Review of the Role of TRAF7 in Brain Endothelial Integrity and Cerebrovascular Aging
Abstract
1. Introduction
2. TRAF7 and Endothelial Function
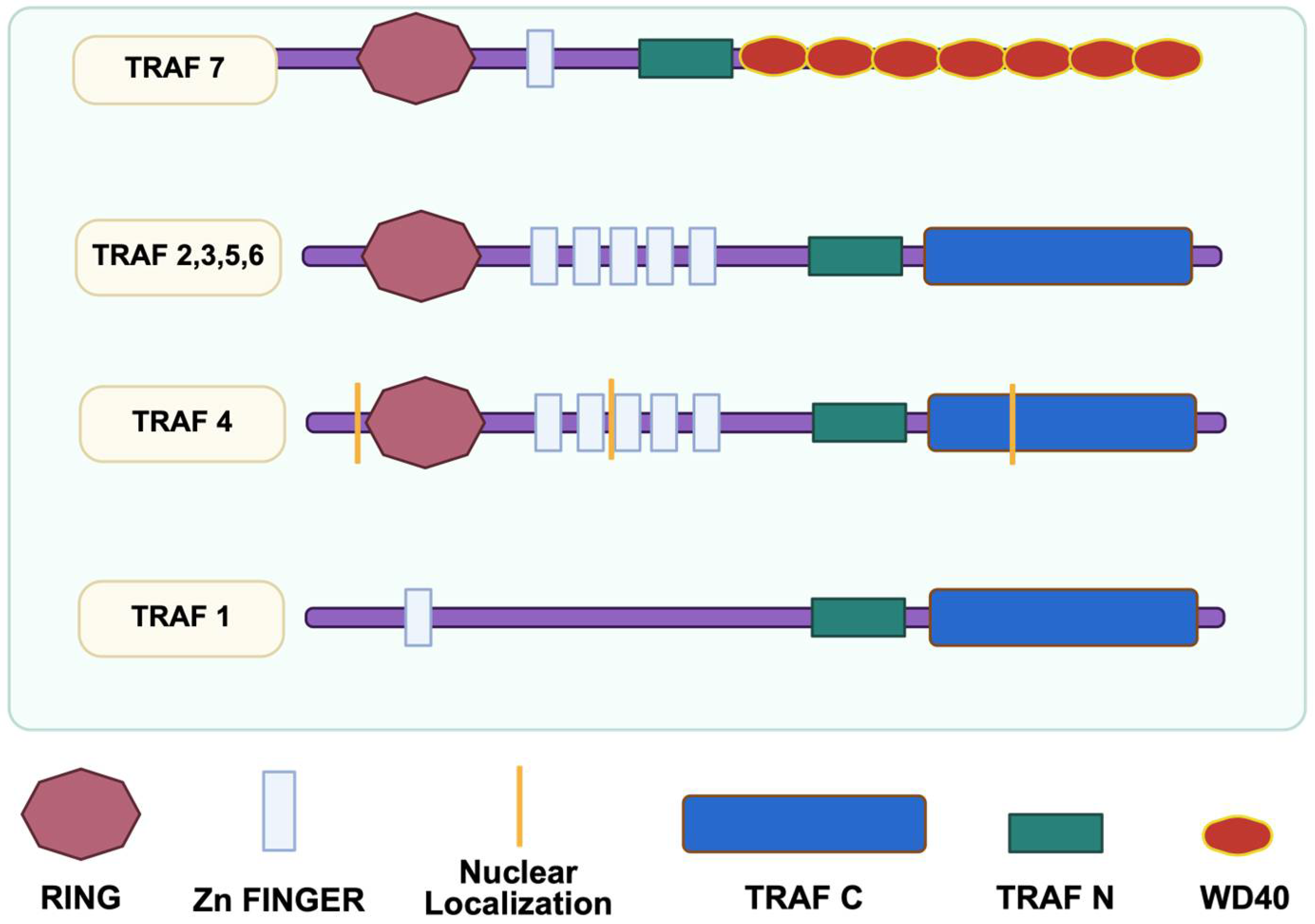
2.1. Overview of the TRAF7 Structure and Signaling Pathways
2.2. Interaction of TRAF7 with Mitogen-Activated Protein Kinases
2.3. Role of TRAF7 in Endothelial Cell Function and Inflammation Regulation
3. TRAF7 in the Context of Cerebrovascular Aging
3.1. Mechanisms of Cerebrovascular Aging and Associated Cognitive Decline
3.2. Impact of Aging on Endothelial Function and BBB Integrity
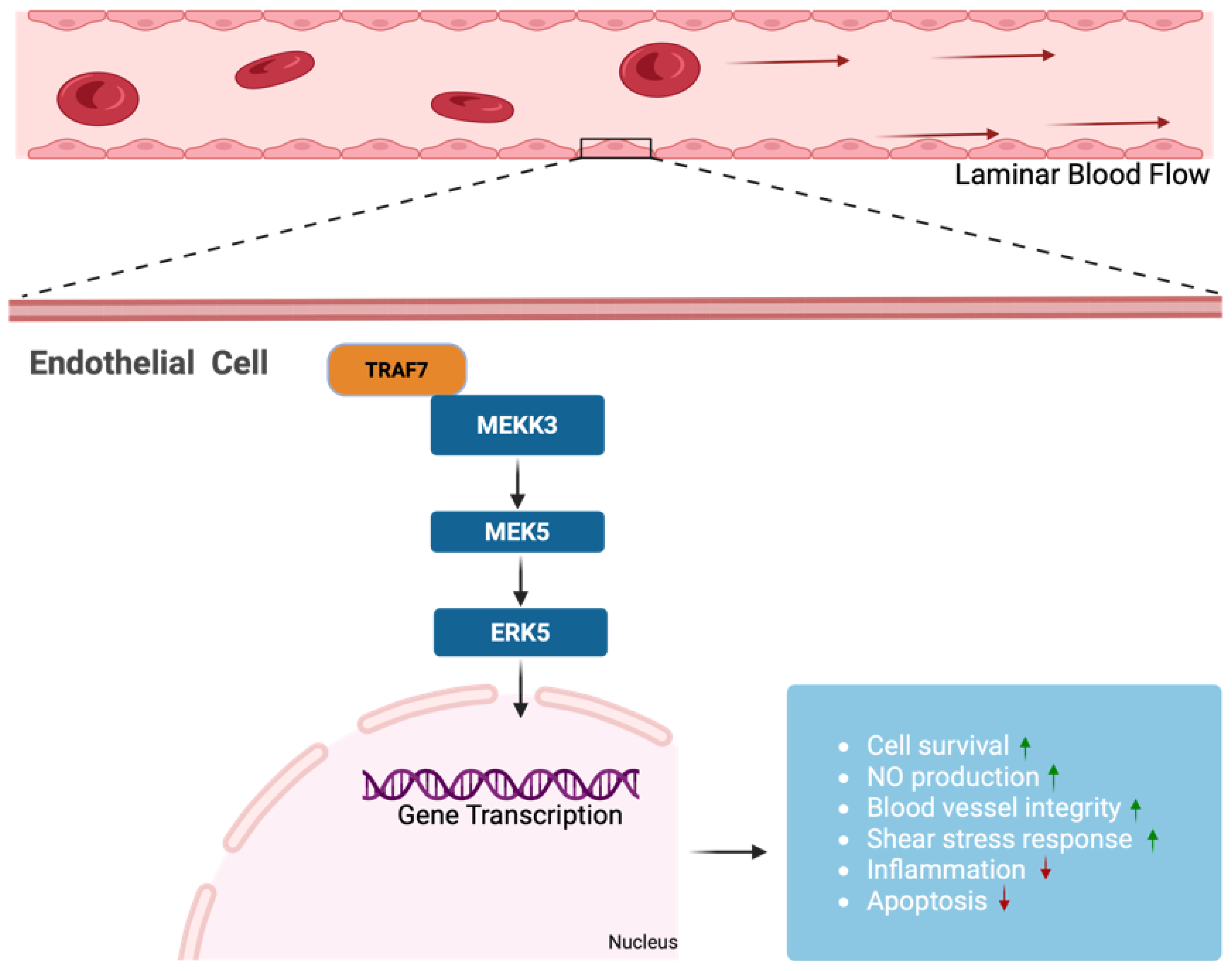
3.3. Role of TRAF7 in Modulating Endothelial Responses to Shear Stress and Inflammation
4. TRAF7 and Vascular Fragility
5. Limitations
6. Future Directions
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kalaria, R.N. Linking cerebrovascular defense mechanisms in brain ageing and Alzheimer’s disease. Neurobiol. Aging 2009, 30, 1512–1514. [Google Scholar] [CrossRef]
- Cho, K. Aging, Cerebrovascular Burden, and Cognitive Decline in New Insight into Cerebrovascular Diseases—An Updated Comprehensive Review; Ambrosi, P.B., Ahmad, R., Abdullahi, A., Agrawal, A., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Davidson, C.G.; Woodford, S.J.; Mathur, S.; Valle, D.B.; Foster, D.; Kioutchoukova, I.; Mahmood, A.; Lucke-Wold, B. Investigation into the vascular contributors to dementia and the associated treatments. Explor. Neurosci. 2023, 2, 224–237. [Google Scholar] [CrossRef]
- Tarumi, T.; Zhang, R. Cerebral blood flow in normal aging adults: Cardiovascular determinants, clinical implications, and aerobic fitness. J. Neurochem. 2018, 144, 595–608. [Google Scholar] [CrossRef]
- Lansdell, T.A.; Chambers, L.C.; Dorrance, A.M. Endothelial Cells and the Cerebral Circulation. Compr. Physiol. 2022, 12, 3449–3508. [Google Scholar] [CrossRef] [PubMed]
- Kallai, A.; Ungvari, A.; Csaban, D.; Orfi, Z.; Lehoczki, A.; Harasztdombi, J.; Yabluchanskiy, A.; Benyo, Z.; Szappanos, A.; Tarantini, S.; et al. Clonal hematopoiesis of indeterminate potential (CHIP) in cerebromicrovascular aging: Implications for vascular contributions to cognitive impairment and dementia (VCID). Geroscience 2025, 47, 2739–2775. [Google Scholar] [CrossRef]
- Negri, S.; Reyff, Z.; Troyano-Rodriguez, E.; Milan, M.; Ihuoma, J.; Tavakol, S.; Shi, H.; Patai, R.; Jiang, R.; Mohon, J.; et al. Endothelial Colony-Forming Cells (ECFCs) in cerebrovascular aging: Focus on the pathogenesis of Vascular Cognitive Impairment and Dementia (VCID), and treatment prospects. Ageing Res. Rev. 2025, 104, 102672. [Google Scholar] [CrossRef]
- Walker, A.E.; Cullen, A.E.; Fico, B.G.; Barnes, J.N. Cerebrovascular Function in Aging. In Masterclass in Neuroendocrinology; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Csiszar, A.; Ungvari, A.; Patai, R.; Gulej, R.; Yabluchanskiy, A.; Benyo, Z.; Kovacs, I.; Sotonyi, P.; Kirkpartrick, A.C.; Prodan, C.I.; et al. Atherosclerotic burden and cerebral small vessel disease: Exploring the link through microvascular aging and cerebral microhemorrhages. Geroscience 2024, 46, 5103–5132. [Google Scholar] [CrossRef]
- Nyul-Toth, A.; Patai, R.; Csiszar, A.; Ungvari, A.; Gulej, R.; Mukli, P.; Yabluchanskiy, A.; Benyo, Z.; Sotonyi, P.; Prodan, C.I.; et al. Linking peripheral atherosclerosis to blood-brain barrier disruption: Elucidating its role as a manifestation of cerebral small vessel disease in vascular cognitive impairment. Geroscience 2024, 46, 6511–6536. [Google Scholar] [CrossRef]
- Zimmerman, B.; Rypma, B.; Gratton, G.; Fabiani, M. Age-related changes in cerebrovascular health and their effects on neural function and cognition: A comprehensive review. Psychophysiology 2021, 58, e13796. [Google Scholar] [CrossRef]
- Lu, H.; Thomas, B.P.; Liu, P. Cerebrovascular Reactivity (CVR) in Aging, Cognitive Impairment, and Dementia. In Cerebrovascular Reactivity; Humana: New York, NY, USA, 2022. [Google Scholar] [CrossRef]
- Ungvari, A.; Gulej, R.; Patai, R.; Papp, Z.; Toth, A.; Szabo, A.A.; Podesser, B.K.; Sotonyi, P.; Benyo, Z.; Yabluchanskiy, A.; et al. Sex-specific mechanisms in vascular aging: Exploring cellular and molecular pathways in the pathogenesis of age-related cardiovascular and cerebrovascular diseases. Geroscience 2025, 47, 301–337. [Google Scholar] [CrossRef]
- Tarantini, S.; Fulop, G.A.; Kiss, T.; Farkas, E.; Zolei-Szenasi, D.; Galvan, V.; Toth, P.; Csiszar, A.; Ungvari, Z.; Yabluchanskiy, A. Demonstration of impaired neurovascular coupling responses in TG2576 mouse model of Alzheimer’s disease using functional laser speckle contrast imaging. Geroscience 2017, 39, 465–473. [Google Scholar] [CrossRef]
- Patai, R.; Kiss, T.; Gulej, R.; Nyul-Toth, A.; Csik, B.; Chandragiri, S.S.; Shanmugarama, S.; Tarantini, S.; Ungvari, A.; Pacher, P.; et al. Transcriptomic profiling of senescence effects on blood-brain barrier-related gene expression in brain capillary endothelial cells in a mouse model of paclitaxel-induced chemobrain. Geroscience 2025, 47, 3677–3691. [Google Scholar] [CrossRef]
- Ungvari, Z.; Kaley, G.; de Cabo, R.; Sonntag, W.E.; Csiszar, A. Mechanisms of vascular aging: New perspectives. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 1028–1041. [Google Scholar] [CrossRef]
- McDonald, W.M. Overview of Neurocognitive Disorders. Focus (Am. Psychiatr. Publ.) 2017, 15, 4–12. [Google Scholar] [CrossRef]
- Murman, D.L. The Impact of Age on Cognition. Semin. Hear. 2015, 36, 111–121. [Google Scholar] [CrossRef]
- Yang, T.; Sun, Y.; Lu, Z.; Leak, R.K.; Zhang, F. The impact of cerebrovascular aging on vascular cognitive impairment and dementia. Ageing Res. Rev. 2017, 34, 15–29. [Google Scholar] [CrossRef]
- Seidel, G.A.; Giovannetti, T.; Libon, D.J. Cerebrovascular disease and cognition in older adults. Curr. Top. Behav. Neurosci. 2012, 10, 213–241. [Google Scholar] [CrossRef]
- Kalaria, R.N. Cerebrovascular disease and mechanisms of cognitive impairment: Evidence from clinicopathological studies in humans. Stroke 2012, 43, 2526–2534. [Google Scholar] [CrossRef]
- Bakhtiari, A.; Vestergaard, M.B.; Benedek, K.; Fagerlund, B.; Mortensen, E.L.; Osler, M.; Lauritzen, M.; Larsson, H.B.W.; Lindberg, U. Changes in hippocampal volume during a preceding 10-year period do not correlate with cognitive performance and hippocampal blood-brain barrier permeability in cognitively normal late-middle-aged men. Geroscience 2023, 45, 1161–1175. [Google Scholar] [CrossRef]
- Gulej, R.; Nyul-Toth, A.; Csik, B.; Patai, R.; Petersen, B.; Negri, S.; Chandragiri, S.S.; Shanmugarama, S.; Mukli, P.; Yabluchanskiy, A.; et al. Young blood-mediated cerebromicrovascular rejuvenation through heterochronic parabiosis: Enhancing blood-brain barrier integrity and capillarization in the aged mouse brain. Geroscience 2024, 46, 4415–4442. [Google Scholar] [CrossRef]
- Ting, K.K.; Coleman, P.; Kim, H.J.; Zhao, Y.; Mulangala, J.; Cheng, N.C.; Li, W.; Gunatilake, D.; Johnstone, D.M.; Loo, L.; et al. Vascular senescence and leak are features of the early breakdown of the blood-brain barrier in Alzheimer’s disease models. Geroscience 2023, 45, 3307–3331. [Google Scholar] [CrossRef]
- Zachariou, V.; Pappas, C.; Bauer, C.E.; Shao, X.; Liu, P.; Lu, H.; Wang, D.J.J.; Gold, B.T. Regional differences in the link between water exchange rate across the blood-brain barrier and cognitive performance in normal aging. Geroscience 2024, 46, 265–282. [Google Scholar] [CrossRef]
- Graves, S.I.; Baker, D.J. Implicating endothelial cell senescence to dysfunction in the ageing and diseased brain. Basic. Clin. Pharmacol. Toxicol. 2020, 127, 102–110. [Google Scholar] [CrossRef]
- Bai, T.; Yu, S.; Feng, J. Advances in the Role of Endothelial Cells in Cerebral Small Vessel Disease. Front. Neurol. 2022, 13, 861714. [Google Scholar] [CrossRef]
- Walchli, T.; Ghobrial, M.; Schwab, M.; Takada, S.; Zhong, H.; Suntharalingham, S.; Vetiska, S.; Gonzalez, D.R.; Wu, R.; Rehrauer, H.; et al. Single-cell atlas of the human brain vasculature across development, adulthood and disease. Nature 2024, 632, 603–613. [Google Scholar] [CrossRef]
- McConnell, H.L.; Mishra, A. Cells of the Blood-Brain Barrier: An Overview of the Neurovascular Unit in Health and Disease. Methods Mol. Biol. 2022, 2492, 3–24. [Google Scholar] [CrossRef]
- Garcia, F.J.; Sun, N.; Lee, H.; Godlewski, B.; Mathys, H.; Galani, K.; Zhou, B.; Jiang, X.; Ng, A.P.; Mantero, J.; et al. Single-cell dissection of the human brain vasculature. Nature 2022, 603, 893–899. [Google Scholar] [CrossRef]
- Patai, R.; Csik, B.; Nyul-Toth, A.; Gulej, R.; Vali Kordestan, K.; Chandragiri, S.S.; Shanmugarama, S.; Tarantini, S.; Mukli, P.; Ungvari, A.; et al. Persisting blood-brain barrier disruption following cisplatin treatment in a mouse model of chemotherapy-associated cognitive impairment. Geroscience 2025, 47, 3835–3847. [Google Scholar] [CrossRef]
- Fekete, M.; Varga, P.; Ungvari, Z.; Fekete, J.T.; Buda, A.; Szappanos, A.; Lehoczki, A.; Mozes, N.; Grosso, G.; Godos, J.; et al. The role of the Mediterranean diet in reducing the risk of cognitive impairement, dementia, and Alzheimer’s disease: A meta-analysis. Geroscience 2025, 47, 3111–3130. [Google Scholar] [CrossRef]
- da, C.P.-L.A.C.; Szarvas, Z.; Peterfi, A.; Kaposzta, Z.; Mukli, P.; Shahriari, A.; Muranyi, M.; Pinto, C.B.; Owens, C.D.; Adams, C.; et al. Time-restricted eating for prevention of age-related vascular cognitive decline in older adults: A protocol for a single-arm open-label interventional trial. PLoS ONE 2024, 19, e0314871. [Google Scholar] [CrossRef]
- da, C.P.-L.A.C.; Pinto, C.B.; Mukli, P.; Peterfi, A.; Kaposzta, Z.; Owens, C.D.; Szarvas, Z.; Muranyi, M.; Adams, C.; Shahriari, A.; et al. Energy metabolism dysregulation, cerebrovascular aging, and time-restricted eating: Current evidence and proof-of-concept findings. PNAS Nexus 2024, 3, pgae505. [Google Scholar] [CrossRef]
- Quick, S.; Moss, J.; Rajani, R.M.; Williams, A. A Vessel for Change: Endothelial Dysfunction in Cerebral Small Vessel Disease. Trends Neurosci. 2021, 44, 289–305. [Google Scholar] [CrossRef]
- Ashby, J.W.; Mack, J.J. Endothelial Control of Cerebral Blood Flow. Am. J. Pathol. 2021, 191, 1906–1916. [Google Scholar] [CrossRef]
- Hainsworth, A.H.; Oommen, A.T.; Bridges, L.R. Endothelial cells and human cerebral small vessel disease. Brain Pathol. 2015, 25, 44–50. [Google Scholar] [CrossRef]
- Cohen, R.A. The role of nitric oxide and other endothelium-derived vasoactive substances in vascular disease. Prog. Cardiovasc. Dis. 1995, 38, 105–128. [Google Scholar] [CrossRef]
- Ainslie, P.N.; Ogoh, S. Regulation of cerebral blood flow in mammals during chronic hypoxia: A matter of balance. Exp. Physiol. 2010, 95, 251–262. [Google Scholar] [CrossRef]
- Laina, A.; Stellos, K.; Stamatelopoulos, K. Vascular ageing: Underlying mechanisms and clinical implications. Exp. Gerontol. 2018, 109, 16–30. [Google Scholar] [CrossRef]
- Csiszar, A.; Tarantini, S.; Yabluchanskiy, A.; Ungvari, Z. PCSK9: An emerging player in cardiometabolic aging and its potential as a therapeutic target and biomarker. Geroscience 2024, 46, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Zotti, T.; Vito, P.; Stilo, R. The seventh ring: Exploring TRAF7 functions. J. Cell. Physiol. 2012, 227, 1280–1284. [Google Scholar] [CrossRef]
- Zotti, T.; Scudiero, I.; Vito, P.; Stilo, R. The Emerging Role of TRAF7 in Tumor Development. J. Cell. Physiol. 2017, 232, 1233–1238. [Google Scholar] [CrossRef]
- Bradley, J.R.; Pober, J.S. Tumor necrosis factor receptor-associated factors (TRAFs). Oncogene 2001, 20, 6482–6491. [Google Scholar] [CrossRef]
- Xu, L.G.; Li, L.Y.; Shu, H.B. TRAF7 potentiates MEKK3-induced AP1 and CHOP activation and induces apoptosis. J. Biol. Chem. 2004, 279, 17278–17282. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, T.; Bauch, A.; Ruffner, H.; Angrand, P.O.; Bergamini, G.; Croughton, K.; Cruciat, C.; Eberhard, D.; Gagneur, J.; Ghidelli, S.; et al. A physical and functional map of the human TNF-alpha/NF-kappa B signal transduction pathway. Nat. Cell Biol. 2004, 6, 97–105. [Google Scholar] [CrossRef]
- Scudiero, I.; Zotti, T.; Ferravante, A.; Vessichelli, M.; Reale, C.; Masone, M.C.; Leonardi, A.; Vito, P.; Stilo, R. Tumor necrosis factor (TNF) receptor-associated factor 7 is required for TNFalpha-induced Jun NH2-terminal kinase activation and promotes cell death by regulating polyubiquitination and lysosomal degradation of c-FLIP protein. J. Biol. Chem. 2012, 287, 6053–6061. [Google Scholar] [CrossRef]
- Tsitsikov, E.N.; Phan, K.P.; Liu, Y.; Tsytsykova, A.V.; Kinter, M.; Selland, L.; Garman, L.; Griffin, C.; Dunn, I.F. TRAF7 is an essential regulator of blood vessel integrity during mouse embryonic and neonatal development. iScience 2023, 26, 107474. [Google Scholar] [CrossRef]
- Castilla-Vallmanya, L.; Selmer, K.K.; Dimartino, C.; Rabionet, R.; Blanco-Sanchez, B.; Yang, S.; Reijnders, M.R.F.; van Essen, A.J.; Oufadem, M.; Vigeland, M.D.; et al. Phenotypic spectrum and transcriptomic profile associated with germline variants in TRAF7. Genet. Med. 2020, 22, 1215–1226. [Google Scholar] [CrossRef]
- Shirakura, K.; Ishiba, R.; Kashio, T.; Funatsu, R.; Tanaka, T.; Fukada, S.I.; Ishimoto, K.; Hino, N.; Kondoh, M.; Ago, Y.; et al. The Robo4-TRAF7 complex suppresses endothelial hyperpermeability in inflammation. J. Cell Sci. 2019, 132, jcs220228. [Google Scholar] [CrossRef]
- Okada, Y. Potential Therapeutic Strategies and Drugs That Target Vascular Permeability in Severe Infectious Diseases. Biol. Pharm. Bull. 2024, 47, 549–555. [Google Scholar] [CrossRef]
- Tsitsikov, E.N.; Hameed, S.; Tavakol, S.A.; Stephens, T.M.; Tsytsykova, A.V.; Garman, L.; Bi, W.L.; Dunn, I.F. Specific gene expression signatures of low grade meningiomas. Front. Oncol. 2023, 13, 1126550. [Google Scholar] [CrossRef]
- Schapira, M.; Tyers, M.; Torrent, M.; Arrowsmith, C.H. WD40 repeat domain proteins: A novel target class? Nat. Rev. Drug Discov. 2017, 16, 773–786. [Google Scholar] [CrossRef]
- Yang, J.; Lin, Y.; Guo, Z.; Cheng, J.; Huang, J.; Deng, L.; Liao, W.; Chen, Z.; Liu, Z.; Su, B. The essential role of MEKK3 in TNF-induced NF-kappaB activation. Nat. Immunol. 2001, 2, 620–624. [Google Scholar] [CrossRef]
- Yoshida, H.; Jono, H.; Kai, H.; Li, J.D. The tumor suppressor cylindromatosis (CYLD) acts as a negative regulator for toll-like receptor 2 signaling via negative cross-talk with TRAF6 AND TRAF7. J. Biol. Chem. 2005, 280, 41111–41121. [Google Scholar] [CrossRef]
- Jones, C.A.; London, N.R.; Chen, H.; Park, K.W.; Sauvaget, D.; Stockton, R.A.; Wythe, J.D.; Suh, W.; Larrieu-Lahargue, F.; Mukouyama, Y.S.; et al. Robo4 stabilizes the vascular network by inhibiting pathologic angiogenesis and endothelial hyperpermeability. Nat. Med. 2008, 14, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.W.; Mathivet, T.; Larrivee, B.; Tong, R.K.; Kowalski, J.; Pibouin-Fragner, L.; Bouvree, K.; Stawicki, S.; Nicholes, K.; Rathore, N.; et al. Robo4 maintains vessel integrity and inhibits angiogenesis by interacting with UNC5B. Dev. Cell 2011, 20, 33–46. [Google Scholar] [CrossRef]
- Shirakura, K.; Ishiba, R.; Kashio, T.; Sakai, M.; Fukushima, Y.; Yamamoto, N.; Manabe, S.; Shigesada, N.; Tanaka, T.; Hino, N.; et al. Endothelial Robo4 regulates IL-6 production by endothelial cells and monocytes via a crosstalk mechanism in inflammation. Biochem. Biophys. Res. Commun. 2018, 495, 801–806. [Google Scholar] [CrossRef]
- Suchting, S.; Heal, P.; Tahtis, K.; Stewart, L.M.; Bicknell, R. Soluble Robo4 receptor inhibits in vivo angiogenesis and endothelial cell migration. FASEB J. 2005, 19, 121–123. [Google Scholar] [CrossRef]
- Yang, S.H.; Sharrocks, A.D.; Whitmarsh, A.J. MAP kinase signalling cascades and transcriptional regulation. Gene 2013, 513, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.; Narang, H. The complexity of mitogen-activated protein kinases (MAPKs) made simple. Cell. Mol. Life Sci. 2008, 65, 3525–3544. [Google Scholar] [CrossRef]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef]
- Wang, X.; Tournier, C. Regulation of cellular functions by the ERK5 signalling pathway. Cell. Signal. 2006, 18, 753–760. [Google Scholar] [CrossRef]
- Hayashi, M.; Kim, S.W.; Imanaka-Yoshida, K.; Yoshida, T.; Abel, E.D.; Eliceiri, B.; Yang, Y.; Ulevitch, R.J.; Lee, J.D. Targeted deletion of BMK1/ERK5 in adult mice perturbs vascular integrity and leads to endothelial failure. J. Clin. Investig. 2004, 113, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Boerm, M.; McCarty, M.; Bucana, C.; Fidler, I.J.; Zhuang, Y.; Su, B. Mekk3 is essential for early embryonic cardiovascular development. Nat. Genet. 2000, 24, 309–313. [Google Scholar] [CrossRef]
- Hayashi, M.; Lee, J.D. Role of the BMK1/ERK5 signaling pathway: Lessons from knockout mice. J. Mol. Med. 2004, 82, 800–808. [Google Scholar] [CrossRef]
- Wang, X.; Merritt, A.J.; Seyfried, J.; Guo, C.; Papadakis, E.S.; Finegan, K.G.; Kayahara, M.; Dixon, J.; Boot-Handford, R.P.; Cartwright, E.J.; et al. Targeted deletion of mek5 causes early embryonic death and defects in the extracellular signal-regulated kinase 5/myocyte enhancer factor 2 cell survival pathway. Mol. Cell. Biol. 2005, 25, 336–345. [Google Scholar] [CrossRef]
- Sohn, S.J.; Sarvis, B.K.; Cado, D.; Winoto, A. ERK5 MAPK regulates embryonic angiogenesis and acts as a hypoxia-sensitive repressor of vascular endothelial growth factor expression. J. Biol. Chem. 2002, 277, 43344–43351. [Google Scholar] [CrossRef]
- Regan, C.P.; Li, W.; Boucher, D.M.; Spatz, S.; Su, M.S.; Kuida, K. Erk5 null mice display multiple extraembryonic vascular and embryonic cardiovascular defects. Proc. Natl. Acad. Sci. USA 2002, 99, 9248–9253. [Google Scholar] [CrossRef]
- Roberts, O.L.; Holmes, K.; Muller, J.; Cross, D.A.; Cross, M.J. ERK5 and the regulation of endothelial cell function. Biochem. Soc. Trans. 2009, 37 Pt 6, 1254–1259. [Google Scholar] [CrossRef]
- Fisher, O.S.; Deng, H.; Liu, D.; Zhang, Y.; Wei, R.; Deng, Y.; Zhang, F.; Louvi, A.; Turk, B.E.; Boggon, T.J.; et al. Structure and vascular function of MEKK3-cerebral cavernous malformations 2 complex. Nat. Commun. 2015, 6, 7937. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.; Malik, A.B. Signaling mechanisms regulating endothelial permeability. Physiol. Rev. 2006, 86, 279–367. [Google Scholar] [CrossRef] [PubMed]
- Pober, J.S.; Sessa, W.C. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 2007, 7, 803–815. [Google Scholar] [CrossRef]
- He, H.; Wu, Z.; Li, S.; Chen, K.; Wang, D.; Zou, H.; Chen, H.; Li, Y.; Liu, Z.; Qu, C. TRAF7 enhances ubiquitin-degradation of KLF4 to promote hepatocellular carcinoma progression. Cancer Lett. 2020, 469, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kang, J.; Liu, N.; Huang, J.; Zhang, X.; Pang, K.; Zhang, S.; Wang, M.; Zhao, Y.; Dong, S.; et al. Hydrogen sulfide improves endothelial barrier function by modulating the ubiquitination degradation of KLF4 through TRAF7 S-sulfhydration in diabetic aorta. Free Radic. Biol. Med. 2024, 216, 118–138. [Google Scholar] [CrossRef]
- Song, X.; Hu, R.; Chen, Y.; Xiao, M.; Zhang, H.; Wu, S.; Lu, Q. The structure of TRAF7 coiled-coil trimer provides insight into its function in zebrafish embryonic development. J. Mol. Cell Biol. 2024, 16, mjad083. [Google Scholar] [CrossRef]
- Chen, M.; Shi, S.; Zhao, J.; Pan, Q.; Huang, C.; Shen, Q.; Liu, Z. Propofol inhibits cell apoptosis and inflammatory response in ox-LDL-induced human umbilical vein endothelial cells through the modulation of the circ_0003645/miR-149-3p/TRAF7 axis. Clin. Hemorheol. Microcirc. 2023, 84, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Shirakura, K.; Takayama, Y.; Muatsui, M.; Watanabe, Y.; Yamamoto, T.; Takahashi, J.; Tanaka, S.; Hino, N.; Doi, T.; et al. Endothelial ROBO4 suppresses PTGS2/COX-2 expression and inflammatory diseases. Commun. Biol. 2024, 7, 599. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Shen, Q.; Pivetti, C.D.; Lee, E.S.; Wu, M.H.; Yuan, S.Y. Molecular mechanisms of endothelial hyperpermeability: Implications in inflammation. Expert. Rev. Mol. Med. 2009, 11, e19. [Google Scholar] [CrossRef]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory Mechanisms Contributing to Endothelial Dysfunction. Biomedicines 2021, 9, 781. [Google Scholar] [CrossRef]
- Rodrigues, S.F.; Granger, D.N. Blood cells and endothelial barrier function. Tissue Barriers 2015, 3, e978720. [Google Scholar] [CrossRef]
- Godo, S.; Shimokawa, H. Endothelial Functions. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e108–e114. [Google Scholar] [CrossRef]
- Claesson-Welsh, L.; Dejana, E.; McDonald, D.M. Permeability of the Endothelial Barrier: Identifying and Reconciling Controversies. Trends Mol. Med. 2021, 27, 314–331. [Google Scholar] [CrossRef]
- Michiels, C. Endothelial cell functions. J. Cell. Physiol. 2003, 196, 430–443. [Google Scholar] [CrossRef] [PubMed]
- Raz, L.; Knoefel, J.; Bhaskar, K. The neuropathology and cerebrovascular mechanisms of dementia. J. Cereb. Blood Flow. Metab. 2016, 36, 172–186. [Google Scholar] [CrossRef]
- Wang, F.; Cao, Y.; Ma, L.; Pei, H.; Rausch, W.D.; Li, H. Dysfunction of Cerebrovascular Endothelial Cells: Prelude to Vascular Dementia. Front. Aging Neurosci. 2018, 10, 376. [Google Scholar] [CrossRef] [PubMed]
- Janaszak-Jasiecka, A.; Ploska, A.; Wieronska, J.M.; Dobrucki, L.W.; Kalinowski, L. Endothelial dysfunction due to eNOS uncoupling: Molecular mechanisms as potential therapeutic targets. Cell. Mol. Biol. Lett. 2023, 28, 21. [Google Scholar] [CrossRef]
- Haley, M.J.; Lawrence, C.B. The blood-brain barrier after stroke: Structural studies and the role of transcytotic vesicles. J. Cereb. Blood Flow. Metab. 2017, 37, 456–470. [Google Scholar] [CrossRef]
- Nahirney, P.C.; Reeson, P.; Brown, C.E. Ultrastructural analysis of blood-brain barrier breakdown in the peri-infarct zone in young adult and aged mice. J. Cereb. Blood Flow. Metab. 2016, 36, 413–425. [Google Scholar] [CrossRef]
- van der Loo, B.; Labugger, R.; Skepper, J.N.; Bachschmid, M.; Kilo, J.; Powell, J.M.; Palacios-Callender, M.; Erusalimsky, J.D.; Quaschning, T.; Malinski, T.; et al. Enhanced peroxynitrite formation is associated with vascular aging. J. Exp. Med. 2000, 192, 1731–1744. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.J. Normal Aging Induces Changes in the Brain and Neurodegeneration Progress: Review of the Structural, Biochemical, Metabolic, Cellular, and Molecular Changes. Front. Aging Neurosci. 2022, 14, 931536. [Google Scholar] [CrossRef]
- Izzo, C.; Carrizzo, A.; Alfano, A.; Virtuoso, N.; Capunzo, M.; Calabrese, M.; De Simone, E.; Sciarretta, S.; Frati, G.; Oliveti, M.; et al. The Impact of Aging on Cardio and Cerebrovascular Diseases. Int. J. Mol. Sci. 2018, 19, 481. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Tarantini, S.; Donato, A.J.; Galvan, V.; Csiszar, A. Mechanisms of Vascular Aging. Circ. Res. 2018, 123, 849–867. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Sonntag, W.E.; Csiszar, A. Mitochondria and aging in the vascular system. J. Mol. Med. 2010, 88, 1021–1027. [Google Scholar] [CrossRef]
- Santisteban, M.M.; Iadecola, C. The pathobiology of neurovascular aging. Neuron 2025, 113, 49–70. [Google Scholar] [CrossRef] [PubMed]
- Andonian, B.J.; Hippensteel, J.A.; Abuabara, K.; Boyle, E.M.; Colbert, J.F.; Devinney, M.J.; Faye, A.S.; Kochar, B.; Lee, J.; Litke, R.; et al. Inflammation and aging-related disease: A transdisciplinary inflammaging framework. Geroscience 2025, 47, 515–542. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69 (Suppl. 1), S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, A.; Ungvari, Z.; Koller, A.; Edwards, J.G.; Kaley, G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol. Genom. 2004, 17, 21–30. [Google Scholar] [CrossRef]
- Kallai, A.; Ungvari, Z.; Fekete, M.; Maier, A.B.; Mikala, G.; Andrikovics, H.; Lehoczki, A. Genomic instability and genetic heterogeneity in aging: Insights from clonal hematopoiesis (CHIP), monoclonal gammopathy (MGUS), and monoclonal B-cell lymphocytosis (MBL). Geroscience 2025, 47, 703–720. [Google Scholar] [CrossRef]
- Ren, P.; Zhang, J.; Vijg, J. Somatic mutations in aging and disease. Geroscience 2024, 46, 5171–5189. [Google Scholar] [CrossRef]
- Stock, A.J.; Ayyar, S.; Kashyap, A.; Wang, Y.; Yanai, H.; Starost, M.F.; Tanaka-Yano, M.; Bodogai, M.; Sun, C.; Wang, Y.; et al. Boosting NAD ameliorates hematopoietic impairment linked to short telomeres in vivo. Geroscience 2023, 45, 2213–2228. [Google Scholar] [CrossRef]
- Khan, E. An examination of the blood-brain barrier in health and disease. Br. J. Nurs. 2005, 14, 509–513. [Google Scholar] [CrossRef]
- Nippert, A.R.; Chiang, P.P.; Newman, E.A. Whisker-evoked neurovascular coupling is preserved during hypoglycemia in mouse cortical arterioles and capillaries. J. Cereb. Blood Flow. Metab. 2024, 44, 155–168. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, W.; Kan, Y.; Ren, C.; Ji, X. From Mechanisms to Medicine: Neurovascular Coupling in the Diagnosis and Treatment of Cerebrovascular Disorders: A Narrative Review. Cells 2024, 14, 16. [Google Scholar] [CrossRef]
- Donato, A.J.; Morgan, R.G.; Walker, A.E.; Lesniewski, L.A. Cellular and molecular biology of aging endothelial cells. J. Mol. Cell. Cardiol. 2015, 89 Pt B, 122–135. [Google Scholar] [CrossRef]
- Knopp, R.C.; Erickson, M.A.; Rhea, E.M.; Reed, M.J.; Banks, W.A. Cellular senescence and the blood-brain barrier: Implications for aging and age-related diseases. Exp. Biol. Med. 2023, 248, 399–411. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Baker, D.J.; Tachibana, M.; Liu, C.C.; van Deursen, J.M.; Brott, T.G.; Bu, G.; Kanekiyo, T. Vascular Cell Senescence Contributes to Blood-Brain Barrier Breakdown. Stroke 2016, 47, 1068–1077. [Google Scholar] [CrossRef]
- Che, J.; Sun, Y.; Deng, Y.; Zhang, J. Blood-brain barrier disruption: A culprit of cognitive decline? Fluids Barriers CNS 2024, 21, 63. [Google Scholar] [CrossRef]
- French, S.R.; Meyer, B.P.; Arias, J.C.; Levendovzsky, S.R.; Weinkauf, C.C. Biomarkers of blood-brain barrier and neurovascular unit integrity in human cognitive impairment and dementia. Alzheimers Dement. 2025, 21, e70104. [Google Scholar] [CrossRef]
- Enciu, A.M.; Gherghiceanu, M.; Popescu, B.O. Triggers and effectors of oxidative stress at blood-brain barrier level: Relevance for brain ageing and neurodegeneration. Oxid. Med. Cell. Longev. 2013, 2013, 297512. [Google Scholar] [CrossRef]
- Kim, S.; Jung, U.J.; Kim, S.R. Role of Oxidative Stress in Blood-Brain Barrier Disruption and Neurodegenerative Diseases. Antioxidants 2024, 13, 1462. [Google Scholar] [CrossRef] [PubMed]
- Gulej, R.; Nyul-Toth, A.; Csik, B.; Petersen, B.; Faakye, J.; Negri, S.; Chandragiri, S.S.; Mukli, P.; Yabluchanskiy, A.; Conley, S.; et al. Rejuvenation of cerebromicrovascular function in aged mice through heterochronic parabiosis: Insights into neurovascular coupling and the impact of young blood factors. Geroscience 2024, 46, 327–347. [Google Scholar] [CrossRef] [PubMed]
- Kieronska-Rudek, A.; Kij, A.; Bar, A.; Kurpinska, A.; Mohaissen, T.; Grosicki, M.; Stojak, M.; Sternak, M.; Buczek, E.; Proniewski, B.; et al. Phylloquinone improves endothelial function, inhibits cellular senescence, and vascular inflammation. Geroscience 2024, 46, 4909–4935. [Google Scholar] [CrossRef] [PubMed]
- Pospiech, E.; Bar, A.; Pisarek-Pacek, A.; Karas, A.; Branicki, W.; Chlopicki, S. Epigenetic clock in the aorta and age-related endothelial dysfunction in mice. Geroscience 2024, 46, 3993–4002. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Mediterranean dietary pattern, inflammation and endothelial function: A systematic review and meta-analysis of intervention trials. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 929–939. [Google Scholar] [CrossRef]
- Shannon, O.M.; Mendes, I.; Kochl, C.; Mazidi, M.; Ashor, A.W.; Rubele, S.; Minihane, A.M.; Mathers, J.C.; Siervo, M. Mediterranean Diet Increases Endothelial Function in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Nutr. 2020, 150, 1151–1159. [Google Scholar] [CrossRef]
- Clark, P.R.; Jensen, T.J.; Kluger, M.S.; Morelock, M.; Hanidu, A.; Qi, Z.; Tatake, R.J.; Pober, J.S. MEK5 is activated by shear stress, activates ERK5 and induces KLF4 to modulate TNF responses in human dermal microvascular endothelial cells. Microcirculation 2011, 18, 102–117. [Google Scholar] [CrossRef]
- Kashio, T.; Shirakura, K.; Kinoshita, M.; Morita, M.; Ishiba, R.; Muraoka, K.; Kanbara, T.; Tanaka, M.; Funatsu, R.; Hino, N.; et al. HDAC inhibitor, MS-275, increases vascular permeability by suppressing Robo4 expression in endothelial cells. Tissue Barriers 2021, 9, 1911195. [Google Scholar] [CrossRef] [PubMed]
- Gissler, M.C.; Stachon, P.; Wolf, D.; Marchini, T. The Role of Tumor Necrosis Factor Associated Factors (TRAFs) in Vascular Inflammation and Atherosclerosis. Front. Cardiovasc. Med. 2022, 9, 826630. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Lin, D.; Zhong, Z.; Ye, Q. Roles of TRAFs in Ischemia-Reperfusion Injury. Front. Cell Dev. Biol. 2020, 8, 586487. [Google Scholar] [CrossRef]
- Ungvari, Z.; Buffenstein, R.; Austad, S.N.; Podlutsky, A.; Kaley, G.; Csiszar, A. Oxidative stress in vascular senescence: Lessons from successfully aging species. Front. Biosci. 2008, 13, 5056–5070. [Google Scholar] [CrossRef]
- Ungvari, Z.; Tarantini, S.; Kirkpatrick, A.C.; Csiszar, A.; Prodan, C.I. Cerebral microhemorrhages: Mechanisms, consequences, and prevention. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H1128–H1143. [Google Scholar] [CrossRef]
- Ungvari, Z.; Tarantini, S.; Sorond, F.; Merkely, B.; Csiszar, A. Mechanisms of Vascular Aging, A Geroscience Perspective: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Balbi, M.; Ghosh, M.; Longden, T.A.; Jativa Vega, M.; Gesierich, B.; Hellal, F.; Lourbopoulos, A.; Nelson, M.T.; Plesnila, N. Dysfunction of mouse cerebral arteries during early aging. J. Cereb. Blood Flow. Metab. 2015, 35, 1445–1453. [Google Scholar] [CrossRef]
- Capdeville, M.; Coutard, M.; Osborne-Pellegrin, M.J. Spontaneous rupture of the internal elastic lamina in the rat: The manifestation of a genetically determined factor which may be linked to vascular fragility. Blood Vessel. 1989, 26, 197–212. [Google Scholar] [CrossRef]
- Donato, A.J.; Machin, D.R.; Lesniewski, L.A. Mechanisms of Dysfunction in the Aging Vasculature and Role in Age-Related Disease. Circ. Res. 2018, 123, 825–848. [Google Scholar] [CrossRef]
- Liu, Y.; Bloom, S.I.; Donato, A.J. The role of senescence, telomere dysfunction and shelterin in vascular aging. Microcirculation 2019, 26, e12487. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, B.; Yu, Y.; Gao, W.; Liu, W.; Chen, L.; Xia, Z.; Cao, Q. Vascular Aging in Ischemic Stroke. J. Am. Heart Assoc. 2024, 13, e033341. [Google Scholar] [CrossRef]
- Faakye, J.; Nyul-Toth, A.; Gulej, R.; Csik, B.; Tarantini, S.; Shanmugarama, S.; Prodan, C.; Mukli, P.; Yabluchanskiy, A.; Conley, S.; et al. Imaging the time course, morphology, neuronal tissue compression, and resolution of cerebral microhemorrhages in mice using intravital two-photon microscopy: Insights into arteriolar, capillary, and venular origin. Geroscience 2023, 45, 2851–2872. [Google Scholar] [CrossRef]
- Caminiti, R.; Carresi, C.; Mollace, R.; Macri, R.; Scarano, F.; Oppedisano, F.; Maiuolo, J.; Serra, M.; Ruga, S.; Nucera, S.; et al. The potential effect of natural antioxidants on endothelial dysfunction associated with arterial hypertension. Front. Cardiovasc. Med. 2024, 11, 1345218. [Google Scholar] [CrossRef] [PubMed]
- Medina-Leyte, D.J.; Zepeda-Garcia, O.; Dominguez-Perez, M.; Gonzalez-Garrido, A.; Villarreal-Molina, T.; Jacobo-Albavera, L. Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Approaches. Int. J. Mol. Sci. 2021, 22, 3850. [Google Scholar] [CrossRef] [PubMed]
- Su, J.B. Vascular endothelial dysfunction and pharmacological treatment. World J. Cardiol. 2015, 7, 719–741. [Google Scholar] [CrossRef]
- Real, M.G.C.; Falcione, S.R.; Boghozian, R.; Clarke, M.; Todoran, R.; St Pierre, A.; Zhang, Y.; Joy, T.; Jickling, G.C. Endothelial Cell Senescence Effect on the Blood-Brain Barrier in Stroke and Cognitive Impairment. Neurology 2024, 103, e210063. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Jiang, H.; Wei, H.; Zhou, Y.; Ji, X.; Zhou, C. Endothelial Senescence in Neurological Diseases. Aging Dis. 2023, 14, 2153–2166. [Google Scholar] [CrossRef] [PubMed]

| Disorder/Model(s) | Findings | Limitations/Clinical Relevance | Reference |
|---|---|---|---|
| No disorder Human tissues HEK293 cells | Human TRAF7 mRNA detected in (highest to lowest) skeletal muscle, heart, brain, kidney, placenta, spleen, colon, small intestine, brain, thymus, lung, leukocytes TRAF7 binds MEKK3 TRAF7 interaction with AP1 and CHOP | Not a study of a disease process, only characterization of some TRAF7 interactions. Cell culture results may not reflect tissue-specific TRAF7 interactions. | Xu, L-G et al., 2004 [45] |
| No disorder A529 cells HeLa cells HEK293 cells | TRAF7 activates NF-κB promoter. TRAF7 enhances TRAF6 activation of NF-κB promoter. TRAF7 participates in phosphorylation of IκBα and p38 and induction of TNF-α, IL-1β, and IL-8. CYLD inhibits TRAF6 and TRAF7 ubiquitination. | Not a study of a disease process, only characterization of some TRAF7 interactions. Cell culture results may not reflect tissue-specific TRAF7 interactions. | Yoshida H et al., 2005 [55] |
| No disorder HEK293 cells HeLa cells Mouse embryonic fibroblasts | TRAF7 facilitates TNF-α activation of AP1 promoter TRAF7 facilitates TNF-α-mediated phosphorylation of JNK. TRAF7 ubiquitinates c-FLIPL, but not JNK. | Not a study of a disease process, only characterization of some TRAF7 interactions. Cell culture results may not reflect tissue-specific TRAF7 interactions. | Scudiero I et al., 2012 [47] |
| Hepatocellular carcinoma (HCC) PLC5 cells HepG2 cells MHCC97H cells MHCC97L cells HEK293 cells Patient tissue samples–tumor and healthy tissue | Increased TRAF7 mRNA in HCC compared to normal tissues HCC with higher TRAF7 expression had less disease-free survival and less overall survival. In HCC cells: TRAF7 expression negatively correlated with KLF4 expression. KLF4 expression was reduced by TRAF7 ubiquitination of KLF4. TNF-α decreased TRAF7 expression, which inhibited KLF4 degradation. IL-6 increased TRAF7 expression, which facilitated KLF4 degradation. TRAF7 did not affect apoptosis. TRAF7 promoted metastasis. In normal cells: TRAF7 increased KLF4 ubiquitination. TRAF7 binds KLF4 via the RING finger and coiled-coil domains. The N-terminal of KLF4 (1–60 amino acids) is required for TRAF7 ubiquitination. | Mechanism of KLF4 regulation by TRAF7 in HCC may not apply to other cancers. The interaction in normal cell lines was due to overexpression or knockdown of expression; the TRAF7-KLF4 interaction may not be physiologically relevant for oncogenesis. | He H et al., 2020 [74] |
| Diabetes (Type 1 or 2) db/db or wild type mice Streptozotocin (STZ) treated Wistar rats Mouse aortic endothelial cells (MAECs) | Db/db mice (aorta) had higher TRAF7 expression, which was reversed by NaSH. Wistar rats treated with STZ and fed a high-fat diet had increased TRAF7 expression (aorta) that was inhibited by NaSH treatment. In MAECs, treatment with high glucose and palmitate (a model of diabetes) increased TRAF7 expression and was reversed with NaSH. TRAF7 ubiquitination of KLF4 was decreased by NASH. In the diabetes cell culture model, TRAF7 decreased Nrf2, VE-cadherin, β-catenin, and occludin expression. H2S sulfhydration of TRAF7 inhibits modulation of KLF4. | Animal models of diabetes have limited translation to chronic diabetes. The majority of the mechanism was determined using MAECs, which are specialized and may not represent the physiology of other endothelial cells. Uncertain if the MAEC diabetic model is relevant to chronic diabetes. | Li Q et al., 2024 [75] |
| No disorder Floxed (fl) TRAF7 mice TRAF7fl/fl:E2a-Cre mice TRAF7fl/fl:Tie2-Cre TRAF7fl/fl:Cdh5 (PAC)-CreERT2 Human umbilical vein endothelial cells (HUVECs) HEK293 cells | Global TRAF7 deletion was embryonic lethal due to developmental heart defects. Endothelial TRAF7 deletion was embryonic lethal. Postnatal endothelial TRAF7 deletion caused lethal brain hemorrhage. TRAF7 bound MEKK3, MEKK2, MEK5, and SCRIB. MEKK3 and MEK5 bound to the C-terminal WD40 domain of TRAF7. MEK5 and SCRIB bound to the RING and zinc finger domains of TRAF7. Phosphorylation of ERK5 increased in HUVECs exposed to shear stress mediated by TRAF7, SCRIB, and MEKK3. Expression of KLF2 and KLF4 was also increased in HUVECs exposed to shear stress. | Not a study of a disease process, only characterization of some TRAF7 interactions. The lethal TRAF7 deletion in the mouse model does not reproduce the clinical TRAF7 syndrome, which causes developmental delay and other abnormalities. Cell culture results may not reflect tissue-specific TRAF7 interactions. The shear stress cell culture model may have limited translatability to clinical vascular disorders. | Tsitsikov E et al., 2023 [48] |
| No disorder Wild-type or red fluorescent protein-expressing zebrafish | TRAF7 expression in zebrafish can be monitored throughout development, with the highest expression in the brain. Knockdown of TRAF7 causes abnormal development in zebrafish. The coiled-coil domain of TRAF7 is necessary for zebrafish development. | Not a study of a disease process, only characterization of some TRAF7 interactions. Zebrafish are commonly used to study developmental biology since they are transparent and can be used in large numbers. Zebrafish biology may not accurately model human biology/disease. | Song X et al., 2024 [76] |
| Atherosclerosis (AS) Serum samples from healthy volunteers and AS patients HUVEC model of AS induced by oxidized low-density lipoprotein (ox-LDL) | In HUVECs, ox-LDL caused dose and time-dependent decreases in cell viability and increases in apoptosis; propofol treatment reversed the ox-LDL effects. Circular RNA, Circ_0003645, expression was increased in AS serum and ox-LDL-treated HUVECs. Propofol treatment reduced Circ_0003645 in ox-LDL-treated HUVECs. miR-149-3p was decreased in AS serum and HUVECs treated with ox-LDL, due to Circ_0003645, and restored with propofol treatment. TRAF7 increased in AS serum and ox-LDL-treated HUVECs and decreased by propofol treatment. miR-149-3p decreased TRAF7 expression, and the effect was reversed by Circ_0003645-induced decrease in miR-149-3p. | While a mechanism for the modulation of TRAF7 in a HUVEC model of AS was developed, this same model was not evaluated in cells from AS patients. The ox-LDL model of AS in HUVEC may not accurately model chronic disease. | Chen M et al., 2023 [77] |
| Inflammation-Induced Endothelial Hyperpermeability Robo4 knockout mice HUVECs HEK293 cells COS-7 cells | In Robo4-/- mice, lipopolysaccharide (LPS)-induced permeability in the heart, lung, and small intestine. Robo4 inhibits TNF-α-induced endothelial hyperpermeability. Robo4 increases VE-cadherin localization to endothelial cell junctions. TRAF7 interacts with the C-terminal of Robo4. TRAF7 is necessary for Robo4 inhibition of endothelial hyperpermeability. | Cell culture results may not reflect tissue-specific TRAF7 interactions. The importance of Robo4 signaling in inflammation-induced hyperpermeability in clinical disorders is unknown. | Shirakura K et al., 2019 [50] |
| No disorder HEK293 cells | Analysis of the TNF-α-NF-κB signaling pathway. TRAF7 was identified as reducing NF-κB activation. MEKK3 phosphorylates and ubiquitinates TRAF7. TRAF7 WD40 domain interacts with MEKK3. TRAF7 coiled-coil domain is necessary for TRAF7 homodimerization. TRAF7-MEKK3 interaction activates NF-κB, JNK, p38. | Cell culture results may not reflect tissue-specific TRAF7 interactions. Modulation of inflammatory signaling may be relevant for treating chronic inflammatory diseases. | Bouwmeester T et al., 2004 [46] |
| TRAF7 Syndrome Genetic testing of patients Skin biopsy fibroblast cell culture | 45 patients with TRAF7 germline variants were identified. All variants were within the WD40 region of TRAF7. mRNA expression of selected differentially expressed genes were verified. | Many of the germline variants were unique, indicating there is not a single key variant to target for treatment of the syndrome. | Castilla-Vallmanya L. et al., 2020 [49] |
| Inflammation-Induced Endothelial Hyperpermeability HUVECs HEK293 cells COS-7 cells Robo4 knockout mice –collagen-induced arthritis model | Robo4 and TRAF7 interact to ubiquitinate IQGAP1 to suppress RAC1. RAC1 induces PTGS2 expression, which leads to endothelial hyperpermeability via JNK-AP1 signaling. | Cell culture results may not reflect tissue-specific TRAF7 interactions. The importance of Robo4 signaling in inflammation-induced hyperpermeability in clinical disorders is unknown. | Tanaka M et al., 2024 [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ihuoma, J.; Tavakol, S.; Negri, S.; Ballard, C.; Phan, K.; Orock, A.; Reyff, Z.; Milan, M.; Troyano-Rodriguez, E.; Rudraboina, R.; et al. Review of the Role of TRAF7 in Brain Endothelial Integrity and Cerebrovascular Aging. Life 2025, 15, 1280. https://doi.org/10.3390/life15081280
Ihuoma J, Tavakol S, Negri S, Ballard C, Phan K, Orock A, Reyff Z, Milan M, Troyano-Rodriguez E, Rudraboina R, et al. Review of the Role of TRAF7 in Brain Endothelial Integrity and Cerebrovascular Aging. Life. 2025; 15(8):1280. https://doi.org/10.3390/life15081280
Chicago/Turabian StyleIhuoma, Jennifer, Sherwin Tavakol, Sharon Negri, Cade Ballard, Khanh Phan, Albert Orock, Zeke Reyff, Madison Milan, Eva Troyano-Rodriguez, Rakesh Rudraboina, and et al. 2025. "Review of the Role of TRAF7 in Brain Endothelial Integrity and Cerebrovascular Aging" Life 15, no. 8: 1280. https://doi.org/10.3390/life15081280
APA StyleIhuoma, J., Tavakol, S., Negri, S., Ballard, C., Phan, K., Orock, A., Reyff, Z., Milan, M., Troyano-Rodriguez, E., Rudraboina, R., Csiszar, A., Johnson, A. C., Dunn, I. F., & Tarantini, S. (2025). Review of the Role of TRAF7 in Brain Endothelial Integrity and Cerebrovascular Aging. Life, 15(8), 1280. https://doi.org/10.3390/life15081280







