Cerebral Resistance Artery Histological Remodeling After Training—Sex Differences
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chemicals
2.3. Intensive Swim Training Protocol
2.4. Histology and Immunohistochemistry
2.5. Image Analysis
2.6. Statistical Analysis
3. Results
3.1. Exercise Effect of Pial Arterioles
3.1.1. RF Staining
3.1.2. SMA Staining
3.2. Sex Differences in Pial Arterioles
3.2.1. RF Staining
3.2.2. SMA Staining
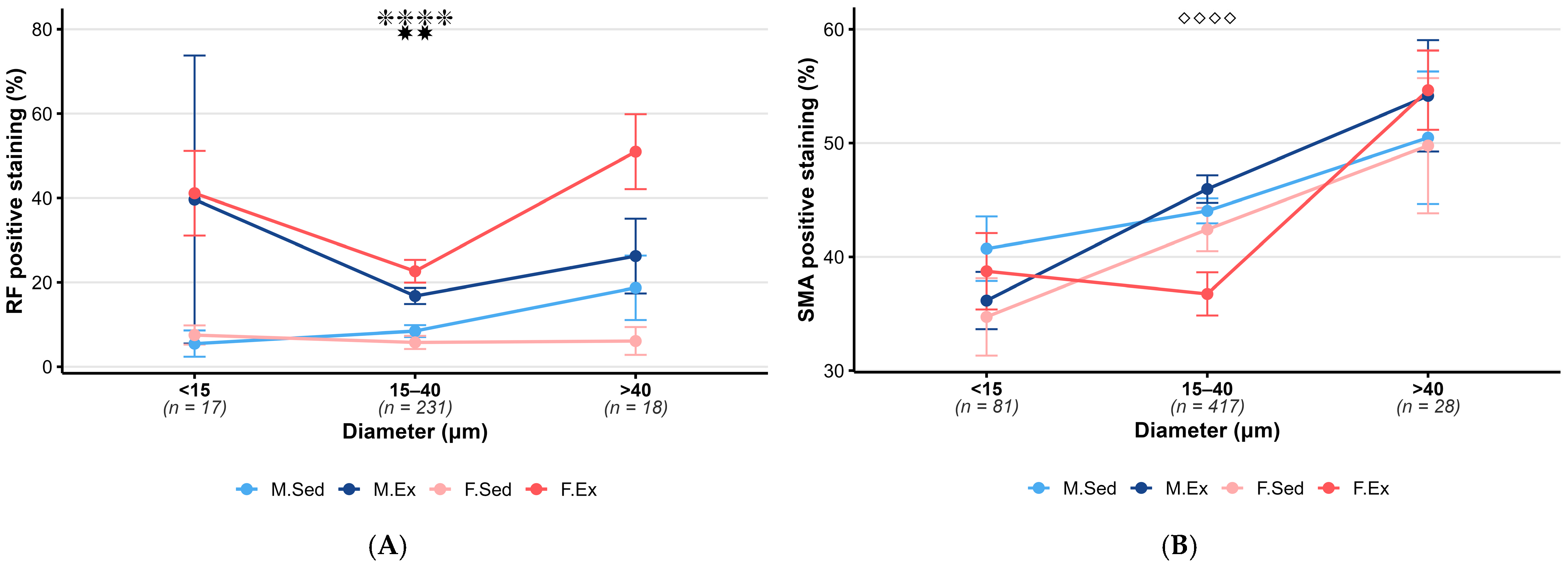
3.3. Exercise Effect of Intracerebral Arterioles
3.3.1. RF Staining
3.3.2. SMA Staining
3.4. Sex Differences in Intracerebral Arterioles
3.4.1. RF Staining
3.4.2. SMA Staining
4. Discussion
4.1. Exercise Differences
4.2. Sex Differences
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brellenthin, A.G.; Lanningham-Foster, L.M.; Kohut, M.L.; Li, Y.; Church, T.S.; Blair, S.N.; Lee, D.C. Comparison of the Cardiovascular Benefits of Resistance, Aerobic, and Combined Exercise (CardioRACE): Rationale, design, and methods. Am. Heart J. 2019, 217, 101–111. [Google Scholar] [CrossRef]
- Querido, J.S.; Sheel, A.W. Regulation of cerebral blood flow during exercise. Sports Med. 2007, 37, 765–782. [Google Scholar] [CrossRef]
- Klein, S.P.; De Sloovere, V.; Meyfroidt, G.; Depreitere, B. Differential Hemodynamic Response of Pial Arterioles Contributes to a Quadriphasic Cerebral Autoregulation Physiology. J. Am. Heart Assoc. 2022, 11, e022943. [Google Scholar] [CrossRef] [PubMed]
- Willie, C.K.; Tzeng, Y.C.; Fisher, J.A.; Ainslie, P.N. Integrative regulation of human brain blood flow. J. Physiol. 2014, 592, 841–859. [Google Scholar] [CrossRef]
- Olivo, G.; Nilsson, J.; Garzón, B.; Lebedev, A.; Wåhlin, A.; Tarassova, O.; Ekblom, M.; Lövdén, M. Immediate effects of a single session of physical exercise on cognition and cerebral blood flow: A randomized controlled study of older adults. Neuroimage 2021, 225, 117500. [Google Scholar] [CrossRef] [PubMed]
- Stimpson, N.J.; Davison, G.; Javadi, A.H. Joggin’ the Noggin: Towards a Physiological Understanding of Exercise-Induced Cognitive Benefits. Neurosci. Biobehav. Rev. 2018, 88, 177–186. [Google Scholar] [CrossRef]
- McGillivray, J.E.; Debruin, H.; Kumbhare, D.; Noseworthy, M.D. The Effect of Exercise on Neural Activation and Cognition: A Review of Task-Based fMRI Studies. Crit. Rev. Biomed. Eng. 2021, 49, 21–52. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, J.; Åberg, M.A.; Schiöler, L.; Nilsson, M.; Wallin, A.; Torén, K.; Kuhn, H.G. Cardiovascular and cognitive fitness at age 18 and risk of early-onset dementia. Brain 2014, 137, 1514–1523. [Google Scholar] [CrossRef]
- Brown, A.D.; McMorris, C.A.; Longman, R.S.; Leigh, R.; Hill, M.D.; Friedenreich, C.M.; Poulin, M.J. Effects of cardiorespiratory fitness and cerebral blood flow on cognitive outcomes in older women. Neurobiol. Aging 2010, 31, 2047–2057. [Google Scholar] [CrossRef]
- Sobol, N.A.; Hoffmann, K.; Vogel, A.; Lolk, A.; Gottrup, H.; Høgh, P.; Hasselbalch, S.G.; Beyer, N. Associations between physical function, dual-task performance and cognition in patients with mild Alzheimer’s disease. Aging Ment. Health 2016, 20, 1139–1146. [Google Scholar] [CrossRef]
- Barnes, J.N.; Pearson, A.G.; Corkery, A.T.; Eisenmann, N.A.; Miller, K.B. Exercise, Arterial Stiffness, and Cerebral Vascular Function: Potential Impact on Brain Health. J. Int. Neuropsychol. Soc. 2021, 27, 761–775. [Google Scholar] [CrossRef]
- DeSouza, C.A.; Shapiro, L.F.; Clevenger, C.M.; Dinenno, F.A.; Monahan, K.D.; Tanaka, H.; Seals, D.R. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 2000, 102, 1351–1357. [Google Scholar] [CrossRef]
- Shibata, S.; Fujimoto, N.; Hastings, J.L.; Carrick-Ranson, G.; Bhella, P.S.; Hearon, C.M., Jr.; Levine, B.D. The effect of lifelong exercise frequency on arterial stiffness. J. Physiol. 2018, 596, 2783–2795. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Dinenno, F.A.; Monahan, K.D.; Clevenger, C.M.; DeSouza, C.A.; Seals, D.R. Aging, habitual exercise, and dynamic arterial compliance. Circulation 2000, 102, 1270–1275. [Google Scholar] [CrossRef]
- Yoshizawa, M.; Maeda, S.; Miyaki, A.; Misono, M.; Saito, Y.; Tanabe, K.; Kuno, S.; Ajisaka, R. Effect of 12 weeks of moderate-intensity resistance training on arterial stiffness: A randomised controlled trial in women aged 32–59 years. Br. J. Sports Med. 2009, 43, 615–618. [Google Scholar] [CrossRef]
- Tanaka, H. Antiaging Effects of Aerobic Exercise on Systemic Arteries. Hypertension 2019, 74, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Clayton, J.A.; Collins, F.S. Policy: NIH to balance sex in cell and animal studies. Nature 2014, 509, 282–283. [Google Scholar] [CrossRef]
- Koh, K.K. Effects of estrogen on the vascular wall: Vasomotor function and inflammation. Cardiovasc. Res. 2002, 55, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Krause, D.N.; Duckles, S.P.; Pelligrino, D.A. Influence of sex steroid hormones on cerebrovascular function. J. Appl. Physiol. (1985) 2006, 101, 1252–1261. [Google Scholar] [CrossRef]
- Arrick, D.M.; Li, C.; Mayhan, W.G. Sex-related differences in reactivity of cerebral arterioles during moderate exercise training. Microcirculation 2016, 23, 549–557. [Google Scholar] [CrossRef]
- Griffith, T.M. Modulation of blood flow and tissue perfusion by endothelium-derived relaxing factor. Exp. Physiol. 1994, 79, 873–913. [Google Scholar] [CrossRef] [PubMed]
- Pak, K.J.; Geary, G.G.; Duckles, S.P.; Krause, D.N. Male-female differences in the relative contribution of endothelial vasodilators released by rat tail artery. Life Sci. 2002, 71, 1633–1642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Thor, D.; Han, X.; Anderson, L.; Rahimian, R. Sex differences in mesenteric endothelial function of streptozotocin-induced diabetic rats: A shift in the relative importance of EDRFs. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H1183–H1198. [Google Scholar] [CrossRef]
- Chrissobolis, S.; Sobey, C.G. Influence of gender on K+-induced cerebral vasodilatation. Stroke 2004, 35, 747–752. [Google Scholar] [CrossRef]
- Alwatban, M.R.; Liu, Y.; Perdomo, S.J.; Ward, J.L.; Vidoni, E.D.; Burns, J.M.; Billinger, S.A. TCD Cerebral Hemodynamic Changes during Moderate-Intensity Exercise in Older Adults. J. Neuroimaging 2020, 30, 76–81. [Google Scholar] [CrossRef]
- Fiuza-Luces, C.; Santos-Lozano, A.; Joyner, M.; Carrera-Bastos, P.; Picazo, O.; Zugaza, J.L.; Izquierdo, M.; Ruilope, L.M.; Lucia, A. Exercise benefits in cardiovascular disease: Beyond attenuation of traditional risk factors. Nat. Rev. Cardiol. 2018, 15, 731–743. [Google Scholar] [CrossRef]
- Paluch, A.E.; Boyer, W.R.; Franklin, B.A.; Laddu, D.; Lobelo, F.; Lee, D.C.; McDermott, M.M.; Swift, D.L.; Webel, A.R.; Lane, A. Resistance Exercise Training in Individuals With and Without Cardiovascular Disease: 2023 Update: A Scientific Statement From the American Heart Association. Circulation 2024, 149, e217–e231. [Google Scholar] [CrossRef]
- Merkely, P.; Bakos, M.; Bányai, B.; Monori-Kiss, A.; Horváth, E.M.; Bognár, J.; Benkő, R.; Oláh, A.; Radovits, T.; Merkely, B.; et al. Sex Differences in Exercise-Training-Related Functional and Morphological Adaptation of Rat Gracilis Muscle Arterioles. Front. Physiol. 2021, 12, 685664. [Google Scholar] [CrossRef]
- Török, M.; Monori-Kiss, A.; Pál, É.; Horváth, E.; Jósvai, A.; Merkely, P.; Barta, B.A.; Mátyás, C.; Oláh, A.; Radovits, T.; et al. Long-term exercise results in morphological and biomechanical changes in coronary resistance arterioles in male and female rats. Biol. Sex Differ. 2020, 11, 7. [Google Scholar] [CrossRef]
- Vezér, M.; Demeter, Á.; Szekeres, M.; Jósvai, A.; Bányai, B.; Oláh, A.; Balogh, F.; Horváth, E.M.; Radovits, T.; Merkely, B.; et al. Sex differences in rat renal arterial responses following exercise training. Am. J. Physiol. Heart Circ. Physiol. 2022, 322, H310–H318. [Google Scholar] [CrossRef] [PubMed]
- Radovits, T.; Oláh, A.; Lux, Á.; Németh, B.T.; Hidi, L.; Birtalan, E.; Kellermayer, D.; Mátyás, C.; Szabó, G.; Merkely, B. Rat model of exercise-induced cardiac hypertrophy: Hemodynamic characterization using left ventricular pressure-volume analysis. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H124–H134. [Google Scholar] [CrossRef]
- Sweeney, P.W.; Walker-Samuel, S.; Shipley, R.J. Insights into cerebral haemodynamics and oxygenation utilising in vivo mural cell imaging and mathematical modelling. Sci. Rep. 2018, 8, 1373. [Google Scholar] [CrossRef]
- Roth, W.; Morgello, S.; Goldman, J.; Mohr, J.P.; Elkind, M.S.; Marshall, R.S.; Gutierrez, J. Histopathological Differences Between the Anterior and Posterior Brain Arteries as a Function of Aging. Stroke 2017, 48, 638–644. [Google Scholar] [CrossRef]
- Hetthéssy, J.R.; Tőkés, A.M.; Kérész, S.; Balla, P.; Dörnyei, G.; Monos, E.; Nádasy, G.L. High pressure-low flow remodeling of the rat saphenous vein wall. Phlebology 2018, 33, 128–137. [Google Scholar] [CrossRef]
- Hadjadj, L.; Monori-Kiss, A.; Horváth, E.M.; Heinzlmann, A.; Magyar, A.; Sziva, R.E.; Miklós, Z.; Pál, É.; Gál, J.; Szabó, I.; et al. Geometric, elastic and contractile-relaxation changes in coronary arterioles induced by Vitamin D deficiency in normal and hyperandrogenic female rats. Microvasc. Res. 2019, 122, 78–84. [Google Scholar] [CrossRef]
- Bliss, E.S.; Wong, R.H.; Howe, P.R.; Mills, D.E. Benefits of exercise training on cerebrovascular and cognitive function in ageing. J. Cereb. Blood Flow Metab. 2021, 41, 447–470. [Google Scholar] [CrossRef]
- Smith, K.J.; Ainslie, P.N. Regulation of cerebral blood flow and metabolism during exercise. Exp. Physiol. 2017, 102, 1356–1371. [Google Scholar] [CrossRef]
- Rzechorzek, W.; Zhang, H.; Buckley, B.K.; Hua, K.; Pomp, D.; Faber, J.E. Aerobic exercise prevents rarefaction of pial collaterals and increased stroke severity that occur with aging. J. Cereb. Blood Flow Metab. 2017, 37, 3544–3555. [Google Scholar] [CrossRef]
- Zhang, P.; Yu, H.; Zhou, N.; Zhang, J.; Wu, Y.; Zhang, Y.; Bai, Y.; Jia, J.; Zhang, Q.; Tian, S.; et al. Early exercise improves cerebral blood flow through increased angiogenesis in experimental stroke rat model. J. Neuroeng. Rehabil. 2013, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Mou, C.; Jiang, Y. Effect of dual task-based training on motor and cognitive function in stroke patients: A systematic review and meta-analysis of randomized controlled trails. BMC Neurol. 2025, 25, 290. [Google Scholar] [CrossRef] [PubMed]
- Coverdale, N.S.; Champagne, A.A.; Allen, M.D.; Tremblay, J.C.; Ethier, T.S.; Fernandez-Ruiz, J.; Marshall, R.A.; MacPherson, R.E.K.; Pyke, K.E.; Cook, D.J.; et al. Brain atrophy, reduced cerebral perfusion, arterial stiffening, and wall thickening with aging coincide with stimulus-specific changes in fMRI-BOLD responses. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2024, 326, R346–R356. [Google Scholar] [CrossRef]
- Iadecola, C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat. Rev. Neurosci. 2004, 5, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Kisler, K.; Nelson, A.R.; Montagne, A.; Zlokovic, B.V. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 2017, 18, 419–434. [Google Scholar] [CrossRef]
- de la Torre, J.C. Is Alzheimer’s disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol. 2004, 3, 184–190. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hainzl, T.; Nádasy, G.L.; Márka, E.R.; Nagy, K.; Kollarics, R.; Tőkés, A.-M.; Oláh, A.; Radovits, T.; Merkely, B.; Ács, N.; et al. Cerebral Resistance Artery Histological Remodeling After Training—Sex Differences. Life 2025, 15, 1304. https://doi.org/10.3390/life15081304
Hainzl T, Nádasy GL, Márka ER, Nagy K, Kollarics R, Tőkés A-M, Oláh A, Radovits T, Merkely B, Ács N, et al. Cerebral Resistance Artery Histological Remodeling After Training—Sex Differences. Life. 2025; 15(8):1304. https://doi.org/10.3390/life15081304
Chicago/Turabian StyleHainzl, Tobias, György L. Nádasy, Emese Róza Márka, Kamilla Nagy, Réka Kollarics, Anna-Mária Tőkés, Attila Oláh, Tamás Radovits, Béla Merkely, Nándor Ács, and et al. 2025. "Cerebral Resistance Artery Histological Remodeling After Training—Sex Differences" Life 15, no. 8: 1304. https://doi.org/10.3390/life15081304
APA StyleHainzl, T., Nádasy, G. L., Márka, E. R., Nagy, K., Kollarics, R., Tőkés, A.-M., Oláh, A., Radovits, T., Merkely, B., Ács, N., Várbíró, S., Jósvai, A., & Török, M. (2025). Cerebral Resistance Artery Histological Remodeling After Training—Sex Differences. Life, 15(8), 1304. https://doi.org/10.3390/life15081304




