Abstract
Dementia is a significant global health issue that is exacerbated by an aging population. Imaging plays an established role in the evaluation of patients with neurocognitive disorders such as dementia. In current clinical practice, magnetic resonance imaging (MRI) and positron emission tomography (PET) are primary imaging modalities used separately but in concert to help diagnose and classify dementia. The clinical applications of PET/MRI hybrid imaging in dementia are an active area of research, particularly given the continued emergence of functional MRI (fMRI) and amyloid PET tracers. This narrative review provides a comprehensive overview of the rationale and current evidence for PET/MRI hybrid dementia imaging from 2018 to 2023. Hybrid imaging offers advantages in the accuracy of characterizing neurodegenerative disorders, and future research will need to address the cost of integrated PET/MRI systems compared to stand-alone scanners, the development of new biomarkers, and image correction techniques.
Keywords:
PET/MRI; hybrid imaging; radiotracers; artificial intelligence; Alzheimer’s disease; dementia; aging 1. Introduction
In recent years, the use of simultaneous positron emission tomography and magnetic resonance imaging (PET/MRI) hybrid imaging has transformed how we understand and image dementia and mild cognitive impairment (MCI). Its role is expected to expand further due to an anticipated increase in the population of elderly patients (greater than or equal to 65 years old). The prevalence of Alzheimer’s disease (AD) is approximately 4% in this population and increases dramatically with age [1]. This global health issue is also compounded by the underdiagnosis of dementia based on current clinical guidelines. Studies have shown a discrepancy between diagnosis rates and the projected increase in the population with dementia in developed and developing nations [2,3]. Ferri et al. summarized that age-specific dementia prevalence of AD should be expected to be similar worldwide, but differences exist due to difficulties establishing social impairment or lower survival [2]. Their team suggests that developing nations with a relatively low base number are expected to see rapid expansion. For example, they estimated that although in 2010 Latin American countries had approximately half as many people with dementia as North America, this number is expected to be in a similar range of 9 million by 2040.
Several bodies of work emphasize the importance of earlier intervention and prevention using common treatments such as acetylcholinesterase inhibitors or monoclonal antibodies against amyloid-beta (Aβ), which can be augmented by simultaneous PET/MRI imaging [4,5]. Amyloid deposition appears to plateau early in the presymptomatic or prodromal phases of AD, according to longitudinal data on individuals undergoing serial amyloid imaging and MR imaging examinations [6]. Individuals diagnosed with AD typically do not show a considerable increase in amyloid over time as determined by PET tracers, which highlights the role of hybrid imaging that can also detect subtle changes on MRI in the preclinical phases of dementia [7]. In addition, the recent introduction of novel anti-amyloid beta monoclonal antibodies represents a paradigm shift in the management of Alzheimer’s dementia, ushering in a new era of targeted therapies. Lecanemab, the first monoclonal antibody to be approved by the United States Food and Drug Administration, has been shown to significantly delay clinical decline and improve functional status among patients with Alzheimer’s dementia. However, the efficacy of lecanemab has only been established in the setting of early-stage disease. The detection of amyloid-beta deposition early in the course of illness is therefore of paramount importance. Advancements in PET/MR have the potential to identify patients with the earliest imaging signs of Alzheimer’s dementia who may benefit from lecanemab and similar anti-amyloid beta agents. Indeed, with the pressing demand to better understand and characterize dementia for this growing demographic, PET/MRI has a growing role in early diagnosis.
Previous review literature on hybrid PET/MR imaging in dementia was mostly limited to time periods before 2020 and has focused on various specifics such as changes in image acquisition settings, post-processing, regions of interest such as the hippocampus, and tracers [8,9,10]. As imaging continues to quickly improve, the purpose of this narrative review is to examine the latest articles published between 2018 and 2023 on the applications of PET/MRI in the characterization of various subtypes of dementia. This article will also summarize significant findings in key brain regions for its continued future use and the overall distribution of scanner field strengths, brands, and tracers in the latest set of studies.
2. Methods
A PubMed inquiry was performed with the MeSH terms (“PET-MR” OR “PET-MRI”) AND (“Dementia” OR “MCI” OR “Mild cognitive impairment” OR “Alzheimer” OR “AD”) on 20 January 2024 with dates restricted to between 2018 and 2023. Common abbreviations were employed to capture papers of interest related to the application of hybrid imaging to subtypes of dementia or MCI. The inclusion criteria used to filter papers for initial eligibility included published in English, sample size of at least ten human subjects, related to MCI or dementia, and employed hybrid PET/MRI imaging. This is a critical distinction since several studies may have listed PET/MRI as a keyword but imaged patients with either modality separately at different time points. Abstracts were also excluded if the objective of this study was to compare the performance of different PET tracers or were from a conference proceeding. Two researchers were assigned to each paper to filter the abstracts and titles using these guidelines. The papers were further divided into categories such as clinical, technical, or review based on the article type displayed on PubMed. Further data extraction was performed on the clinical studies to describe current trends, and each article was read by at least two researchers on the team. Downstream analysis was performed with R (Version 4.2.2, The R Foundation for Statistical Computing, Vienna, Austria) and the following packages: tidyverse (Version 2.0.0) and scales (Version 1.2.1). Figure generation was performed on Adobe Illustrator (Version 26.2.1, Adobe Inc., San Jose, CA, USA).
3. Results
The literature search workflow is summarized in Figure 1. A total of 229 studies were initially identified using the PubMed search. After abstracts were excluded according to the inclusion criteria detailed in Section 2, the number of articles was narrowed to 105. The most common reasons for exclusion in the first screen were for employing MRI or PET separately (n = 50/229) and not being directly applied to the disease of interest (n = 43/229). The least common reasons were non-human studies (n = 8/229), low sample size (n = 13/229), comparing the performance of PET tracers (n = 9/229), and being a conference proceeding (n = 1/229). After attempting access with institutional subscriptions, 101 studies were obtained. After a full-text review, six more studies were removed due to not being applied to MCI or dementia directly or for employing MRI and PET separately. A total of 95 papers were included in this narrative review: 61 clinical, 16 technical, and 18 review manuscripts.
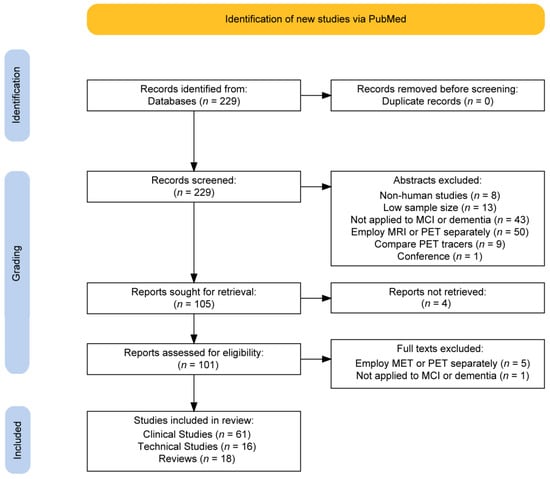
Figure 1.
Flow chart summarizing the selection criteria of retrieved articles from a PubMed database search.
Figure 2 demonstrates the distribution of key characteristics in the trend of clinical studies (n = 60) in the past five years. The most commonly used PET tracer was 18F-FDG (63.9%; n = 39/61) with 11C-PiB (13.1%; n = 8/61) and 18F-Flutematamol (3.3%; n = 2/61) as runner-ups. 15O-Water, 64Cu-ATSM, 18F-THK-5317, 18F-PI-2620, and 18F-Florbetapir were each used in one study (1.6%; n = 1/61). A minority of studies had unspecified tracers (8.2%; n = 5/61). GE Signa was the most commonly used hybrid scanner (39.3%; n = 24/61), and the Siemens Biograph mMR was second (37.7%; n = 23/61). The most used field strength was 3 T (65.6%; n = 40/61), with only one at 7 T (1.6%; n = 1/61). A significant number of articles had unspecified field strengths (31.1%, n = 19/61). Figure 3 shows the trend in the number of articles over time related to the filtered and raw database query. The two years with the highest number of articles among the filtered articles were 2021 (24.6%; n = 15/61) and 2022 (26.2%; n = 16/61). Among the raw articles, 2021 (23.6%; n = 54/229) and 2022 (22.7%; n = 52/229) were also the years with the highest number of initially relevant articles. Both of these subplots in Figure 3 demonstrate an overall positive trend in the number of PET/MRI papers over time. Table 1 summarizes the main features of the eligible clinical studies.
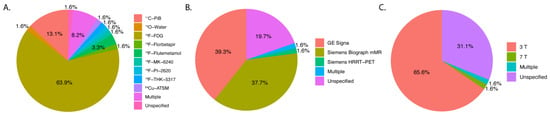
Figure 2.
Pie charts demonstrating the distribution of key features of each clinical article that was filtered (n = 59). (A) PET tracers, (B) PET/MRI brand, and model (C) field strength. Note: Percentages may not sum to 100% due to label rounding.
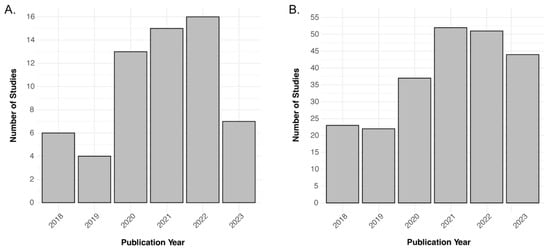
Figure 3.
Bar charts showcasing the trend in the number of articles over the 2018–2023 data range. (A) n = 60 for the filtered articles and (B) n = 229 for the unfiltered database inquiry.

Table 1.
General characteristics of the clinical studies included in the review.
4. Discussion
Overall, the number of reviews after filtering was 19.4% (18/93) of the eligible articles. Common themes across these review articles included the rise of artificial intelligence, technical improvements to PET/MRI imaging, and new and previous regions of interest for evaluating dementia [8,10,72,73,74,75,76,77]. The distribution of these article types remains consistent with this current review and is discussed further in the sections below along with additional insights from the clinical articles sourced in the past five years.
4.1. Artificial Intelligence
More in-depth discussions surrounding artificial intelligence (AI) and machine learning (ML) were present across all article types within this review [78,79,80]. Zhou et al. and Minoshima et al. summarized the common ML techniques used in radiology such as feed-forward neural networks (FFNN) and convolutional neural networks (CNN) [78,79]. This work showed that most AI studies in hybrid imaging employ a CNN or generative adversarial network (GAN) as a part of the classification model. CNNs are useful in automatically extracting spatial features from images and can learn relevant hierarchies, but are not effective in temporal information, which renders this modality ineffective for evaluating the progression of AD over time on volumetric data. GANs can generate synthetic data that can resemble real dementia samples; however, it can be challenging to train and can create unrealistic images. Logan et al. provide a similar explanation of different networks and explain the application of ensemble models which combine the output of different models for a final prediction [80]. Common themes in the review articles remain consistent with each other, such as brain regions of interest in dementia, but with the recent spike in interest in AI, there are more recent studies that discuss how ML improves PET/MRI acquisitions compared to previous periods. As it stands, CNNs typically have the best performance and potential when it comes to neuroimaging analysis and is the go-to model compared to other networks in this review. This model can automatically extract and learn image features and can efficiently analyze large datasets of PET/MRI images. Limitations of CNNs are not unique to this model and affect the field of AI as a whole such as sensitivity to imaging protocols, which can be mitigated by training on various scanners or field strengths, and overfitting if there is limited data. Within this discussion, the technical improvements and clinical classification of dementia subtypes aided by ML advancements will be further detailed.
4.2. Technical Improvements
4.2.1. Attenuation Correction
Technical studies accounted for 19.4% (18/93) of the selected articles. A majority focused on accurate attenuation correction (AC) [39,81,82,83,84,85,86]. Traditionally, a density map for the correction had to be derived from MRI to properly measure the PET tracer activity but was challenging due to the lack of bone signal [87]. Several methods were studied to correct attenuation in a large cohort. Best methods, including RESOLUTE, a well-known segmentation-based AC method, were found to be sensitive to specified MRI sequences and thus could be significantly impacted by newer models or system updates [88]. Ladefegod et al. leveraged CNNs to convert non-attenuation corrected PET images to attenuation and scatter-corrected images, and due to the nature of neural networks, were shown to be more robust to different scanners [39]. Additional studies used deep learning to correlate a longitudinal relaxation rate, which is best evaluated by a T1-weighted study, with Hounsfield units in bone and soft tissues in the brain to perform AC [83]. Outside of ML, a study performed in 2020 by Sgard et al. used a zero echo-time (ZTE) MRI to generate the attenuation map and yielded lower bias and interindividual variability compared to a single-atlas AC method [81]. In addition, a quality assessment study by Øen et al. validated that MRI-based AC (MRAC) performed closely to CT-based AC (CTAC). Scores calculated for FDG uptake showed that the cerebellar region had the smallest mean absolute difference from this paper [82].
4.2.2. Motion Correction
Various methods have been evaluated to correct motion. These range from mechanical methods, such as head restraints, to image-based methods, such as frame-based motion correction (FBMC), which registers the PET images to a reference position so that they are aligned [89]. Two technical articles in this review discussed and quantitatively measured the impact of motion correction on the imaging of dementia patients [90,91]. In a study performed by Tiss et al., using frame-by-frame motion detection reduced the standard deviation in the quantification of tau accumulation by 16% to 49% in areas such as the precuneus and amygdala [90]. Notably, in a study that included 30 subjects with dementia, Chen et al. found that motion correction had the greatest impact in regions with larger amplitude motion, such as the medial orbitofrontal cortex [91].
4.2.3. Tracer and Imaging Techniques
A few articles within this review studied how to comprehensively evaluate the scores of tracer intensity on hybrid imaging [92,93]. One study by Coath et al. focused on using the Centiloid conversion to standardize measurements for amyloid-beta plaque accumulation measured by 18F-Florbetapir across different study cohorts, such as Insight 46 or the Alzheimer’s Disease Neuroimaging Initiative (ADNI) [92]. The standardized uptake value ratio (SUVR) is widely used for extracting data from PET images. This metric is dependent on many variables, including the tracer used, target and reference regions, and image acquisition parameters. The 0 Centiloids (CL) anchor point represents the average amyloid level in young healthy controls while 100 Centiloids correspond to the average level in a typical patient with AD with a generalized linear formula: Centiloids = a × (SUVR) + b. The coefficients are determined through a calibration process with samples on the anchor points. Another project by Ford et al. used a heuristic scoring system to count regions of the brain below a preset z-score threshold using statistical parametric mapping (SPM). This method standardizes and examines brain images on a voxel-by-voxel basis and employs statistical tests such as ANOVA or Student’s t-test to determine significant differences. They found that this scoring system had similar performance compared to the random forest method that was trained on all the z-scores to differentiate frontotemporal lobar degeneration (FTLD) from AD. Using this heuristic method is more accessible than a machine learning tool for labs that lack computational support.
Interestingly, there is an effort to generate zero-dose healthy baselines of FDG PET/MRI images and acquire hybrid imaging at ultra-low doses using GANs and CNNs to help the development of patient safety protocols [94,95,96,97]. Many clinicians interpret the FDG-PET component of hybrid images with healthy controls of a similar demographic. However, diminished tracer uptake is non-specific and may reflect intrinsic anatomical differences or represent sequela of prior neurologic insult, such as chronic microvascular ischemic change. Previous investigations have used variational autoencoders (VAEs), which are a type of neural network that learns to compress the input image data and then reconstruct it back into its original form. The variational component allows the encoder to distribute the input onto a latent space allowing for variability and more control over how the VAE generates a custom image. In one study by Hinge et al., these artificially generated images were compared with healthy controls and had invariance to mean relative percent difference and mean absolute value relative difference [94]. Another set of projects focused on preserving diagnostic quality with shorter scan times with a high-sensitivity time-of-flight (TOF) PET system. Behr et al. showed that the image quality on the TOF PET/MRI images was comparable to or better than PET/CT even with reduced scan times and established that the 4 min acquisition times received the highest scores. Although the brain and parotid glands did not show significant differences in SUVmax, other normal tissues like the humerus, aorta, lung, spleen, and liver showed major differences. This suggests limitations in applying this technology beyond neuroimaging. In another study, ultra-low dose tau accumulation images were enhanced using a GAN [96]. The authors used 5% of the original full dose and found that the ultra-low-dose images had an underestimation in SUVRs in regions with classically higher uptake but that the bias was reduced in the enhanced images, especially in the amyloid-positive healthy population. This is especially useful in tracers that are specific for amyloid-beta plaques such as 18F-Florbetaben since they have a relatively short half-life, synergistically increasing the safety profile of these imaging runs [97]. These findings should be taken into consideration when designing future high-powered studies to reduce subject radiation dose.
Overall, 18F-FDG is the most prevalent tracer used in clinical practice and is the most represented among the articles extracted in this review. It is currently non-specific for AD pathology since hypometabolism can occur in various types of dementia and neurological conditions. However, the use of the tracer in both evaluation of AD and non-AD neurodegenerative conditions remains important since this tracer is widely used in clinical practice and has a wealth of literature for comparison. In addition, most of the tracers specific for amyloid and tau plaques such as 18F-Flutemetamol, 18F-MK-6240, and 18F-PI-2620 are primarily employed in research settings due to developing protocols. 11C-PiB has been well-validated and is highly regarded for its specificity for amyloid-beta plaques, but its short half-life of approximately 20 minutes requires an on-site cyclotron and limits its use in research institutions. On the other hand, 18F-Florbetapir is an agent that binds specifically to amyloid plaques and is approved for clinical use in many countries including the United States to estimate plaque density, making this tracer useful for AD evaluation. It is not approved, however, for confirming a diagnosis of AD. Our findings suggest that a combination of 18F-FDG and 18F-Florbetapir be employed in clinical practice for the characterization of AD, and a combination of 18F-FDG and 11C-PiB to be leveraged at research facilities, where available. For non-AD causes of dementia, 18F-FDG on its own could be appropriate as an initial imaging study in PET/MRI. Regarding variation across imaging techniques, it was shown in a meta-analysis that the accuracy of hybrid imaging markers is dependent on how the tracer is measured and the type of tracer itself [98]. Frisoni et al. stated that 18F-FDG had more variability compared to the amyloid biomarkers due to the lack of a strong consensus on imaging protocols and encouraged clinicians to analyze work using standard diagnostic criteria from the International Working Group and data analysis tools that are well validated such as FreeSurfer [98]. Consequently, PET/MRI performs best as long as there is a reference normative population such as healthy controls present for a baseline comparison within the same study.
4.3. Functional Connectivity
Functional connectivity studies, which revolve around associations between activities of different brain regions and often involve function MRI (fMRI), remain a critical area of focus in PET/MRI. Common networks studied in dementia include the default mode network (DMN), salience network (SN), central executive network (CEN), sensorimotor networks, as well as language and visual networks. The DMN is active when the brain is at wakeful rest, such as daydreaming and recalling memories, while the CEN is involved in high-level cognition, such as problem solving and decision making. The SN is critical for filtering relevant internal and external stimuli and coordinating responses to these stimuli. One team led by Zhang et al. showed that there was lower glucose metabolism observed in the DMN, CEN, and SN in patients with AD compared to the healthy controls [69]. This was further supported by studies that showed that the temporal properties of the DMN were altered in PET/MRI of patients with different stages of AD, meaning that affected patients spent a shorter amount of time in this network [53]. Specific disruptions in the DMN include a reduction in long-range functional connectivity between the medial prefrontal cortex (MPFC) and a decrease in the blood-oxygenation level-dependent (BOLD) functional connectivity within the precuneus of the DMN [32]. Within the SN, patients with posterior cortical atrophy and semantic dementia were shown to have decreased activity from the left superior frontal gyrus to the right anterior insula [21].
Another study by Carlson et al. found that white matter connectivity was impacted in the hippocampus by tau accumulation. This was measured with fractional anisotropy, finding that there were significant elevations in the entorhinal, perirhinal, and cornu ammonis (CA) 1 regions among patients with dementia or MCI. The limbic cortico-striato-thalmo-cortical (CTSC) circuit has been discussed in detail regarding the behavioral variant frontotemporal dementia in both the presymptomatic and symptomatic stages [42]. This analysis found that there was weakened frontostriatal connectivity but enhanced striatothalamic and thalamofrontal connectivity in asymptomatic carriers of a tau protein mutation compared to non-carriers. Common limitations within functional connectivity studies are that most are cross-sectional at a single time point (time of imaging), which does not show disease progression or the evolution of these network connectivity changes.
4.4. Brain Regions of Interest and Biomarkers
4.4.1. Hippocampus
The hippocampus is of interest in hybrid imaging because this area is known to be important in memory and cognition, and volume losses have been associated with MCI and dementia. One study by James et al. found that patients with smaller hippocampal volume at age 70 had a faster search speed decline in the preceding 26 years, which was measured by the response time to identifying a target within a visual set-up [35,99]. For every 1 mL decrease in volume, there was an additional decline of approximately 1 point in search speed per year. Another body of work suggested that amyloid-beta patients with preclinical AD had a smaller presubiculum volume compared to the healthy controls [52]. However, not every study showed consistent results regarding the loss of whole-hippocampal volume. One group showed that although the whole-hippocampal SUV was not statistically different between AD/MCI and controls, there was a substantially lower SUV in the dentate gyrus (DG) in the test group compared to the controls [17]. Despite this variation, the hippocampus remains significant as an anatomical region of interest based on the papers included in this review, and we recommend that this area be evaluated in hybrid imaging studies for conditions along the spectrum of MCI and Alzheimer’s disease.
4.4.2. Gray and White Matter
Studies examining gray and white matter features are critically important to the study of dementia in hybrid imaging since brain atrophy is a common feature in the disease group and the degree of volume differences can distinguish between subtypes of cognitive impairment. In one cross-sectional study, there were significant differences in white matter diffusion metrics such as fractional anisotropy (FA), radial diffusivity (RD), radial kurtosis (RK), and axonal water fraction (AWF) between the amyloid-beta positive- and -negative groups [25]. FA is a scalar value that quantifies the directionality of white matter and ranges from 0 to 1, where 0 represents equal diffusion in all directions while 1 means highly one-directional diffusion. Low values suggest that there is disruption to the fibers. RD is a measure of water diffusion perpendicular to the main axis of diffusion. Increased RD values suggest demyelination. Radial kurtosis characterizes the non-Gaussian behavior of water diffusion and an elevated value means that there is complex tissue microstructure such as axonal damage. Finally, AWF quantifies the fraction of water content associated with axonal space, and increases in AWF are typically associated with a loss of axons.
Outside of diffusion metrics, white matter hyperintensity volume (WMHV) may represent an important marker of dementia; one group found that greater volumes were associated with poorer tracing accuracy in a circle drawing task within patients that had a Familial Alzheimer’s Disease (FAD) mutation and sporadic Alzheimer’s [44]. Gray matter volume losses were also found to be weakly to strongly positively correlated with regional hypometabolism within the different lobes depending on the subtype of dementia. For example, there was moderately significant gray matter loss in the occipital lobes for dementia with Lewy bodies (DLB) and strongly significant gray matter loss in the superior parietal lobule in corticobasal degeneration (CBD) [27].
4.4.3. Biomarker Evaluation
Iron, amyloid-beta, and tau protein accumulation assessments represent important neurodegenerative disease markers and have been used to track disease progression as well as the early detection of dementia. Amyloid and tau accumulation is the most common assessment performed in the evaluation of AD. A significant number of clinical studies within this review show an increased amyloid and tau protein burden in patients with different types of dementia [25,31,35,37,38,55,94]. These subtypes can be further differentiated depending on the local distribution of the amyloid. For example, in patients with early-onset familial AD, it has been shown that there is increased amyloid deposition in the striatum compared to late-onset sporadic AD [55]. Interestingly, amyloid-beta can be identified by tracers that directly bind to them such as Pittsburgh compound B (PiB). Iron plaques have also been shown to correlate to amyloid-beta plaques in specific regions such as the frontal and temporal cortex; however, iron levels alone were not directly linked to cognitive performance [62]. In addition, another study showed increased quantitative susceptibility mapping (QSM), a measure of iron accumulation, in the putamen in patients with AD compared with non-AD patients [61].
4.5. Associated Illnesses
There are numerous causes of cognitive impairment for which PET imaging may play an important role in diagnosis and management, including Parkinson’s disease (PD), normal pressure hydrocephalus (NPH), multiple sclerosis (MS), and Down’s syndrome. Within this review, one analysis of the characteristics of Parkinson’s disease with mild cognitive impairment (PD-MCI) showed that 8/25 patients (32%) had amyloid-beta positivity. The study identified significant deposition in regions like the right caudal and rostral middle frontal cortex, precuneus, and left pars triangularis in the amyloid-beta-positive patients [31]. A study on MS showed that lower myelin content as measured by decreased 11C-PiB uptake was associated with lower cognitive status [15]. For NPH, an investigation led by Mangalore et al. showed hypometabolism in the medial frontal and temporal lobes on PET and sulcal enlargement of the same areas on MRI, suggesting that the increased pressure on the brain alters perfusion and contributes to cognitive impairment [46]. Lastly, Down’s syndrome is unique in the fact that a large percentage of patients with this disease eventually develop cognitive impairment. One ML study used support vector machine (SVM) regression models to show that there was a significant reduction in white matter connectivity in PiB-positive patients with Down’s syndrome compared with PiB-negative patients [14]. Given the ongoing development in the literature for imaging protocols, there is no strong consensus for tracers in approaching other etiologies of dementia beyond AD. However, a similar approach to the one described in Section 4.2.3 can be potentially employed, in which 18F-FDG can be used for the initial study due to its prevalence and 18F-Florbetapir can be added on in clinical practice if there is suspicion of amyloid pathology among the dementia subtypes.
4.6. Scanners
It is critical for future recommendations to evaluate the most common field strengths, model brands, and tracers used in PET/MRI. The most commonly used tracer among the articles reviewed was FDG. 3 T was the most common field strength, which is consistent with the models currently available for commercial sale on the combined PET/MRI imaging systems market. GE and Siemens are the only two producers of integrated imaging systems, which is consistent with an almost even split between the majority of scanner brands extracted from this review.
Only one study within this review noted the advantages of using ultra-high field MRI in combination with PET on the hippocampus compared to the 1.5 T and 3 T strengths [22]. 7 T has been increasingly utilized to identify subtle findings that differentiate disease and physiologic brain states in the setting of Cushing’s disease, epilepsy, dementia, and similar clinical entities [100,101,102]. Choi et al. found that the 7 T integration provided a higher signal-to-noise ratio and stronger spatial resolution which enabled detailed segmentation of the hippocampal subregions that was not possible before. They found that there was significantly less metabolic activity in the middle CA1, posterior CA1, and CA2/3 regions of the left hippocampus and the middle and posterior CA2/3 regions of the right hippocampus in the AD group. This paper also described decreased activity between CA4 and the dentate gyrus (DG) within the hippocampus. Limitations include that 7 T has been mainly restricted to the research setting. However, 7 T MRI is expected to become widely available in the clinical setting in the near future and may allow for the detection of subtle hippocampal aberrations in pre-symptomatic stages of AD.
4.7. Comparison to MRI or PET
Advances in PET/MRI hybrid imaging including the development of new tracers and techniques have been fast-paced and increasingly and rigorously compared to PET or MRI alone. Historically, the first studies on PET/MRI were in the brain, combining PET detection of neurotransmitter activity with MRI detection of white matter damage [103,104,105]. A feasibility analysis performed by Schlemmer et al. in 2008 showed that image quality was comparable to the individual scanners, and the temporal and spatial registration allowed for accurate matching of tracer uptake and MR signal features [103]. Subsequent studies showed that leveraging features from both modalities led to stronger performance compared to each alone [36,106,107]. Dukart et al. in 2011 showed that a support vector machine (SVM) classification was able to discriminate between AD and frontotemporal lobar degeneration (FTLD) using gray-matter information on MRI and fluorodeoxyglucose (FDG) uptake on PET with an accuracy of 94%, which was higher than the model trained on MR information alone [106]. MRI on its own has been shown by prior studies to lack specificity in detecting AD, particularly in the early stages of the disease [108,109]. In a 2007 study by Matsunari et al., voxel-based morphometry (VBM) which is MRI-based had a specificity of 87% for early-onset AD compared to 96% in FDG-PET [108]. In addition, enthusiasm for PET/MRI grew with the increasing use of new tracers beyond FDG such as 11C-Pittsburgh compound-B (PiB). PiB was first introduced in 2004 and binds specifically to amyloid-beta plaques in the brain, allowing for direct quantification of plaque burden in the brain [110]. Certainly, these improvements may improve the specificity of multimodal image acquisition in dementia subtyping while enabling evaluation within a single session for many patients who may otherwise have difficulty tolerating multiple sessions.
PET/MRI has become more financially reasonable for the evaluation of cognitive impairment due to its increased efficacy [72,111]. Manuel et al. estimate that for every five-year delay in dementia onset, there are direct savings of approximately USD 8 billion per year or approximately USD 135,000 per person [112]. Prato et al. estimate savings of USD 115,000 per person assuming that PET/MRI costs approximately USD 2000 per study and approximately one in ten patients have evidence of cognitive impairment on hybrid imaging [72]. Currently, due to the slower-than-anticipated incorporation of hybrid systems, cycle times for newer generation of models may be greater than ten years. Due to this trend, more companies are aiming to build dedicated brain PET inserts that can augment stand-alone MRI systems, which is cheaper than a full hybrid system. Specifically, it costs approximately USD 6 million for a packaged hybrid system versus USD 1.3 million for a PET insert that can be added to a USD 3 million 3 T MRI system, making PET/MRI more attractive as a modality [72]. Increasing adoption of the technology will continue to motivate clinical studies and reviews to more strongly inform the direction of multimodal imaging.
4.8. Limitations
Limitations of this study include that PubMed indexing may require four to twelve weeks to complete for some journals. This may explain why the number of articles in 2023 from this literature search is lower compared to the previous two years. In addition, the narrative review focused on articles from PubMed-indexed articles, which reflects high-quality articles based on journal selection but does not include papers indexed in other databases such as Scopus and Web of Science. For describing macro-trends in the dataset and for an in-depth discussion of common themes, narrative reviews are typically sufficient in broad recommendations. However, potential areas of improvement are to expand the scope of the review systematically to encompass multiple databases and leverage a quality and bias assessment such as the Cochrane risk-of-bias tool [113].
4.9. Ongoing Challenges and Future Directions of Hybrid Imaging
Hybrid imaging will play a central role in the diagnosis of AD and other neurocognitive disorders. The early detection of amyloid-beta deposition is of profound importance in the emerging era of targeted monoclonal antibodies and other therapies. However, there remains a paucity of data about hybrid imaging for the diagnosis and management of FTD, LBD, and other major causes of cognitive decline. Indeed, in our comprehensive review, only eight studies included patients with a suspected diagnosis of FTD and only three included patients with clinical signs of LBD. The development of novel radiotracers and nuclear imaging techniques for FTD is an area of increasing interest; although relatively uncommon, FTD is often characterized by a young age of onset and rapid progression. Hybrid imaging may allow for earlier diagnosis and prompt initiation of neurobehavioral interventions for this rare but devastating condition. In addition, although I-123 ioflupane SPECT imaging and FDG PET/CT are frequently used to confirm a suspected diagnosis of LBD, sensitivity and specificity are somewhat limited. Hybrid imaging represents an opportunity to improve the evaluation of patients with clinical signs and symptoms of LBD. The diagnosis of other causes of neurocognitive impairment, including NPH and CBD, may also be improved with hybrid imaging, although further research is necessary to establish reliable imaging biomarkers for these conditions.
5. Conclusions
This review article summarizes PET/MRI studies from 2018 to 2023 and the advantages and disadvantages of simultaneous imaging within this period. This paper also describes recommendations for future use and the expected advances in hybrid imaging with the addition of new tracers and AI. Future directions of hybrid imaging include incorporating more AI techniques to improve the technical aspects of PET/MRI such as attenuation and motion correction, validating more multi-modal biomarkers to evaluate dementia, and elucidating more regions of interest and functional connectivity networks. Current challenges in the field include the increased cost of integrated PET/MRI systems compared to stand-alone scanners, the challenge of image registration and analysis of two layers of data, and the increased radiation exposure to patients. The existing data suggest that hybrid imaging will play a central role in the diagnosis, management, and monitoring of dementia. Additional research is necessary to establish hybrid imaging techniques for the evaluation of non-AD dementia and develop strategies to facilitate the timely initiation of pharmacologic and neurobehavioral interventions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diagnostics14060585/s1.
Author Contributions
Conception and design, A.G., T.G.C., B.D.B. and J.L. Acquisition of data, J.L., J.R. and K.W. Analysis and interpretation of data, J.L. Drafting this article, A.G., T.G.C., B.D.B., P.E.K., K.W., J.R. and J.L. Critically revising this article, A.G., T.G.C., B.D.B., P.E.K., K.W., J.R. and J.L. Reviewed submitted version of manuscript, A.G., T.G.C., B.D.B., P.E.K., K.W., J.R. and J.L. Approved the final version of the manuscript on behalf of A.G., T.G.C., B.D.B., P.E.K., K.W., J.R. and J.L. Administrative/technical/material support, J.L. and A.G. Study supervision, A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable since this review is based exclusively on secondary research, comprising an analysis and synthesis of previously published data, literature, and findings in the public domain.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented and analyzed in this study are available as Supplementary File S1.
Acknowledgments
The authors would like to express gratitude to the USC Department of Radiology for their support of this review article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| MRI | Magnetic Resonance Imaging |
| MRI-ASL | MRI-Arterial Spin Labeling |
| FDG-PET | Fluorodeoxyglucose Positron-Emission Tomography |
| FTD | Frontotemporal Dementia |
| HC | Healthy Control |
| Aß | Amyloid Beta |
| PD | Parkinson’s Disease |
| DLB | Dementia with Lewy Bodies |
| CSF | Cerebrospinal Fluid |
| P-tau181 | Phosphorylated Tau at position 181 |
| AD | Alzheimer’s Disease |
| MCI | Mild Cognitive Impairment |
| TOF-MRI | Time-of-Flight MRI |
| DTI | Diffusion Tensor Imaging |
| LBD | Lewy Body Dementia |
| CI | Cognitive Impairment |
| eAD | Early Alzheimer’s Disease |
| bvFTD | Behavioral variant Frontotemporal Dementia |
| preAD | Pre-Alzheimer’s Disease |
| DKI | Diffusional Kurtosis Imaging |
| AWF | Axonal Water Fraction |
| CBD | Corticobasal Degeneration |
| FTLD/PPA | Frontotemporal Lobar Degeneration/Primary Progressive Aphasia |
| FC | Functional Connectivity |
| PCA | Posterior Cortical Atrophy |
| SD | Semantic Dementia |
| aMCI | Amnestic Mild Cognitive Impairment |
| SCD | Subjective Cognitive Decline |
| BOLD-FC | Blood Oxygen Level-Dependent Functional Connectivity |
| MMSE | Mini-Mental State Examination |
| NPH | Normal Pressure Hydrocephalus |
| RS-fMRI | Resting-State Functional MRI |
| SUVR | Standardized Uptake Value Ratio |
| EOFAD | Early Onset Familial Alzheimer’s Disease |
| PD-MCI | Parkinson’s Disease with Mild Cognitive Impairment |
| FCSRT | Free and Cued Selective Reminding Test |
| R-MEG | Resting-State Magnetoencephalography |
| FAD | Familial Alzheimer’s Disease |
| WMHV | White Matter Hyperintensity Volume |
| ALS | Amyotrophic Lateral Sclerosis |
References
- Hebert, L.E.; Weuve, J.; Scherr, P.A.; Evans, D.A. Alzheimer Disease in the United States (2010–2050) Estimated Using the 2010 Census. Neurology 2013, 80, 1778–1783. [Google Scholar] [CrossRef] [PubMed]
- Ferri, C.P.; Prince, M.; Brayne, C.; Brodaty, H.; Fratiglioni, L.; Ganguli, M.; Hall, K.; Hasegawa, K.; Hendrie, H.; Huang, Y.; et al. Global Prevalence of Dementia: A Delphi Consensus Study. Lancet 2005, 366, 2112–2117. [Google Scholar] [CrossRef]
- Boustani, M.; Peterson, B.; Hanson, L.; Harris, R.; Lohr, K.N.; U.S. Preventive Services Task Force. Screening for Dementia in Primary Care: A Summary of the Evidence for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2003, 138, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Howard, R.S.; Schneider, L.S. The Current Landscape of Prevention Trials in Dementia. Neurother. J. Am. Soc. Exp. Neurother. 2022, 19, 228–247. [Google Scholar] [CrossRef]
- Petrella, J.R. Neuroimaging and the Search for a Cure for Alzheimer Disease. Radiology 2013, 269, 671–691. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R.; Lowe, V.J.; Weigand, S.D.; Wiste, H.J.; Senjem, M.L.; Knopman, D.S.; Shiung, M.M.; Gunter, J.L.; Boeve, B.F.; Kemp, B.J.; et al. Serial PIB and MRI in Normal, Mild Cognitive Impairment and Alzheimer’s Disease: Implications for Sequence of Pathological Events in Alzheimer’s Disease. Brain 2009, 132, 1355–1365. [Google Scholar] [CrossRef]
- Casamitjana, A.; Petrone, P.; Tucholka, A.; Falcon, C.; Skouras, S.; Molinuevo, J.L.; Vilaplana, V.; Gispert, J.D. Alzheimer’s Disease Neuroimaging Initiative MRI-Based Screening of Preclinical Alzheimer’s Disease for Prevention Clinical Trials. J. Alzheimers Dis. 2018, 64, 1099–1112. [Google Scholar] [CrossRef]
- Yu, B.; Shan, Y.; Ding, J. A Literature Review of MRI Techniques Used to Detect Amyloid-Beta Plaques in Alzheimer’s Disease Patients. Ann. Palliat. Med. 2021, 10, 10062–10074. [Google Scholar] [CrossRef]
- Kas, A.; Rozenblum, L.; Pyatigorskaya, N. Clinical Value of Hybrid PET/MR Imaging: Brain Imaging Using PET/MR Imaging. Magn. Reson. Imaging Clin. N. Am. 2023, 31, 591–604. [Google Scholar] [CrossRef]
- Lorking, N.; Murray, A.D.; O’Brien, J.T. The Use of Positron Emission Tomography/Magnetic Resonance Imaging in Dementia: A Literature Review. Int. J. Geriatr. Psychiatry 2021, 36, 1501–1513. [Google Scholar] [CrossRef]
- Anazodo, U.C.; Finger, E.; Kwan, B.Y.M.; Pavlosky, W.; Warrington, J.C.; Günther, M.; Prato, F.S.; Thiessen, J.D.; St Lawrence, K.S. Using Simultaneous PET/MRI to Compare the Accuracy of Diagnosing Frontotemporal Dementia by Arterial Spin Labelling MRI and FDG-PET. NeuroImage Clin. 2018, 17, 405–414. [Google Scholar] [CrossRef]
- Biundo, R.; Weis, L.; Fiorenzato, E.; Pistonesi, F.; Cagnin, A.; Bertoldo, A.; Anglani, M.; Cecchin, D.; Antonini, A. The Contribution of Beta-Amyloid to Dementia in Lewy Body Diseases: A 1-Year Follow-up Study. Brain Commun. 2021, 3, fcab180. [Google Scholar] [CrossRef]
- Blessing, E.M.; Parekh, A.; Betensky, R.A.; Babb, J.; Saba, N.; Debure, L.; Varga, A.W.; Ayappa, I.; Rapoport, D.M.; Butler, T.A.; et al. Association between Lower Body Temperature and Increased Tau Pathology in Cognitively Normal Older Adults. Neurobiol. Dis. 2022, 171, 105748. [Google Scholar] [CrossRef]
- Brown, S.S.G.; Mak, E.; Clare, I.; Grigorova, M.; Beresford-Webb, J.; Walpert, M.; Jones, E.; Hong, Y.T.; Fryer, T.D.; Coles, J.P.; et al. Support Vector Machine Learning and Diffusion-Derived Structural Networks Predict Amyloid Quantity and Cognition in Adults with Down’s Syndrome. Neurobiol. Aging 2022, 115, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Campanholo, K.R.; Pitombeira, M.S.; Rimkus, C.M.; Mendes, M.F.; Apóstolos-Pereira, S.L.; Busatto Filho, G.; Callegaro, D.; Buchpiguel, C.A.; Duran, F.; De Paula Faria, D. Myelin Imaging Measures as Predictors of Cognitive Impairment in MS Patients: A Hybrid PET-MRI Study. Mult. Scler. Relat. Disord. 2022, 57, 103331. [Google Scholar] [CrossRef] [PubMed]
- Carlson, M.L.; DiGiacomo, P.S.; Fan, A.P.; Goubran, M.; Khalighi, M.M.; Chao, S.Z.; Vasanawala, M.; Wintermark, M.; Mormino, E.; Zaharchuk, G.; et al. Simultaneous FDG-PET/MRI Detects Hippocampal Subfield Metabolic Differences in AD/MCI. Sci. Rep. 2020, 10, 12064. [Google Scholar] [CrossRef]
- Carlson, M.L.; Toueg, T.N.; Khalighi, M.M.; Castillo, J.; Shen, B.; Azevedo, E.C.; DiGiacomo, P.; Mouchawar, N.; Chau, G.; Zaharchuk, G.; et al. Hippocampal Subfield Imaging and Fractional Anisotropy Show Parallel Changes in Alzheimer’s Disease Tau Progression Using Simultaneous Tau-PET/MRI at 3T. Alzheimers Dement. 2021, 13, e12218. [Google Scholar] [CrossRef]
- Ceccarini, J.; Bourgeois, S.; Van Weehaeghe, D.; Goffin, K.; Vandenberghe, R.; Vandenbulcke, M.; Sunaert, S.; Van Laere, K. Direct Prospective Comparison of 18F-FDG PET and Arterial Spin Labelling MR Using Simultaneous PET/MR in Patients Referred for Diagnosis of Dementia. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2142–2154. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Cui, C.; Su, Y.; Jing, D.; Wu, L.; Liang, P.; Liang, Z. Evaluating the Association between Brain Atrophy, Hypometabolism, and Cognitive Decline in Alzheimer’s Disease: A PET/MRI Study. Aging 2021, 13, 7228–7246. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Bi, S.; Shan, Y.; Cui, B.; Yang, H.; Qi, Z.; Zhao, Z.; Han, Y.; Yan, S.; Lu, J. Multiparametric Hippocampal Signatures for Early Diagnosis of Alzheimer’s Disease Using 18 F-FDG PET/MRI Radiomics. CNS Neurosci. Ther. 2023. prepint. [Google Scholar] [CrossRef]
- Chen, Y.; Zeng, Q.; Wang, Y.; Luo, X.; Sun, Y.; Zhang, L.; Liu, X.; Li, K.; Zhang, M.; Peng, G. Characterizing Differences in Functional Connectivity Between Posterior Cortical Atrophy and Semantic Dementia by Seed-Based Approach. Front. Aging Neurosci. 2022, 14, 850977. [Google Scholar] [CrossRef]
- Choi, E.J.; Son, Y.D.; Noh, Y.; Lee, H.; Kim, Y.B.; Park, K.H. Glucose Hypometabolism in Hippocampal Subdivisions in Alzheimer’s Disease: A Pilot Study Using High-Resolution 18F-FDG PET and 7.0-T MRI. J. Clin. Neurol. 2018, 14, 158–164. [Google Scholar] [CrossRef]
- Chu, M.; Liu, L.; Wang, J.; Liu, L.; Kong, Y.; Jing, D.; Xie, K.; Cui, Y.; Cui, B.; Zhang, J.; et al. Investigating the Roles of Anterior Cingulate in Behavioral Variant Frontotemporal Dementia: A PET/MRI Study. J. Alzheimers Dis. 2021, 84, 1771–1779. [Google Scholar] [CrossRef]
- Ding, C.; Du, W.; Zhang, Q.; Wang, L.; Han, Y.; Jiang, J. Coupling Relationship between Glucose and Oxygen Metabolisms to Differentiate Preclinical Alzheimer’s Disease and Normal Individuals. Hum. Brain Mapp. 2021, 42, 5051–5062. [Google Scholar] [CrossRef]
- Dong, J.W.; Jelescu, I.O.; Ades-Aron, B.; Novikov, D.S.; Friedman, K.; Babb, J.S.; Osorio, R.S.; Galvin, J.E.; Shepherd, T.M.; Fieremans, E. Diffusion MRI Biomarkers of White Matter Microstructure Vary Nonmonotonically with Increasing Cerebral Amyloid Deposition. Neurobiol. Aging 2020, 89, 118–128. [Google Scholar] [CrossRef]
- Franceschi, A.M.; Clifton, M.A.; Naser-Tavakolian, K.; Ahmed, O.; Bangiyev, L.; Clouston, S.; Franceschi, D. FDG PET/MRI for Visual Detection of Crossed Cerebellar Diaschisis in Patients With Dementia. Am. J. Roentgenol. 2021, 216, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, A.M.; Naser-Tavakolian, K.; Clifton, M.; Ahmed, O.; Stoffers, K.; Bangiyev, L.; Cruciata, G.; Clouston, S.; Franceschi, D. Hybrid Imaging in Dementia: A Semi-Quantitative (18F)-Fluorodeoxyglucose Positron Emission Tomography/Magnetic Resonance Imaging Approach in Clinical Practice. World J. Nucl. Med. 2021, 20, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, A.M.; Clifton, M.; Naser-Tavakolian, K.; Ahmed, O.; Cruciata, G.; Bangiyev, L.; Clouston, S.; Franceschi, D. (18F)-Fluorodeoxyglucose Positron Emission Tomography/Magnetic Resonance Imaging Assessment of Hypometabolism Patterns in Clinical Phenotypes of Suspected Corticobasal Degeneration. World J. Nucl. Med. 2021, 20, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, A.M.; Naser-Tavakolian, K.; Clifton, M.; Bangiyev, L.; Cruciata, G.; Clouston, S.; Franceschi, D. Metabolic Positron-Emission Tomography/Magnetic Resonance Imaging in Primary Progressive Aphasia and Frontotemporal Lobar Degeneration Subtypes: Reassessment of Expected [18F]-Fluorodeoxyglucose Uptake Patterns. World J. Nucl. Med. 2021, 20, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zhou, Z.; Liu, L.; Zhang, J.; Xie, H.; Zhang, X.; Zhu, M.; Wang, R. Functional Abnormality Associated With Tau Deposition in Alzheimer’s Disease—A Hybrid Positron Emission Tomography/MRI Study. Front. Aging Neurosci. 2021, 13, 758053. [Google Scholar] [CrossRef]
- Garon, M.; Weis, L.; Fiorenzato, E.; Pistonesi, F.; Cagnin, A.; Bertoldo, A.; Anglani, M.; Cecchin, D.; Antonini, A.; Biundo, R. Quantification of Brain β-Amyloid Load in Parkinson’s Disease With Mild Cognitive Impairment: A PET/MRI Study. Front. Neurol. 2021, 12, 760518. [Google Scholar] [CrossRef]
- Göttler, J.; Preibisch, C.; Riederer, I.; Pasquini, L.; Alexopoulos, P.; Bohn, K.P.; Yakushev, I.; Beller, E.; Kaczmarz, S.; Zimmer, C.; et al. Reduced Blood Oxygenation Level Dependent Connectivity Is Related to Hypoperfusion in Alzheimer’s Disease. J. Cereb. Blood Flow Metab. 2019, 39, 1314–1325. [Google Scholar] [CrossRef]
- Holstege, H.; Beker, N.; Dijkstra, T.; Pieterse, K.; Wemmenhove, E.; Schouten, K.; Thiessens, L.; Horsten, D.; Rechtuijt, S.; Sikkes, S.; et al. The 100-plus Study of Cognitively Healthy Centenarians: Rationale, Design and Cohort Description. Eur. J. Epidemiol. 2018, 33, 1229–1249. [Google Scholar] [CrossRef]
- James, S.-N.; Manning, E.N.; Storey, M.; Nicholas, J.M.; Coath, W.; Keuss, S.E.; Cash, D.M.; Lane, C.A.; Parker, T.; Keshavan, A.; et al. Neuroimaging, Clinical and Life Course Correlates of Normal-Appearing White Matter Integrity in 70-Year-Olds. Brain Commun. 2023, 5, fcad225. [Google Scholar] [CrossRef] [PubMed]
- James, S.-N.; Nicholas, J.M.; Lu, K.; Keshavan, A.; Lane, C.A.; Parker, T.; Buchanan, S.M.; Keuss, S.E.; Murray-Smith, H.; Wong, A.; et al. Adulthood Cognitive Trajectories over 26 Years and Brain Health at 70 Years of Age: Findings from the 1946 British Birth Cohort. Neurobiol. Aging 2023, 122, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Kaltoft, N.S.; Marner, L.; Larsen, V.A.; Hasselbalch, S.G.; Law, I.; Henriksen, O.M. Hybrid FDG PET/MRI vs. FDG PET and CT in Patients with Suspected Dementia—A Comparison of Diagnostic Yield and Propagated Influence on Clinical Diagnosis and Patient Management. PLoS ONE 2019, 14, e0216409. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.M.; Sohn, C.-H.; Byun, M.S.; Lee, J.H.; Yi, D.; Lee, Y.; Lee, J.-Y.; Kim, Y.K.; Sohn, B.K.; Yoo, R.-E.; et al. Prediction of Amyloid Positivity in Mild Cognitive Impairment Using Fully Automated Brain Segmentation Software. Neuropsychiatr. Dis. Treat. 2020, 16, 1745–1754. [Google Scholar] [CrossRef] [PubMed]
- Kritikos, M.; Franceschi, A.M.; Vaska, P.; Clouston, S.A.P.; Huang, C.; Salerno, M.; Deri, Y.; Tang, C.; Pellecchia, A.; Santiago-Michels, S.; et al. Assessment of Alzheimer’s Disease Imaging Biomarkers in World Trade Center Responders with Cognitive Impairment at Midlife. World J. Nucl. Med. 2022, 21, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Ladefoged, C.N.; Hansen, A.E.; Henriksen, O.M.; Bruun, F.J.; Eikenes, L.; Øen, S.K.; Karlberg, A.; Højgaard, L.; Law, I.; Andersen, F.L. AI-Driven Attenuation Correction for Brain PET/MRI: Clinical Evaluation of a Dementia Cohort and Importance of the Training Group Size. NeuroImage 2020, 222, 117221. [Google Scholar] [CrossRef]
- Lagarde, J.; Olivieri, P.; Tonietto, M.; Tissot, C.; Rivals, I.; Gervais, P.; Caillé, F.; Moussion, M.; Bottlaender, M.; Sarazin, M. Tau-PET Imaging Predicts Cognitive Decline and Brain Atrophy Progression in Early Alzheimer’s Disease. J. Neurol. Neurosurg. Psychiatry 2022, 93, 459–467. [Google Scholar] [CrossRef]
- Liu, L.; Chu, M.; Nie, B.; Liu, L.; Xie, K.; Cui, Y.; Kong, Y.; Chen, Z.; Nan, H.; Chen, K.; et al. Reconfigured Metabolism Brain Network in Asymptomatic Microtubule-Associated Protein Tau Mutation Carriers: A Graph Theoretical Analysis. Alzheimers Res. Ther. 2022, 14, 52. [Google Scholar] [CrossRef]
- Liu, L.; Chu, M.; Nie, B.; Jiang, D.; Xie, K.; Cui, Y.; Liu, L.; Kong, Y.; Chen, Z.; Nan, H.; et al. Altered Metabolic Connectivity within the Limbic Cortico-Striato-Thalamo-Cortical Circuit in Presymptomatic and Symptomatic Behavioral Variant Frontotemporal Dementia. Alzheimers Res. Ther. 2023, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, S.; Chu, M.; Wang, J.; Xie, K.; Cui, Y.; Ma, J.; Nan, H.; Cui, C.; Qiao, H.; et al. Involvement of Striatal Motoric Subregions in Familial Frontotemporal Dementia with Parkinsonism Harboring the C9orf72 Repeat Expansions. NPJ Park. Dis. 2022, 8, 128. [Google Scholar] [CrossRef]
- Lu, K.; Nicholas, J.M.; Weston, P.S.J.; Stout, J.C.; O’Regan, A.M.; James, S.-N.; Buchanan, S.M.; Lane, C.A.; Parker, T.D.; Keuss, S.E.; et al. Visuomotor Integration Deficits Are Common to Familial and Sporadic Preclinical Alzheimer’s Disease. Brain Commun. 2021, 3, fcab003. [Google Scholar] [CrossRef]
- Maleki Balajoo, S.; Rahmani, F.; Khosrowabadi, R.; Meng, C.; Eickhoff, S.B.; Grimmer, T.; Zarei, M.; Drzezga, A.; Sorg, C.; Tahmasian, M. Decoupling of Regional Neural Activity and Inter-Regional Functional Connectivity in Alzheimer’s Disease: A Simultaneous PET/MR Study. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3173–3185. [Google Scholar] [CrossRef] [PubMed]
- Mangalore, S.; Vankayalapati, S.; Gupta, A.K. Hydrocephalic Dementia: Revisited with Multimodality Imaging and toward a Unified Imaging Approach. J. Neurosci. Rural Pract. 2021, 12, 412–418. [Google Scholar] [CrossRef]
- Marchitelli, R.; Aiello, M.; Cachia, A.; Quarantelli, M.; Cavaliere, C.; Postiglione, A.; Tedeschi, G.; Montella, P.; Milan, G.; Salvatore, M.; et al. Simultaneous Resting-State FDG-PET/fMRI in Alzheimer Disease: Relationship between Glucose Metabolism and Intrinsic Activity. NeuroImage 2018, 176, 246–258. [Google Scholar] [CrossRef]
- Mukku, S.S.R.; Sivakumar, P.T.; Nagaraj, C.; Mangalore, S.; Harbishettar, V.; Varghese, M. Clinical Utility of 18F-FDG-PET/MRI Brain in Dementia: Preliminary Experience from a Geriatric Clinic in South India. Asian J. Psychiatry 2019, 44, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Okazawa, H.; Ikawa, M.; Jung, M.; Maruyama, R.; Tsujikawa, T.; Mori, T.; Rahman, M.G.M.; Makino, A.; Kiyono, Y.; Kosaka, H. Multimodal Analysis Using [11C]PiB-PET/MRI for Functional Evaluation of Patients with Alzheimer’s Disease. EJNMMI Res. 2020, 10, 30. [Google Scholar] [CrossRef]
- Okazawa, H.; Ikawa, M.; Tsujikawa, T.; Mori, T.; Makino, A.; Kiyono, Y.; Nakamoto, Y.; Kosaka, H.; Yoneda, M. Cerebral Oxidative Stress in Early Alzheimer’s Disease Evaluated by 64Cu-ATSM PET/MRI: A Preliminary Study. Antioxidants 2022, 11, 1022. [Google Scholar] [CrossRef]
- Okazawa, H.; Ikawa, M.; Tsujikawa, T.; Makino, A.; Mori, T.; Kiyono, Y.; Kosaka, H. Noninvasive Measurement of [11C]PiB Distribution Volume Using Integrated PET/MRI. Diagnostics 2020, 10, 993. [Google Scholar] [CrossRef]
- Parker, T.D.; Cash, D.M.; Lane, C.A.S.; Lu, K.; Malone, I.B.; Nicholas, J.M.; James, S.-N.; Keshavan, A.; Murray-Smith, H.; Wong, A.; et al. Hippocampal Subfield Volumes and Pre-Clinical Alzheimer’s Disease in 408 Cognitively Normal Adults Born in 1946. PLoS ONE 2019, 14, e0224030. [Google Scholar] [CrossRef]
- Puttaert, D.; Coquelet, N.; Wens, V.; Peigneux, P.; Fery, P.; Rovai, A.; Trotta, N.; Sadeghi, N.; Coolen, T.; Bier, J.-C.; et al. Alterations in Resting-State Network Dynamics along the Alzheimer’s Disease Continuum. Sci. Rep. 2020, 10, 21990. [Google Scholar] [CrossRef]
- Puttaert, D.; Wens, V.; Fery, P.; Rovai, A.; Trotta, N.; Coquelet, N.; De Breucker, S.; Sadeghi, N.; Coolen, T.; Goldman, S.; et al. Decreased Alpha Peak Frequency Is Linked to Episodic Memory Impairment in Pathological Aging. Front. Aging Neurosci. 2021, 13, 711375. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Fu, L.; Wang, R.; Lyu, J.; Ma, H.; Zhan, M.; Zhou, A.; Wang, F.; Zuo, X.; Wei, C. Prominent Striatum Amyloid Retention in Early-Onset Familial Alzheimer’s Disease With PSEN1 Mutations: A Pilot PET/MR Study. Front. Aging Neurosci. 2021, 13, 732159. [Google Scholar] [CrossRef] [PubMed]
- Riederer, I.; Bohn, K.P.; Preibisch, C.; Wiedemann, E.; Zimmer, C.; Alexopoulos, P.; Förster, S. Alzheimer Disease and Mild Cognitive Impairment: Integrated Pulsed Arterial Spin-Labeling MRI and 18F-FDG PET. Radiology 2018, 288, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Saka, E.; Atay, L.O.; Akdemir, U.O.; Yetim, E.; Balci, E.; Arsava, E.M.; Topcuoglu, M.A. Cerebral Vasomotor Reactivity across the Continuum of Subjective Cognitive Impairment, Amnestic Mild Cognitive Impairment and Probable Alzheimer’s Dementia: A Transcranial Doppler and PET/MRI Study. J. Cereb. Blood Flow Metab. 2023, 43, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Scherr, M.; Utz, L.; Tahmasian, M.; Pasquini, L.; Grothe, M.J.; Rauschecker, J.P.; Grimmer, T.; Drzezga, A.; Sorg, C.; Riedl, V. Effective Connectivity in the Default Mode Network Is Distinctively Disrupted in Alzheimer’s Disease-A Simultaneous Resting-State FDG-PET/fMRI Study. Hum. Brain Mapp. 2021, 42, 4134–4143. [Google Scholar] [CrossRef] [PubMed]
- Sekine, T.; Buck, A.; Delso, G.; Kemp, B.; Ter Voert, E.E.G.W.; Huellner, M.; Veit-Haibach, P.; Kaushik, S.; Wiesinger, F.; Warnock, G.; et al. The Impact of Atlas-Based MR Attenuation Correction on the Diagnosis of FDG-PET/MR for Alzheimer’s Diseases- A Simulation Study Combining Multi-Center Data and ADNI-Data. PLoS ONE 2020, 15, e0233886. [Google Scholar] [CrossRef] [PubMed]
- Ssali, T.; Narciso, L.; Hicks, J.; Liu, L.; Jesso, S.; Richardson, L.; Günther, M.; Konstandin, S.; Eickel, K.; Prato, F.; et al. Concordance of Regional Hypoperfusion by pCASL MRI and 15O-Water PET in Frontotemporal Dementia: Is pCASL an Efficacious Alternative? NeuroImage Clin. 2022, 33, 102950. [Google Scholar] [CrossRef] [PubMed]
- Tiepolt, S.; Rullmann, M.; Jochimsen, T.H.; Gertz, H.-J.; Schroeter, M.L.; Patt, M.; Sabri, O.; Barthel, H. Quantitative Susceptibility Mapping in β-Amyloid PET-Stratified Patients with Dementia and Healthy Controls—A Hybrid PET/MRI Study. Eur. J. Radiol. 2020, 131, 109243. [Google Scholar] [CrossRef] [PubMed]
- van Bergen, J.M.G.; Li, X.; Quevenco, F.C.; Gietl, A.F.; Treyer, V.; Meyer, R.; Buck, A.; Kaufmann, P.A.; Nitsch, R.M.; van Zijl, P.C.M.; et al. Simultaneous Quantitative Susceptibility Mapping and Flutemetamol-PET Suggests Local Correlation of Iron and β-Amyloid as an Indicator of Cognitive Performance at High Age. NeuroImage 2018, 174, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Vanderlinden, G.; Ceccarini, J.; Vande Casteele, T.; Michiels, L.; Lemmens, R.; Triau, E.; Serdons, K.; Tournoy, J.; Koole, M.; Vandenbulcke, M.; et al. Spatial Decrease of Synaptic Density in Amnestic Mild Cognitive Impairment Follows the Tau Build-up Pattern. Mol. Psychiatry 2022, 27, 4244–4251. [Google Scholar] [CrossRef] [PubMed]
- Vanhaute, H.; Ceccarini, J.; Michiels, L.; Koole, M.; Sunaert, S.; Lemmens, R.; Triau, E.; Emsell, L.; Vandenbulcke, M.; Van Laere, K. In Vivo Synaptic Density Loss Is Related to Tau Deposition in Amnestic Mild Cognitive Impairment. Neurology 2020, 95, e545–e553. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, H.; Wang, M.; Brendel, M.; Rominger, A.; Shi, K.; Han, Y.; Jiang, J. A Metabolism-Functional Connectome Sparse Coupling Method to Reveal Imaging Markers for Alzheimer’s Disease Based on Simultaneous PET/MRI Scans. Hum. Brain Mapp. 2023, 44, 6020–6030. [Google Scholar] [CrossRef]
- Yan, S.; Zheng, C.; Cui, B.; Qi, Z.; Zhao, Z.; An, Y.; Qiao, L.; Han, Y.; Zhou, Y.; Lu, J. Multiparametric Imaging Hippocampal Neurodegeneration and Functional Connectivity with Simultaneous PET/MRI in Alzheimer’s Disease. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2440–2452. [Google Scholar] [CrossRef]
- Yang, F.; Jiang, J.; Alberts, I.; Wang, M.; Li, T.; Sun, X.; Rominger, A.; Zuo, C.; Shi, K. Combining PET with MRI to Improve Predictions of Progression from Mild Cognitive Impairment to Alzheimer’s Disease: An Exploratory Radiomic Analysis Study. Ann. Transl. Med. 2022, 10, 513. [Google Scholar] [CrossRef]
- Zanovello, M.; Sorarù, G.; Campi, C.; Anglani, M.; Spimpolo, A.; Berti, S.; Bussè, C.; Mozzetta, S.; Cagnin, A.; Cecchin, D. Brain Stem Glucose Hypermetabolism in Amyotrophic Lateral Sclerosis/Frontotemporal Dementia and Shortened Survival: An 18F-FDG PET/MRI Study. J. Nucl. Med. 2022, 63, 777–784. [Google Scholar] [CrossRef]
- Zhang, M.; Guan, Z.; Zhang, Y.; Sun, W.; Li, W.; Hu, J.; Li, B.; Ye, G.; Meng, H.; Huang, X.; et al. Disrupted Coupling between Salience Network Segregation and Glucose Metabolism Is Associated with Cognitive Decline in Alzheimer’s Disease—A Simultaneous Resting-State FDG-PET/fMRI Study. NeuroImage Clin. 2022, 34, 102977. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, W.; Guan, Z.; Hu, J.; Li, B.; Ye, G.; Meng, H.; Huang, X.; Lin, X.; Wang, J.; et al. Simultaneous PET/fMRI Detects Distinctive Alterations in Functional Connectivity and Glucose Metabolism of Precuneus Subregions in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 737002. [Google Scholar] [CrossRef]
- Zorzi, G.; Cecchin, D.; Bussè, C.; Perini, G.; Corbetta, M.; Cagnin, A. Changes of Metabolic Connectivity in Dementia with Lewy Bodies with Visual Hallucinations: A 18F-Fluorodeoxyglucose Positron Emission Tomography/Magnetic Resonance Study. Brain Connect. 2021, 11, 518–528. [Google Scholar] [CrossRef]
- Prato, F.S.; Pavlosky, W.F.; Foster, S.C.; Thiessen, J.D.; Beaujot, R.P. Screening for Dementia Caused by Modifiable Lifestyle Choices Using Hybrid PET/MRI. J. Alzheimers Dis. Rep. 2019, 3, 31–45. [Google Scholar] [CrossRef]
- Dupont, P. A Role of PET/MR Imaging in Dementia? Semin. Nucl. Med. 2021, 51, 296–302. [Google Scholar] [CrossRef]
- Frantellizzi, V.; Conte, M.; De Vincentis, G. Hybrid Imaging of Vascular Cognitive Impairment. Semin. Nucl. Med. 2021, 51, 286–295. [Google Scholar] [CrossRef]
- Mainta, I.C.; Vargas, M.I.; Trombella, S.; Frisoni, G.B.; Unschuld, P.G.; Garibotto, V. Hybrid PET-MRI in Alzheimer’s Disease Research. Methods Mol. Biol. 2018, 1750, 185–200. [Google Scholar] [CrossRef]
- Shepherd, T.M.; Nayak, G.K. Clinical Use of Integrated Positron Emission Tomography-Magnetic Resonance Imaging for Dementia Patients. Top. Magn. Reson. Imaging 2019, 28, 299–310. [Google Scholar] [CrossRef]
- Calabria, F.; Leporace, M.; Cimini, A.; Ricci, M.; Travascio, L.; Bagnato, A. Positron Emission Tomography Molecular Imaging of the Major Neurodegenerative Disorders: Overview and Pictorial Essay, from a Nuclear Medicine Center’s Perspective. J. Integr. Neurosci. 2023, 22, 172. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, Q.; Zhang, Y.; Zheng, J.; Yang, Y.; Du, X.; Feng, H.; Zhang, S. Application of Deep Learning for Prediction of Alzheimer’s Disease in PET/MR Imaging. Bioengineering 2023, 10, 1120. [Google Scholar] [CrossRef] [PubMed]
- Minoshima, S.; Cross, D. Application of Artificial Intelligence in Brain Molecular Imaging. Ann. Nucl. Med. 2022, 36, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Logan, R.; Williams, B.G.; Ferreira da Silva, M.; Indani, A.; Schcolnicov, N.; Ganguly, A.; Miller, S.J. Deep Convolutional Neural Networks With Ensemble Learning and Generative Adversarial Networks for Alzheimer’s Disease Image Data Classification. Front. Aging Neurosci. 2021, 13, 720226. [Google Scholar] [CrossRef] [PubMed]
- Sgard, B.; Khalifé, M.; Bouchut, A.; Fernandez, B.; Soret, M.; Giron, A.; Zaslavsky, C.; Delso, G.; Habert, M.-O.; Kas, A. ZTE MR-Based Attenuation Correction in Brain FDG-PET/MR: Performance in Patients with Cognitive Impairment. Eur. Radiol. 2020, 30, 1770–1779. [Google Scholar] [CrossRef] [PubMed]
- Øen, S.K.; Keil, T.M.; Berntsen, E.M.; Aanerud, J.F.; Schwarzlmüller, T.; Ladefoged, C.N.; Karlberg, A.M.; Eikenes, L. Quantitative and Clinical Impact of MRI-Based Attenuation Correction Methods in [18F]FDG Evaluation of Dementia. EJNMMI Res. 2019, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ying, C.; Binkley, M.M.; Juttukonda, M.R.; Flores, S.; Laforest, R.; Benzinger, T.L.S.; An, H. Deep Learning-Based T1-Enhanced Selection of Linear Attenuation Coefficients (DL-TESLA) for PET/MR Attenuation Correction in Dementia Neuroimaging. Magn. Reson. Med. 2021, 86, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Blanc-Durand, P.; Khalife, M.; Sgard, B.; Kaushik, S.; Soret, M.; Tiss, A.; El Fakhri, G.; Habert, M.-O.; Wiesinger, F.; Kas, A. Attenuation Correction Using 3D Deep Convolutional Neural Network for Brain 18F-FDG PET/MR: Comparison with Atlas, ZTE and CT Based Attenuation Correction. PLoS ONE 2019, 14, e0223141. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, A.M.; Abballe, V.; Raad, R.A.; Nelson, A.; Jackson, K.; Babb, J.; Vahle, T.; Fenchel, M.; Zhan, Y.; Valadez, G.H.; et al. Visual Detection of Regional Brain Hypometabolism in Cognitively Impaired Patients Is Independent of Positron Emission Tomography-Magnetic Resonance Attenuation Correction Method. World J. Nucl. Med. 2018, 17, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.; Han, P.K.; Johnson, K.A.; El Fakhri, G.; Ma, C.; Li, Q. Attenuation Correction Using Deep Learning and Integrated UTE/Multi-Echo Dixon Sequence: Evaluation in Amyloid and Tau PET Imaging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
- Vandenberghe, S.; Marsden, P.K. PET-MRI: A Review of Challenges and Solutions in the Development of Integrated Multimodality Imaging. Phys. Med. Biol. 2015, 60, R115. [Google Scholar] [CrossRef]
- Ladefoged, C.N.; Benoit, D.; Law, I.; Holm, S.; Kjær, A.; Højgaard, L.; Hansen, A.E.; Andersen, F.L. Region Specific Optimization of Continuous Linear Attenuation Coefficients Based on UTE (RESOLUTE): Application to PET/MR Brain Imaging. Phys. Med. Biol. 2015, 60, 8047. [Google Scholar] [CrossRef]
- Nazarparvar, B.; Shamsaei, M.; Rajabi, H. Correction of Head Movements in Positron Emission Tomography Using Point Source Tracking System: A Simulation Study. Ann. Nucl. Med. 2012, 26, 7–15. [Google Scholar] [CrossRef]
- Tiss, A.; Marin, T.; Chemli, Y.; Spangler-Bickell, M.; Gong, K.; Lois, C.; Petibon, Y.; Landes, V.; Grogg, K.; Normandin, M.; et al. Impact of Motion Correction on [18F]-MK6240 Tau PET Imaging. Phys. Med. Biol. 2023, 68, 105015. [Google Scholar] [CrossRef]
- Chen, K.T.; Salcedo, S.; Chonde, D.B.; Izquierdo-Garcia, D.; Levine, M.A.; Price, J.C.; Dickerson, B.C.; Catana, C. MR-Assisted PET Motion Correction in Simultaneous PET/MRI Studies of Dementia Subjects. J. Magn. Reson. Imaging 2018, 48, 1288–1296. [Google Scholar] [CrossRef]
- Coath, W.; Modat, M.; Cardoso, M.J.; Markiewicz, P.J.; Lane, C.A.; Parker, T.D.; Keshavan, A.; Buchanan, S.M.; Keuss, S.E.; Harris, M.J.; et al. Operationalizing the Centiloid Scale for [18F]Florbetapir PET Studies on PET/MRI. Alzheimers Dement. 2023, 15, e12434. [Google Scholar] [CrossRef] [PubMed]
- Ford, J.N.; Sweeney, E.M.; Skafida, M.; Glynn, S.; Amoashiy, M.; Lange, D.J.; Lin, E.; Chiang, G.C.; Osborne, J.R.; Pahlajani, S.; et al. Heuristic Scoring Method Utilizing FDG-PET Statistical Parametric Mapping in the Evaluation of Suspected Alzheimer Disease and Frontotemporal Lobar Degeneration. Am. J. Nucl. Med. Mol. Imaging 2021, 11, 313–326. [Google Scholar] [PubMed]
- Hinge, C.; Henriksen, O.M.; Lindberg, U.; Hasselbalch, S.G.; Højgaard, L.; Law, I.; Andersen, F.L.; Ladefoged, C.N. A Zero-Dose Synthetic Baseline for the Personalized Analysis of [18F]FDG-PET: Application in Alzheimer’s Disease. Front. Neurosci. 2022, 16, 1053783. [Google Scholar] [CrossRef] [PubMed]
- Behr, S.C.; Bahroos, E.; Hawkins, R.A.; Nardo, L.; Ravanfar, V.; Capbarat, E.V.; Seo, Y. Quantitative and Visual Assessments toward Potential Sub-mSv or Ultrafast FDG PET Using High-Sensitivity TOF PET in PET/MRI. Mol. Imaging Biol. 2018, 20, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.T.; Tesfay, R.; Koran, M.E.I.; Ouyang, J.; Shams, S.; Young, C.B.; Davidzon, G.; Liang, T.; Khalighi, M.; Mormino, E.; et al. Generative Adversarial Network-Enhanced Ultra-Low-Dose [18F]-PI-2620 τ PET/MRI in Aging and Neurodegenerative Populations. Am. J. Neuroradiol. 2023, 44, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.T.; Gong, E.; de Carvalho Macruz, F.B.; Xu, J.; Boumis, A.; Khalighi, M.; Poston, K.L.; Sha, S.J.; Greicius, M.D.; Mormino, E.; et al. Ultra–Low-Dose 18F-Florbetaben Amyloid PET Imaging Using Deep Learning with Multi-Contrast MRI Inputs. Radiology 2019, 290, 649–656. [Google Scholar] [CrossRef]
- Frisoni, G.B.; Bocchetta, M.; Chételat, G.; Rabinovici, G.D.; de Leon, M.J.; Kaye, J.; Reiman, E.M.; Scheltens, P.; Barkhof, F.; Black, S.E.; et al. Imaging Markers for Alzheimer Disease: Which vs. How. Neurology 2013, 81, 487–500. [Google Scholar] [CrossRef]
- Salthouse, T.A. Aging and Measures of Processing Speed. Biol. Psychol. 2000, 54, 35–54. [Google Scholar] [CrossRef]
- Lee, J.; Li, C.; Liu, C.-S.J.; Shiroishi, M.; Carmichael, J.D.; Zada, G.; Patel, V. Ultra-High Field 7 T MRI Localizes Regional Brain Volume Recovery Following Corticotroph Adenoma Resection and Hormonal Remission in Cushing’s Disease: A Case Series. Surg. Neurol. Int. 2022, 13, 239. [Google Scholar] [CrossRef]
- Park, J.E.; Cheong, E.-N.; Jung, D.E.; Shim, W.H.; Lee, J.S. Utility of 7 Tesla Magnetic Resonance Imaging in Patients With Epilepsy: A Systematic Review and Meta-Analysis. Front. Neurol. 2021, 12, 621936. [Google Scholar] [CrossRef]
- McKiernan, E.F.; O’Brien, J.T. 7T MRI for Neurodegenerative Dementias in Vivo: A Systematic Review of the Literature. J. Neurol. Neurosurg. Psychiatry 2017, 88, 564–574. [Google Scholar] [CrossRef]
- Schlemmer, H.-P.W.; Pichler, B.J.; Schmand, M.; Burbar, Z.; Michel, C.; Ladebeck, R.; Jattke, K.; Townsend, D.; Nahmias, C.; Jacob, P.K.; et al. Simultaneous MR/PET Imaging of the Human Brain: Feasibility Study. Radiology 2008, 248, 1028–1035. [Google Scholar] [CrossRef]
- Pavese, N.; Simpson, B.S.; Metta, V.; Ramlackhansingh, A.; Chaudhuri, K.R.; Brooks, D.J. [18F]FDOPA Uptake in the Raphe Nuclei Complex Reflects Serotonin Transporter Availability. A Combined [18F]FDOPA and [11C]DASB PET Study in Parkinson’s Disease. NeuroImage 2012, 59, 1080–1084. [Google Scholar] [CrossRef]
- Banaszek, A.; Bladowska, J.; Pokryszko-Dragan, A.; Podemski, R.; Sąsiadek, M.J. Evaluation of the Degradation of the Selected Projectile, Commissural and Association White Matter Tracts Within Normal Appearing White Matter in Patients with Multiple Sclerosis Using Diffusion Tensor MR Imaging—A Preliminary Study. Pol. J. Radiol. 2015, 80, 457–463. [Google Scholar] [CrossRef]
- Dukart, J.; Mueller, K.; Horstmann, A.; Barthel, H.; Möller, H.E.; Villringer, A.; Sabri, O.; Schroeter, M.L. Combined Evaluation of FDG-PET and MRI Improves Detection and Differentiation of Dementia. PLoS ONE 2011, 6, e18111. [Google Scholar] [CrossRef]
- Shaffer, J.L.; Petrella, J.R.; Sheldon, F.C.; Choudhury, K.R.; Calhoun, V.D.; Coleman, R.E.; Doraiswamy, P.M.; Alzheimer’s Disease Neuroimaging Initiative. Predicting Cognitive Decline in Subjects at Risk for Alzheimer Disease by Using Combined Cerebrospinal Fluid, MR Imaging, and PET Biomarkers. Radiology 2013, 266, 583–591. [Google Scholar] [CrossRef]
- Matsunari, I.; Samuraki, M.; Chen, W.-P.; Yanase, D.; Takeda, N.; Ono, K.; Yoshita, M.; Matsuda, H.; Yamada, M.; Kinuya, S. Comparison of 18F-FDG PET and Optimized Voxel-Based Morphometry for Detection of Alzheimer’s Disease: Aging Effect on Diagnostic Performance. J. Nucl. Med. 2007, 48, 1961–1970. [Google Scholar] [CrossRef] [PubMed]
- Ossenkoppele, R.; Smith, R.; Ohlsson, T.; Strandberg, O.; Mattsson, N.; Insel, P.S.; Palmqvist, S.; Hansson, O. Associations between Tau, Aβ, and Cortical Thickness with Cognition in Alzheimer Disease. Neurology 2019, 92, e601–e612. [Google Scholar] [CrossRef] [PubMed]
- Suppiah, S.; Didier, M.-A.; Vinjamuri, S. The Who, When, Why, and How of PET Amyloid Imaging in Management of Alzheimer’s Disease—Review of Literature and Interesting Images. Diagnostics 2019, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Barthel, H.; Schroeter, M.L.; Hoffmann, K.-T.; Sabri, O. PET/MR in Dementia and Other Neurodegenerative Diseases. Semin. Nucl. Med. 2015, 45, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Manuel, D.G.; Garner, R.; Finès, P.; Bancej, C.; Flanagan, W.; Tu, K.; Reimer, K.; Chambers, L.W.; Bernier, J. Alzheimer’s and Other Dementias in Canada, 2011 to 2031: A Microsimulation Population Health Modeling (POHEM) Study of Projected Prevalence, Health Burden, Health Services, and Caregiving Use. Popul. Health Metr. 2016, 14, 37. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).