Neuroprotective Effect Against Ischemic Stroke of the Novel Functional Drink Containing Anthocyanin and Dietary Fiber Enriched-Functional Ingredient from the Mixture of Banana and Germinated Jasmine Rice
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of an Anthocyanin and Dietary Fiber-Enriched Functional Drink from Banana and Black Jasmine Rice
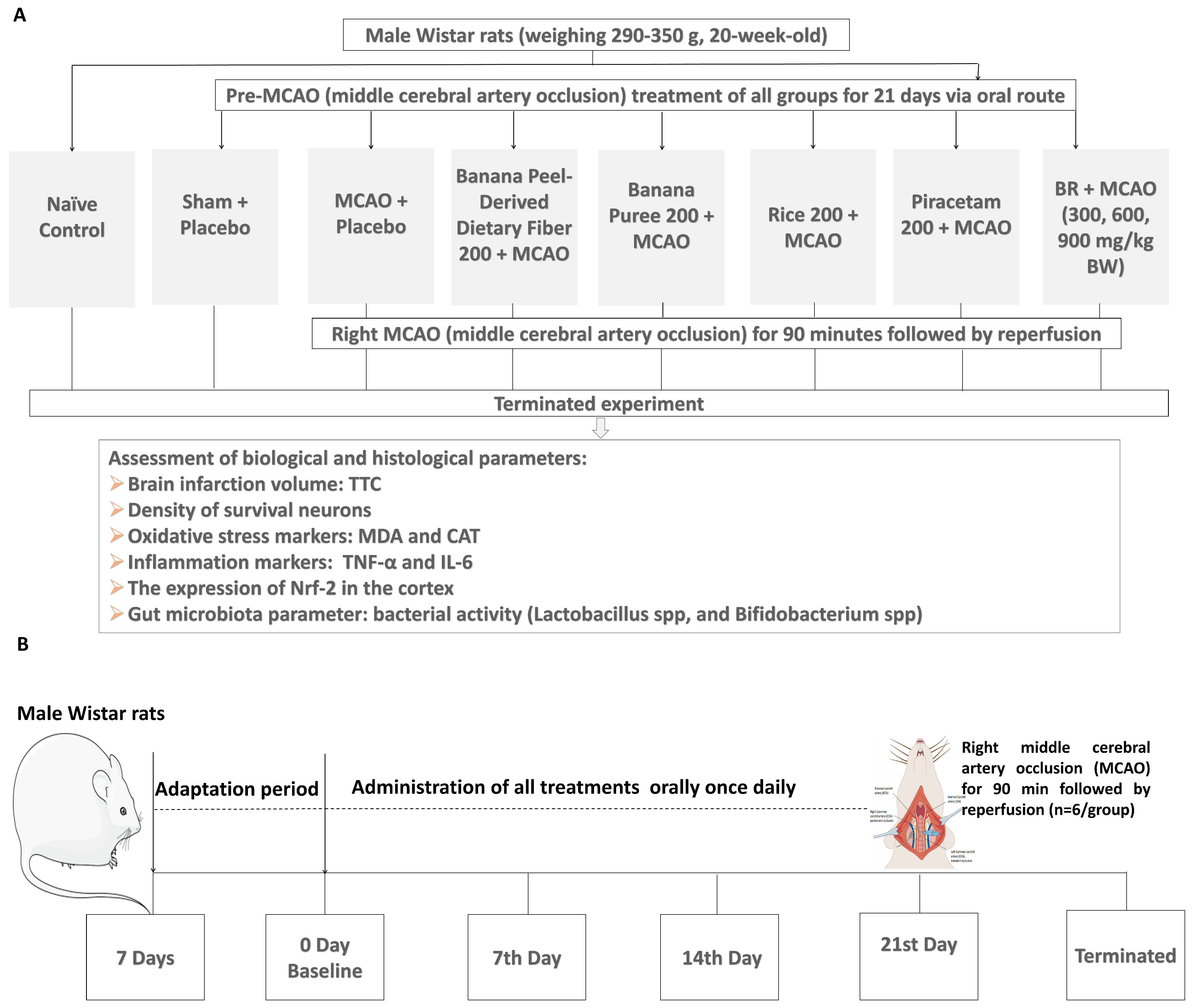
2.2. Study Design
- Group I (Naïve Control): all rats in this group received no treatment and served as the negative control group.
- Group II (Sham + vehicle): the rats in this group were orally given placebo treatment and subjected to sham operation. This group was designed to determine the effect of the operation procedures and placebo on the observed parameters, particularly brain damage and dysfunction, in this study.
- Group III (MCAO + Vehicle): the experimental rats received a placebo and were subjected to a temporary occlusion of the right middle cerebral artery for 90 min (MCAO/IR). This group provided the information regarding the effect of MCAO/IR and placebo on the observed parameters, particularly brain damage and dysfunction, in this study.
- Group IV (Banana peel-derived dietary fiber 200 + MCAO): the animals in this group were orally administered banana peel-derived dietary fiber at the same dose as that presented in the functional drink at a dose of 200 mg/kg body weight (BW) and received MCAO/IR. This group was designed to provide information about the effect of banana peel-derived dietary fiber alone on the observed parameters, particularly brain damage and dysfunction in this study.
- Group V (Banana puree 200 + MCAO): all rats in this group were orally administered banana pulp puree at the same dose as that presented in the functional drink at a dose of 200 mg/kg BW and received MCAO/IR. Information obtained from this group emphasized the effect of banana puree alone on the observed parameters. particularly brain damage and dysfunction in this study.
- Group VI (Rice 200 + MCAO): the experimental rats in this group received germinated black Jasmine rice extract at the same dose as that presented in the functional drink at a dose of 200 mg/kg BW and were subjected to MCAO/IR. The effects of germinated black Jasmine rice extract alone on the observed parameters, particularly brain damage and dysfunction, were obtained from this group.
- Group VII (Piracetam 200 + MCAO): this group served as the positive control group. All experimental animals were orally administered piracetam at a dose of 200 mg/kg BW and subjected to MCAO/IR. This group served as the positive control or was treated with a standard drug targeting cerebral blood flow enhancement [29].
- Group VIII–X (BR + MCAO): rats in groups VIII, IX, and X were orally administered the functional drink containing the functional ingredient from banana and black jasmine rice (BR) at the doses of 300, 600, and 900 ma/kg BW, respectively. These doses were selected based on the transference of in vitro data regarding antioxidant activity to the in vivo experiment. Following the treatment, they were subjected to MCAO/IR. These groups were experimental groups.
2.3. The Temporary Occlusion of Middle Cerebral Artery Induced by Ischemia-Reperfusion (MCAO/IR)
2.4. Brain Water Content Assessment
2.5. Neuronal Density Assessment
2.6. Determination of Oxidative Stress Markers
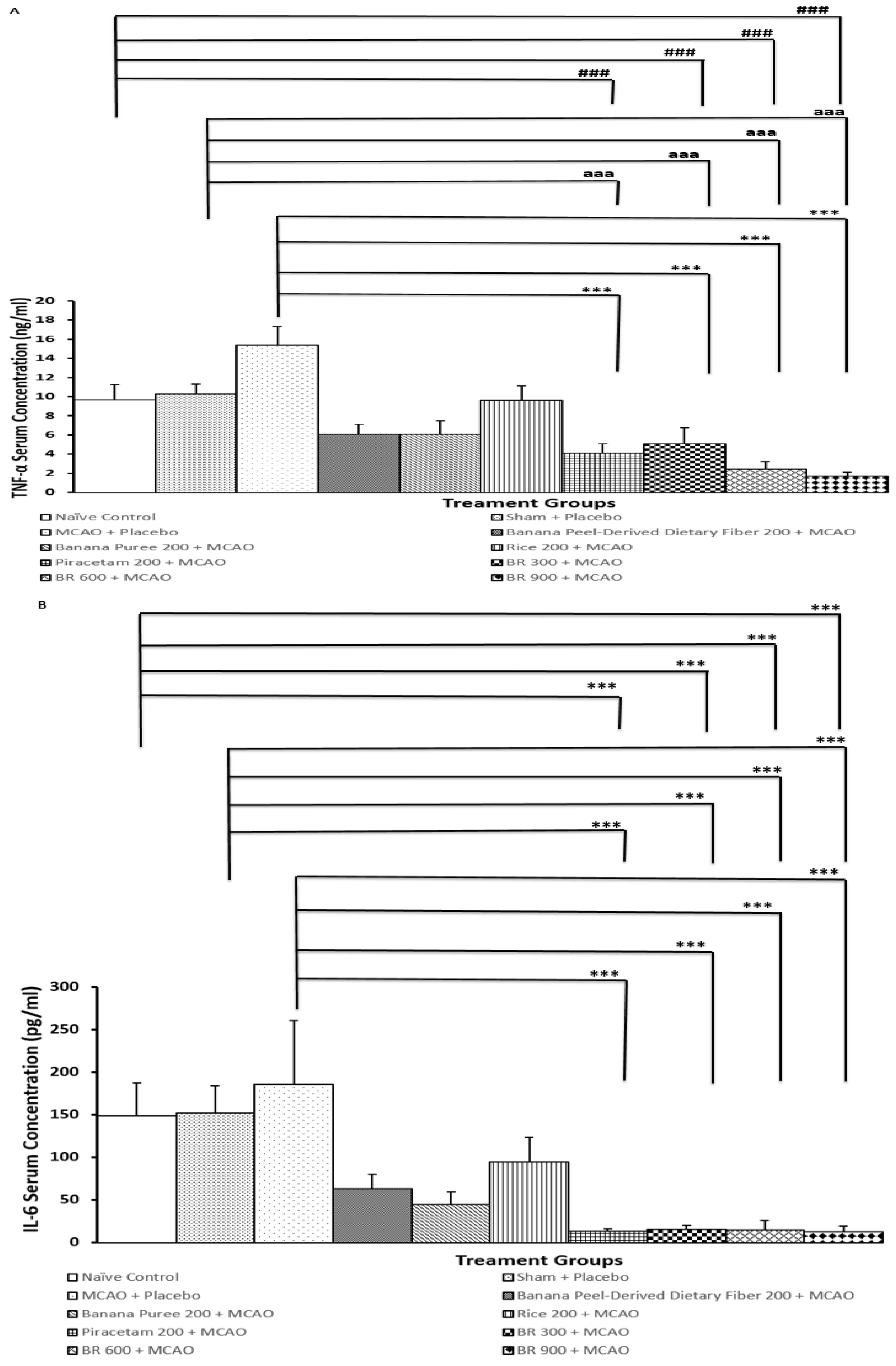
2.7. Assessment of Inflammatory and Immune Markers in Serum
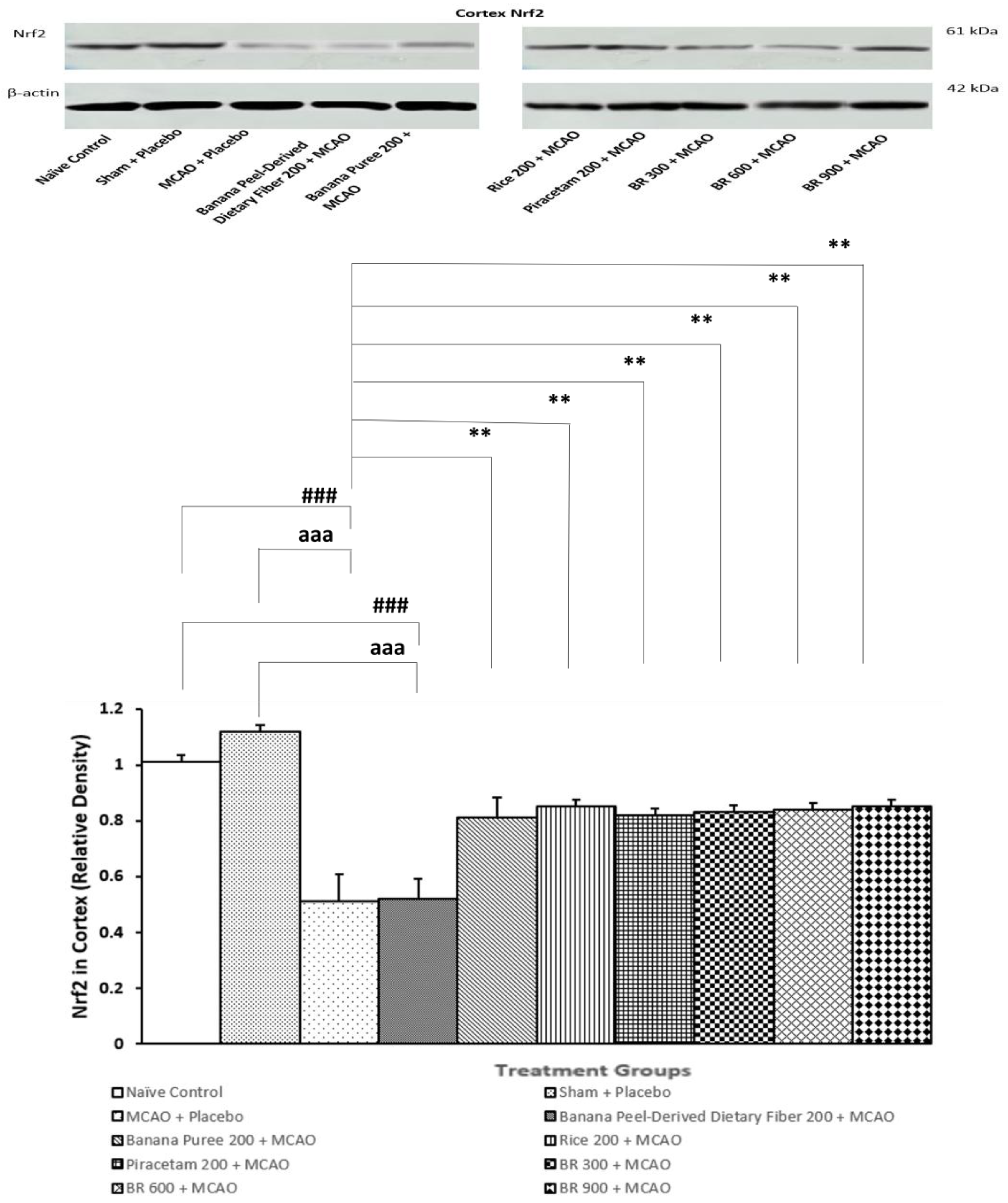
2.8. Assessment of Nrf2 Expression in Cortex
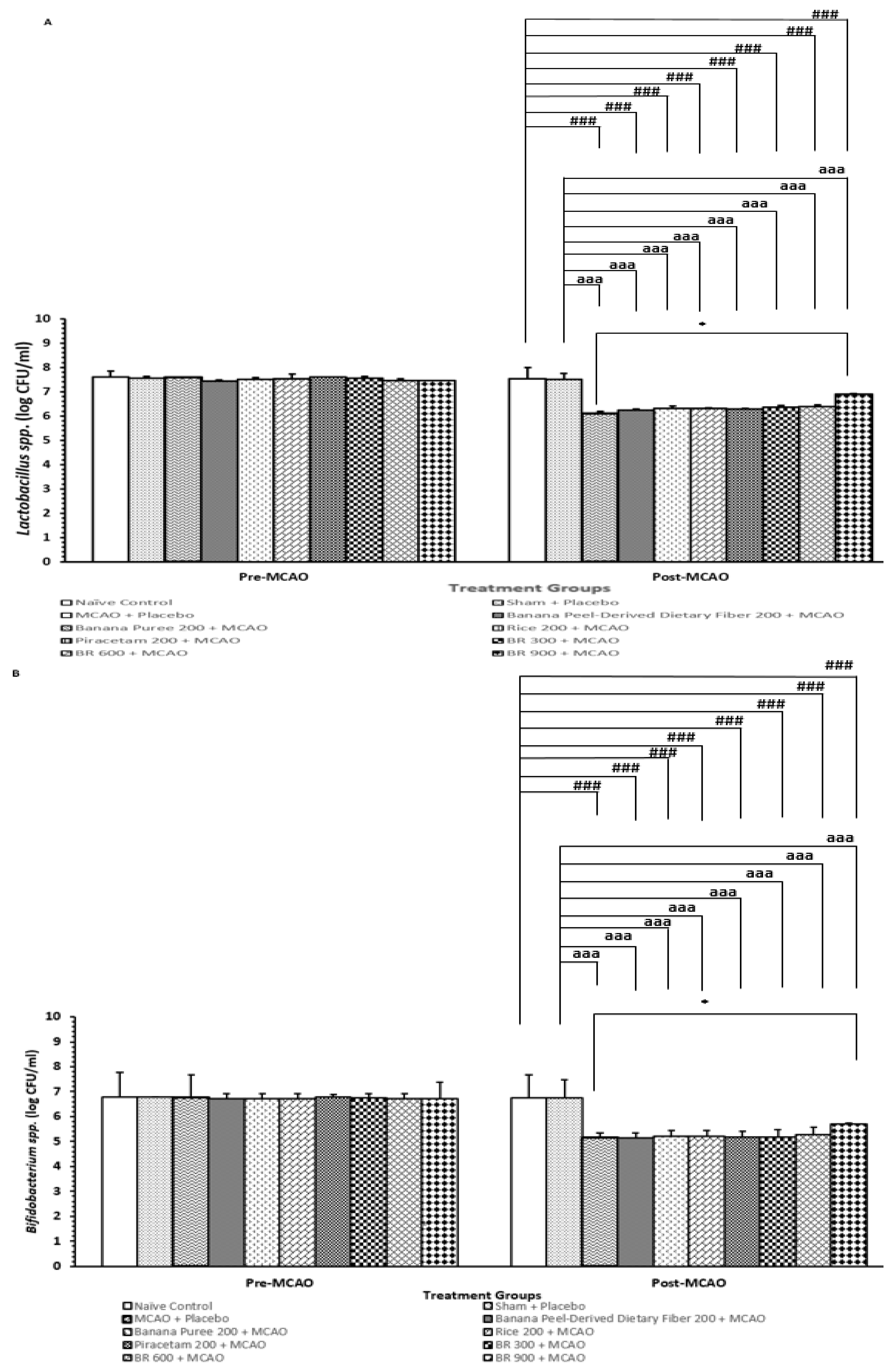
2.9. Determination of Lactobacillus and Bifidobacterium in the Fecal Samples
2.10. Statistical Analysis
3. Results
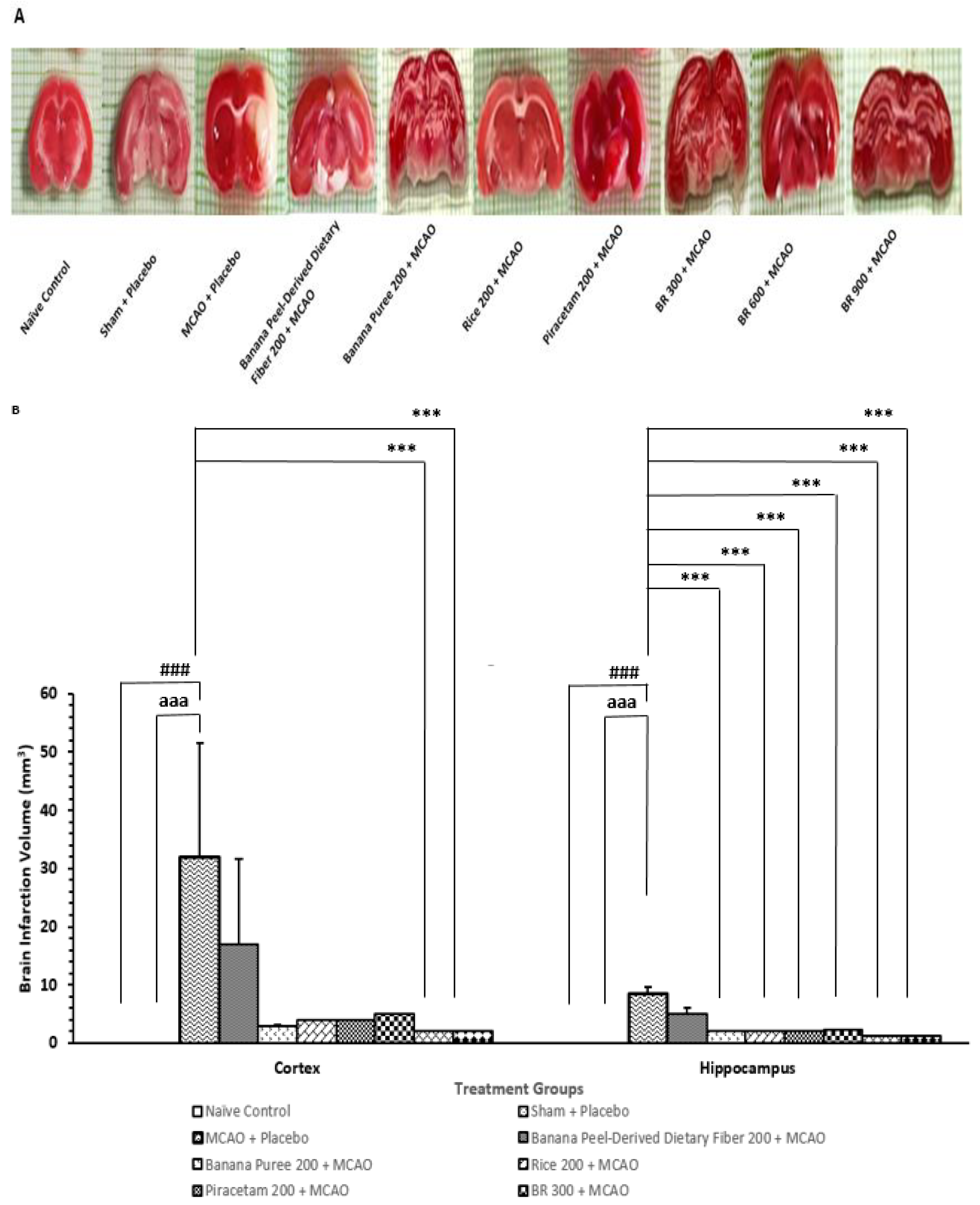
3.1. Changes in Brain Infarcted Volume
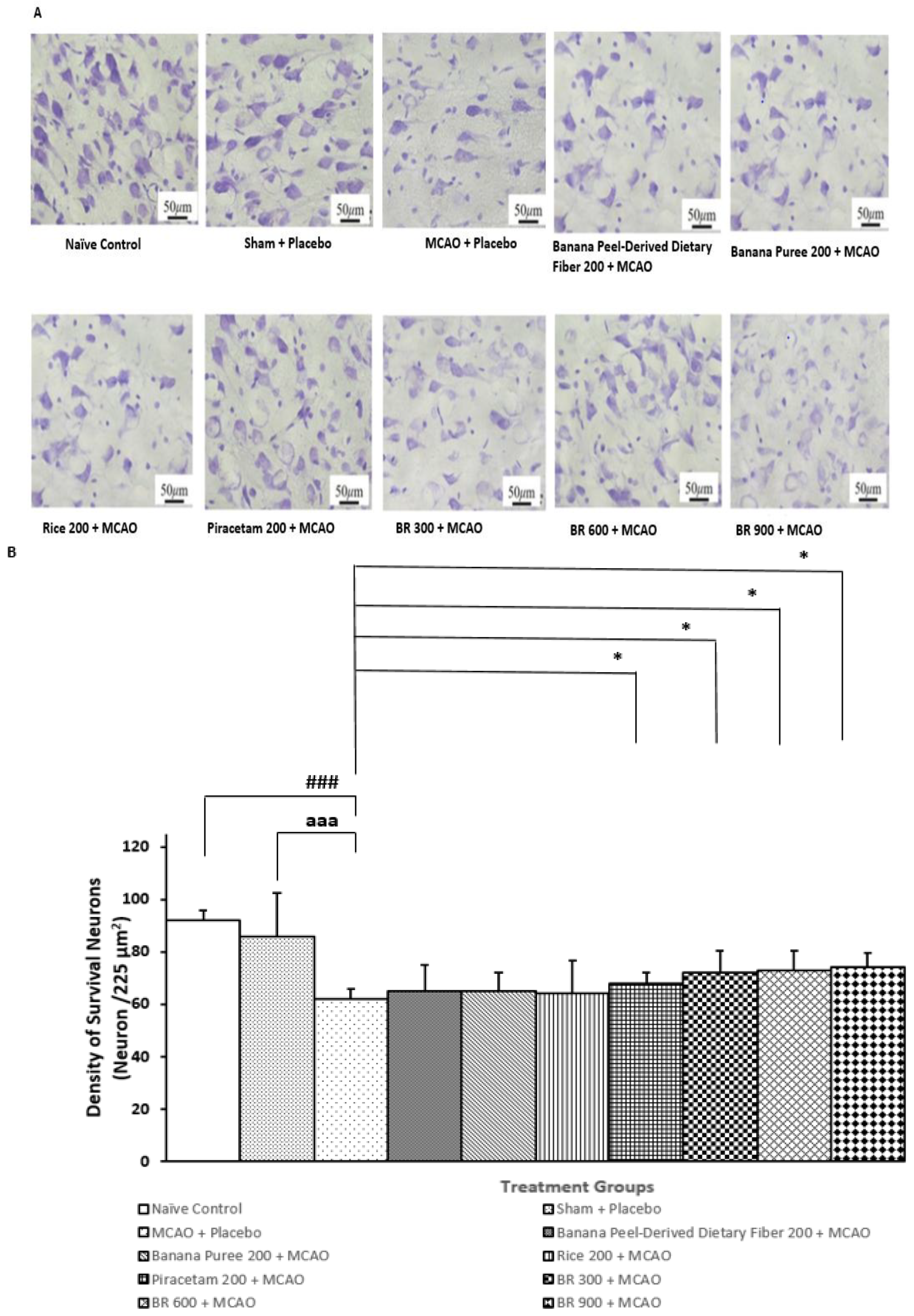
3.2. Changes in Density of the Survival Neurons in Cortex
3.3. Changes in Inflammatory Markers in Serum
3.4. Changes in Oxidative Stress Markers
3.5. Assessment of Nrf2 Expression in Cortex
3.6. Changes in Lactobacillus and Bifidobacterium spp.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- eClinicalMedicine. The rising global burden of stroke. EClinicalMedicine 2023, 59, 102028. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.; Sacco, R.L.; Hacke, W.; Fisher, M.; Pandian, J.; Lindsay, P. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int. J. Stroke 2022, 17, 18–29. [Google Scholar] [CrossRef]
- Leng, T.; Xiong, Z.G. Treatment for ischemic stroke: From thrombolysis to thrombectomy and remaining challenges. Brain Circ. 2019, 5, 8–11. [Google Scholar] [CrossRef]
- Goyal, M.; Almekhlafi, M.; Menon, B.; Hill, M.; Fargen, K.; Parsons, M.; Bang, O.Y.; Siddiqui, A.; Andersson, T.; Mendes, V.; et al. Challenges of acute endovascular stroke trials. Stroke 2014, 45, 3116–3122. [Google Scholar] [CrossRef][Green Version]
- Elendu, C.; Amaechi, D.C.; Elendu, T.C.; Ibhiedu, J.O.; Egbunu, E.O.; Ndam, A.R.; Ogala, F.; Ologunde, T.; Peterson, J.C.; Boluwatife, A.I.; et al. Stroke and cognitive impairment: Understanding the connection and managing symptoms. Ann. Med. Surg. 2023, 85, 6057–6066. [Google Scholar] [CrossRef]
- Miyawaki, Y.; Otani, T.; Morioka, S. Agency judgments in post-stroke patients with sensorimotor deficits. PLoS ONE 2020, 15, e0230603. [Google Scholar] [CrossRef] [PubMed]
- Bártlová, S.; Šedová, L.; Havierniková, L.; Hudáčková, A.; Dolák, F.; Sadílek, P. Quality of Life of Post-stroke Patients. Zdr. Varst. 2022, 61, 101–108. [Google Scholar] [CrossRef]
- Morone, G.; Pichiorri, F. Post-Stroke Rehabilitation: Challenges and New Perspectives. J. Clin. Med. 2023, 12, 550. [Google Scholar] [CrossRef]
- Kalavina, R.; Chisati, E.; Mlenzana, N.; Wazakili, M. The challenges and experiences of stroke patients and their spouses in Blantyre, Malawi. Malawi Med. J. 2019, 31, 112–117. [Google Scholar] [CrossRef]
- Yperzeele, L.; Van Hooff, R.J.; De Smedt, A.; Valenzuela Espinoza, A.; Van de Casseye, R.; Hubloue, I.; De Keyser, J.; Brouns, R. Prehospital stroke care: Limitations of current interventions and focus on new developments. Cerebrovasc. Dis. 2014, 38, 1–9. [Google Scholar] [CrossRef]
- Hurd, M.D.; Goel, I.; Sakai, Y.; Teramura, Y. Current status of ischemic stroke treatment: From thrombolysis to potential regenerative medicine. Regen. Ther. 2021, 18, 408–417. [Google Scholar] [CrossRef]
- Wu, L.; Xiong, X.; Wu, X.; Ye, Y.; Jian, Z.; Zhi, Z.; Gu, L. Targeting Oxidative Stress and Inflammation to Prevent Ischemia-Reperfusion Injury. Front. Mol. Neurosci. 2020, 13, 28. [Google Scholar] [CrossRef]
- Li, Z.; Bi, R.; Sun, S.; Chen, S.; Chen, J.; Hu, B.; Jin, H. The Role of Oxidative Stress in Acute Ischemic Stroke-Related Thrombosis. Oxid. Med. Cell. Longev. 2022, 2022, 8418820. [Google Scholar] [CrossRef]
- Szepanowski, R.D.; Haupeltshofer, S.; Vonhof, S.E.; Frank, B.; Kleinschnitz, C.; Casas, A.I. Thromboinflammatory challenges in stroke pathophysiology. Semin. Immunopathol. 2023, 45, 389–410. [Google Scholar] [CrossRef]
- Nieswandt, B.; Kleinschnitz, C.; Stoll, G. Ischaemic stroke: A thrombo-inflammatory disease? J. Physiol. 2011, 589, 4115–4123. [Google Scholar] [CrossRef]
- Yu, Z.; Ke, L.; Lu, T.; Li, L.; Gu, H.; Rao, P. Implementing a food first strategy can transform preventive healthcare. NPJ Sci. Food 2024, 8, 57. [Google Scholar] [CrossRef]
- English, C.; MacDonald-Wicks, L.; Patterson, A.; Attia, J.; Hankey, G.J. The role of diet in secondary stroke prevention. Lancet Neurol. 2021, 20, 150–160. [Google Scholar] [CrossRef]
- Hu, D.; Huang, J.; Wang, Y.; Zhang, D.; Qu, Y. Fruits and vegetables consumption and risk of stroke: A meta-analysis of prospective cohort studies. Stroke 2014, 45, 1613–1619. [Google Scholar] [CrossRef]
- Vokó, Z.; Hollander, M.; Hofman, A.; Koudstaal, P.J.; Breteler, M.M. Dietary antioxidants and the risk of ischemic stroke: The Rotterdam Study. Neurology 2003, 61, 1273–1275. [Google Scholar] [CrossRef]
- Li, D.B.; Hao, Q.Q.; Hu, H.L. The relationship between dietary fibre and stroke: A meta-analysis. J. Stroke Cerebrovasc. Dis. 2023, 32, 107144. [Google Scholar] [CrossRef]
- Panda, D.K.; Jyotirmayee, B.; Mahalik, G. Blackrice: A review from its history to chemicalmakeup to health advantages, nutritionalproperties and dietary uses. Plant Sci. Today 2022, 9 (Suppl. S3), 1–15. [Google Scholar] [CrossRef]
- Das, M.; Dash, U.; Mahanand, S.S.; Nayak, P.K.; Kesavan, R.K. Black rice: A comprehensive review on its bioactive compounds, potential health benefits and food applications. Food Chem. Adv. 2023, 3, 100462. [Google Scholar] [CrossRef]
- Patil, S.B.; Khan, M.K. Germinated brown rice as a value added rice product: A review. J. Food Sci. Technol. 2011, 48, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Samad, N.; Muneer, A.; Ullah, N.; Zaman, A.; Ayaz, M.M.; Ahmad, I. Banana fruit pulp and peel involved in antianxiety and antidepressant effects while invigorate memory performance in male mice: Possible role of potential antioxidants. Pak. J. Pharm. Sci. 2017, 30 (Suppl. S3), 989–995. [Google Scholar]
- Amini Khoozani, A.; Birch, J.; Bekhit, A.E.A. Production, application and health effects of banana pulp and peel flour in the food industry. J. Food Sci. Technol. 2019, 56, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Akram, T.; Mustafa, S.; Ilyas, K.; Tariq, M.R.; Ali, S.W.; Ali, S.; Shafiq, M.; Rao, M.; Safdar, W.; Iftikhar, M.; et al. Supplementation of banana peel powder for the development of functional broiler nuggets. PeerJ 2022, 10, e14364. [Google Scholar] [CrossRef]
- Anandhi, P.; Rajeshkumar, S. Banana peel extract has antimicrobial, antioxidant, anti-inflammatory, and wound healing potential—A review. J. Surv. Fish. Sci. 2023, 10 (Suppl. S1), 39–48. [Google Scholar]
- Balajee, V.; Kumar, L.; Kumar, R.; Shanmugam, R. Antioxidant and Anti-inflammatory Properties of the Two Varieties of Musa acuminata: An In Vitro Study. Cureus 2023, 15, e51260. [Google Scholar]
- Kessler, J.; Thiel, A.; Karbe, H.; Heiss, W.D. Piracetam improves activated blood flow and facilitates rehabilitation of poststroke aphasic patients. Stroke 2000, 31, 2112–2116. [Google Scholar] [CrossRef]
- Lin, W.; Venkatesan, R.; Gurleyik, K.; He, Y.Y.; Powers, W.J.; Hsu, C.Y. An absolute measurement of brain water content using magnetic resonance imaging in two focal cerebral ischemic rat models. J. Cereb. Blood Flow. Metab. 2000, 20, 37–44. [Google Scholar] [CrossRef]
- Kirisattayakul, W.; Wattanathorn, J.; Iamsaard, S.; Jittiwat, J.; Suriharn, B.; Lertrat, K. Neuroprotective and Memory-Enhancing Effect of the Combined Extract of Purple Waxy Corn Cob and Pandan in Ovariectomized Rats. Oxid. Med. Cell. Longev. 2017, 2017, 5187102. [Google Scholar] [CrossRef]
- Wattanathorn, J.; Ohnon, W.; Thukhammee, W.; Muchmapura, S.; Wannanon, P.; Tong-Un, T. Cerebroprotective Effect against Cerebral Ischemia of the Combined Extract of Oryza sativa and Anethum graveolens in Metabolic Syndrome Rats. Oxid. Med. Cell. Longev. 2019, 2019, 9658267. [Google Scholar] [CrossRef]
- Medrano-Martorell, S.; Pumar-Pérez, M.; González-Ortiz, S.; Capellades-Font, J. A review of the anatomy of the middle cerebral artery for the era of thrombectomy: A radiologic tool based on CT angiography and perfusion CT. Radiologia 2021, 63, 505–511. [Google Scholar] [CrossRef]
- Chauhan, P.; Jethwa, K.; Rathawa, A.; Chauhan, G.; Mehra, S. The Anatomy of the Hippocampus. In Cerebral Ischemia [Internet]; Pluta, R., Ed.; Exon Publications: Brisbane, Australia, 2021; Chapter 2. Available online: https://www.ncbi.nlm.nih.gov/books/NBK575732/ (accessed on 28 June 2025).
- Xu, W.D.; Yang, C.; Huang, A.F. The role of Nrf2 in immune cells and inflammatory autoimmune diseases: A comprehensivereview. Expert. Opin. Ther. Targets 2024, 28, 789–806. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, J.; Su, X.; Lin, X.; Zhu, L.; Zhuang, Z.; Liu, C.; Zhu, Z.; Zeng, Y. Nrf2 regulates the expression of NOX1 in TNF-α-induced A549 cells. Allergol. Immunopathol. 2023, 51, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Becerril, T.; Rodríguez-Cruz, M.; Villa-Morales, J.; Sánchez-Mendoza, C.R.; Galeazzi-Aguilar, J.E. Circulating Nrf2, Glutathione, and Malondialdehyde Correlate with Disease Severity in Duchenne Muscular Dystrophy. Antioxidants 2023, 12, 871. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Xiong, X.; Zhu, H.; Chen, R.; Zhang, S.; Chen, G.; Jian, Z. Nrf2 Regulates Oxidative Stress and Its Role in Cerebral Ischemic Stroke. Antioxidants 2022, 11, 2377. [Google Scholar] [CrossRef]
- Schilichting, C.L.; Lima, K.C.; Cestari, L.A., Jr.; Sekiyama, J.Y.; Silva, F.M.; Milani, H. Validation of a simple and inexpensive method for the quantitation of infarct in the rat brain. Braz. J. Med. Biol. Res. 2004, 37, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Zhang, X.; Li, P.; Li, H.; Feng, G.; Wang, G. Bifidobacterium bifidum supplementation improves ischemic stroke outcomes in elderly patients: A retrospective study. Medicine 2024, 103, e37682. [Google Scholar] [CrossRef]
- Long, J.; Wang, J.; Li, Y.; Chen, S. Gut microbiota in ischemic stroke: Where we stand and challenges ahead. Front. Nutr. 2022, 9, 1008514. [Google Scholar] [CrossRef] [PubMed]
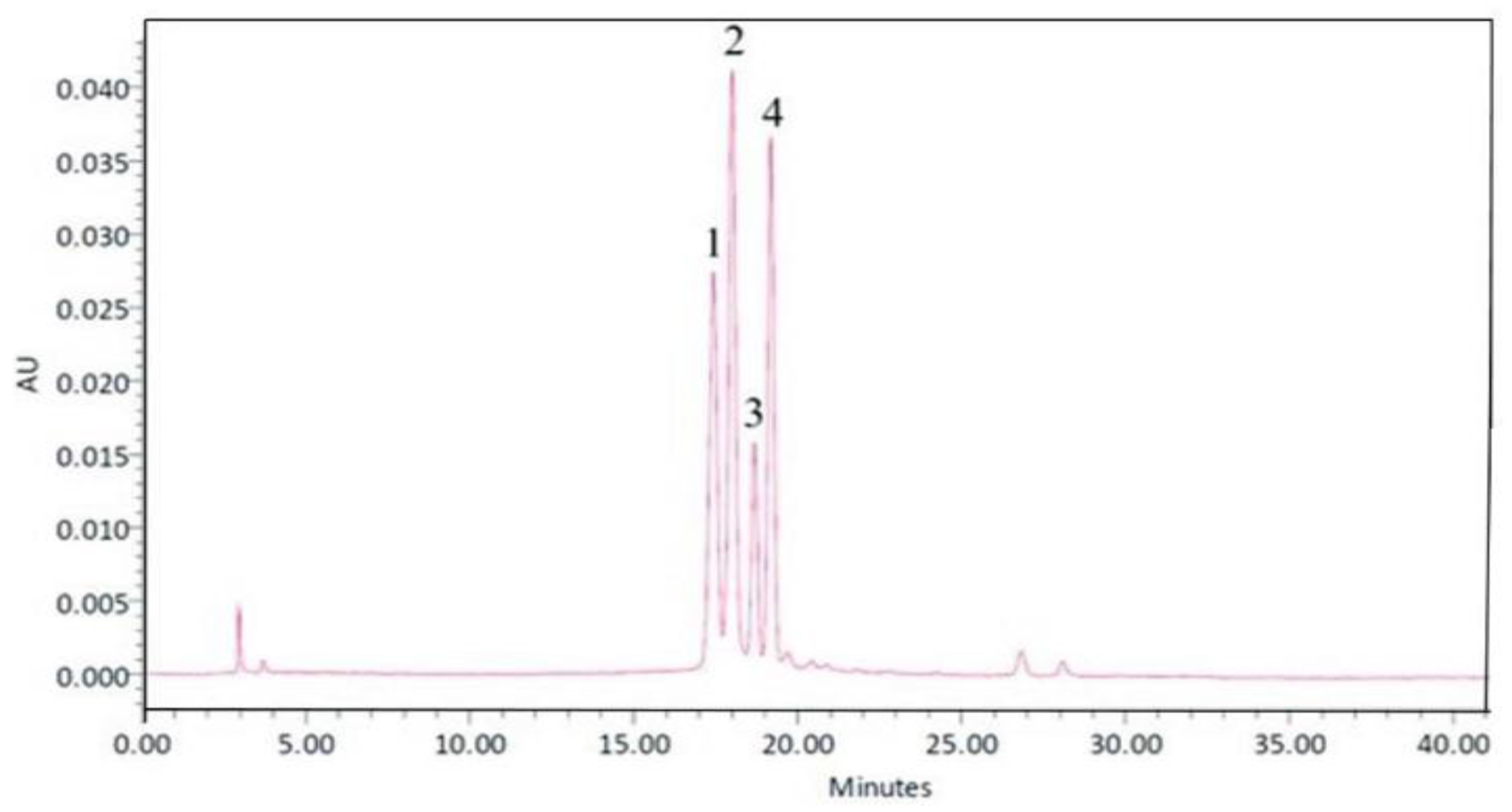
| Groups | Cortex MDA (μmol/L) | Cortex Catalase (kU/L) |
|---|---|---|
| Naïve Control | 5.31 ± 1.1 a,** | 217. 99 ± 10.17 aaa,*** |
| Sham + Placebo | 12.71 ± 0.25 ### | 147.65 ± 1.16 ### |
| MCAO + Placebo | 14.83 ± 0.33 ### | 145.50 ± 1.41 ### |
| Banana Peel-Derived Dietary Fiber 200 + MCAO | 10.15 ± 1.97 | 155.41 ± 1.36 ## |
| Rice 200 + MCAO | 10.55 ± 1.76 | 183.04 ± 1.84 ##,a,** |
| Banana Puree 200 + MCAO | 10.64 ± 1.63 | 165.84 ± 4.84 ### |
| Piracetam 200 + MCAO | 7.16 ± 0.15 * | 178.16 ± 5.61 ##,*,a |
| BR 300 + MCAO | 7.61 ± 0.31 * | 173.84 ± 6.10 ##,*,a |
| BR 600 + MCAO | 6.94 ± 0.37 * | 177.55 ± 16.03 #,a,** |
| BR 900 + MCAO | 5.42 ± 0.78 ** | 194.73 ± 10.34 aaa,*** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhammad, M.; Wattanathorn, J.; Thukham-mee, W.; Phuthong, S.; Muchimapura, S. Neuroprotective Effect Against Ischemic Stroke of the Novel Functional Drink Containing Anthocyanin and Dietary Fiber Enriched-Functional Ingredient from the Mixture of Banana and Germinated Jasmine Rice. Life 2025, 15, 1222. https://doi.org/10.3390/life15081222
Muhammad M, Wattanathorn J, Thukham-mee W, Phuthong S, Muchimapura S. Neuroprotective Effect Against Ischemic Stroke of the Novel Functional Drink Containing Anthocyanin and Dietary Fiber Enriched-Functional Ingredient from the Mixture of Banana and Germinated Jasmine Rice. Life. 2025; 15(8):1222. https://doi.org/10.3390/life15081222
Chicago/Turabian StyleMuhammad, Mubarak, Jintanaporn Wattanathorn, Wipawee Thukham-mee, Sophida Phuthong, and Supaporn Muchimapura. 2025. "Neuroprotective Effect Against Ischemic Stroke of the Novel Functional Drink Containing Anthocyanin and Dietary Fiber Enriched-Functional Ingredient from the Mixture of Banana and Germinated Jasmine Rice" Life 15, no. 8: 1222. https://doi.org/10.3390/life15081222
APA StyleMuhammad, M., Wattanathorn, J., Thukham-mee, W., Phuthong, S., & Muchimapura, S. (2025). Neuroprotective Effect Against Ischemic Stroke of the Novel Functional Drink Containing Anthocyanin and Dietary Fiber Enriched-Functional Ingredient from the Mixture of Banana and Germinated Jasmine Rice. Life, 15(8), 1222. https://doi.org/10.3390/life15081222







