 | 55 | 0 | 1 | 99% |
| Ro52 | 0.969 | 0.910–1.000 | <0.001 | 26 | 42 | 0 | 1 | 99% |
| Ro60 | 0.911 | 0.812–1.000 | <0.001 | 27 | 39 | 0 | 3 | 96% |
| U1-RNP | 0.527 | 0.201–0.854 | <0.001 | 4 | 59 | 5 | 1 | 91% |
| Jo-1 | 0.939 | 0.821–1.000 | <0.001 | 9 | 59 | 1 | 0 | 99% |
| SAMPLE | ID SAMPLE | DISCORDANT ANTIBODY | FEIA (U/mL) TermoFisher | AMiDot (AU/mL) A. Menarini Diagnostics | ZENIT PRIME (AU/mL) A. Menarini Diagnostics | LIA (AU) Euroimmun | CLINICAL DIAGNOSIS |
|---|---|---|---|---|---|---|---|
| 1 | 4340714165 | Scl-70 | POS (69) | NEG (9.63) | POS (121.7) | POS (53) | SSc |
| 2 | 4340714165 | Jo-1 | NEG (0.4) | POS (15.48) | POS (49.5) | POS (160) | SSc |
| 3 | 4340122320 | SSB/La | POS (33) | NEG (0.93) | POS (16.3) | POS (59) | SjS |
| 4 | 4340125659 | SSB/La | POS (118) | NEG (2.36) | POS (82.24) | POS (51) | SjS |
| 5 | 4340125661 | SSB/La | POS (18) | NEG (5.74) | NEG (7.6) | POS (19) | SjS |
| 6 | 4340125688 | SSB/La | NEG (9.1) | POS (15.34) | NEG (1.9) | POS (27) | SjS |
| 7 | 4340125761 | SSB/La | POS (69) | NEG (0.14) | POS (24) | POS (31) | SjS |
| 8 | 4340126222 | SSB/La | POS (18) | NEG (<0.01) | NEG (0) | POS (19) | SjS |
| 9 | 4345098797 | SSB/La | POS (18) | NEG (4.13) | NEG (8.6) | POS (19) | SjS |
| 10 | 4340126647 | Ro60 | POS (16) | NEG (7.74) | POS (20.3) | POS (46) | SSc |
| 11 | 4340127827 | Ro60 | POS (104) | NEG (7.27) | NEG (6.7) | POS (65) | SLE |
| 12 | 4340083266 | Ro60 | POS (26) | NEG (5.03) | Insufficient Sample | NEG (14) | SSc |
| 13 | 4340126585 | Ro52 | POS (14) | NEG (4.63) | NEG (4.4) | POS (91) | SjS |
| 14 | 4340126638 | U1-RNP | POS (15) | NEG (<0.01) | POS (14.4) | nd | SLE |
| 15 | 4340718515 | U1-RNP | NEG (2.6) | POS (31.01) | POS (31.4) | nd | SSc |
| 16 | 4340715372 | U1-RNP | NEG (1.8) | POS (13.22) | POS (27.6) | nd | SSc |
| 17 | 4340083266 | U1-RNP | NEG (1.4) | POS (12.43) | NEG (1.4) | nd | SSc |
| 18 | 4327002104 | U1-RNP | NEG (9.2) | POS (14.08) | POS (18.9) | nd | PM/DM |
| 19 | 4340095147 | U1-RNP | NEG (1.2) | POS (10.98) | NEG (5.9) | nd | PM/DM |
| ANTIGEN | ||||||||
|---|---|---|---|---|---|---|---|---|
| ASSAY (METHODS) | MANUFACTURER | Scl-70 | CENP B | Jo-1 | Ro60 | Ro52 | SSB/La | U1-RNP |
| AMiDot | A. Menarini Diagnostics | N | R | R | R | R | R | R |
| LIA | Euroimmun | N | R | N | N | R | N | na |
| FEIA | TermoFisher Scientific | R | R | R | R | R | R | R |
| CLIA | A. Menarini Diagnostics | R | R | R | R | R | R | R |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Infantino, M.; Pavia, F.; Grossi, V.; Lari, B.; Benucci, M.; Li Gobbi, F.; Pancani, S.; Manfredi, M. Evaluation of a New Multiparametric Microdot Array-Based Immunoassay Panel for Systemic Autoimmune Disease Diagnosis. J. Pers. Med. 2024, 14, 607. https://doi.org/10.3390/jpm14060607
Infantino M, Pavia F, Grossi V, Lari B, Benucci M, Li Gobbi F, Pancani S, Manfredi M. Evaluation of a New Multiparametric Microdot Array-Based Immunoassay Panel for Systemic Autoimmune Disease Diagnosis. Journal of Personalized Medicine. 2024; 14(6):607. https://doi.org/10.3390/jpm14060607
Chicago/Turabian StyleInfantino, Maria, Francesca Pavia, Valentina Grossi, Barbara Lari, Maurizio Benucci, Francesca Li Gobbi, Silvia Pancani, and Mariangela Manfredi. 2024. "Evaluation of a New Multiparametric Microdot Array-Based Immunoassay Panel for Systemic Autoimmune Disease Diagnosis" Journal of Personalized Medicine 14, no. 6: 607. https://doi.org/10.3390/jpm14060607
APA StyleInfantino, M., Pavia, F., Grossi, V., Lari, B., Benucci, M., Li Gobbi, F., Pancani, S., & Manfredi, M. (2024). Evaluation of a New Multiparametric Microdot Array-Based Immunoassay Panel for Systemic Autoimmune Disease Diagnosis. Journal of Personalized Medicine, 14(6), 607. https://doi.org/10.3390/jpm14060607






