Secondary Metabolite Profiling of Satureja aintabensis P.H. Davis and Satureja spicigera (K. Koch) Boiss. by LC-HRMS and Evaluation of Antioxidant and Anticholinergic Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Preparation of Plant Extracts
2.4. LC-HRMS Analysis
2.5. Antioxidant Activities
2.5.1. DPPH Scavenging Assay
2.5.2. ABTS+ Scavenging Activity
2.5.3. DMPD+ Scavenging Activity
2.5.4. Cupric Ion (Cu2+) Reducing Ability Assay (CUPRAC)
2.5.5. Ferric Ion (Fe3+) Reducing Ability Assay
2.6. Anticholinergic Assays
2.7. Statistical Analyses
3. Results
3.1. LC-HRMS Analysis Results
3.2. Reducing Ability Results
3.3. Radical Scavenging Ability Results
3.4. AChE and BChE Inhibition Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| AChE | Acetylcholinesterase enzyme |
| BChE | Butyrylcholinesterase enzyme |
| BHA | Butylated hydroxyanisole |
| BHT | Butylated hydroxytoluene |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| ABTS | 2,2-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid |
| DMPD | N,N-dimethyl-p-phenylenediamine |
| CUPRAC | Cupric Ions (Cu2+) Reducing Ability Assay |
| IC50 | Half-maximal inhibitory concentration |
References
- Harley, R.M.; Atkins, S.; Budantsev, A.; Cantino, P.H.; Conn, B.; Grayer, R.; Harley, M.M.; Kok, R.; Krestovskaja, T.; Morales, A.; et al. The Families and Genera of Vascular Plants. In Labiatae; Kadereit, J.W., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; Volume 7, pp. 167–275. [Google Scholar] [CrossRef]
- Celep, F.; Dirmenci, T. Systematic and bio-geographic overview of Lamiaceae in Turkey. Nat. Volatiles Essent. Oils 2017, 4, 14–27. [Google Scholar]
- Dirmenci, T.; Yazıcı, T.; Özcan, T.; Çelenk, Ç.; Martin, E. A new species and a new natural hybrid of Origanum L. (Lamiaceae) from the west of Turkey. Turk. J. Bot. 2018, 42, 73–90. [Google Scholar] [CrossRef]
- Polat, R.; Satıl, F. An ethnobotanical survey of medicinal plants in Edremit Gulf (Balıkesir–Turkey). J. Ethnopharmacol. 2012, 139, 626–641. [Google Scholar] [CrossRef]
- Gürbüz, İ.; Gençler-Özkan, A.M.; Akaydın, G.; Salihoğlu, E.; Günbatan, T.; Yeşilada, E. Folk medicine in Düzce Province (Turkey). Turk. J. Bot. 2019, 43, 769–784. [Google Scholar] [CrossRef]
- Govaerts, R. (Ed.) WCVP: World Checklist of Vascular Plants. Facilitated by the Royal Botanic Gardens, Kew. [WWW Document]. Available online: http://sftp.kew.org/pub/data-repositories/WCVP/ (accessed on 21 May 2024).
- Davis, P.H. Satureja L. In Flora of Turkey and East Aegean Islands; Davis, P.H., Ed.; Edinburgh University Press: Edinburgh, UK, 1982; Volume 7, pp. 314–322. [Google Scholar]
- Duman, H.; Dirmenci, T.; Özcan, T. A new annual Satureja (Lamiaceae) species from Turkey with molecular evidence, and lectotypification of two species. Turk. J. Bot. 2023, 47, 61–72. [Google Scholar] [CrossRef]
- Baydar, H.; Sağdiç, O.; Özkan, G.; Karadoğan, T. Antibacterial activity and composition of essential oils from Origanum, Thymbra and Satureja species with commercial importance in Turkey. Food Control 2004, 15, 169–172. [Google Scholar] [CrossRef]
- Kurkcuoglu, M.; Tumen, G.; Baser, K.H.C. Essential oil constituents of Satureja boissieri from Turkey. Chem. Nat. Comp. 2001, 37, 329–331. [Google Scholar] [CrossRef]
- Satıl, F.; Dirmenci, T.; Tümen, G.; Turan, Y. Commercial and ethnic uses of Satureja (Sivri Kekik) species in Turkey. Ekoloji 2008, 67, 1–7. [Google Scholar] [CrossRef]
- Selvi, S.; Polat, R.; Çakılcıoğlu, U.; Celep, F.; Dirmenci, T. An ethnobotanical review on medicinal plants of the Lamiaceae family in Turkey. Turk. J. Bot. 2012, 46, 283–332. [Google Scholar] [CrossRef]
- Jafari, F.; Ghavidel, F.; Zarshenas, M.M. A critical overview on the pharmacological and clinical aspects of popular Satureja species. J. Acupunct. Meridian Stud. 2016, 9, 118–127. [Google Scholar] [CrossRef]
- Kartal, M.; Yildiz, A.N.; İnal, E.; Kınoglu, B.K.; Dirmenci, T.; Gören, A.C. Review on the Biological Activities and Phytochemistry of the Genus Satureja. Rec. Nat. Prod. 2025, 19, 400–427. [Google Scholar] [CrossRef]
- Kınoğlu, B.K.; Dirmenci, T.; Alwasel, S.H.; Gülçin, İ.; Gören, A.C. Quantification of main secondary metabolites of Satureja icarica PH Davis (Lamiaceae) by LC-HRMS and evaluation of antioxidant capacities. J. Chem. Metrol. 2023, 17, 199–214. [Google Scholar] [CrossRef]
- Karageçili, H.; Gülçin, İ. The Lamiaceae family plants ethnobotanical properties, ethnopharmacological uses, phytochemical studies and their utilization in public or current clinical practices: A review. Rec. Nat. Prod. 2025, 19, 467–488. [Google Scholar] [CrossRef]
- Bizim Bitkiler. Available online: https://bizimbitkiler.org.tr/yeni/demos/technical/ (accessed on 7 May 2025).
- Royal Botanical Gardens KEW, Plants of the World Online. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:457859-1/images (accessed on 7 May 2025).
- Sampson, B.J.; Tabanca, N.; Kirimer, N.E.; Demirci, B.; Baser, K.H.C.; Khan, I.A.; Spiers, J.M.; Wedge, D.E. Insecticidal activity of 23 essential oils and their major compounds against adult Lipaphis pseudobrassicae (Davis) (Aphididae: Homoptera). Pest Manag. Sci. Former. Pest Sci. 2005, 61, 1122–1128. [Google Scholar] [CrossRef]
- Azaz, A.D.; Kürkcüoglu, M.; Satil, F.; Can Baser, K.H.; Tümen, G. In vitro antimicrobial activity and chemical composition of some Satureja essential oils. Flavour Frag. J. 2005, 20, 587–591. [Google Scholar] [CrossRef]
- Askun, T.; Tekwu, E.M.; Satil, F.; Modanlioglu, S.; Aydeniz, H. Preliminary antimycobacterial study on selected Turkish plants (Lamiaceae) against Mycobacterium tuberculosis and search for some phenolic constituents. BMC Complement. Altern. Med. 2013, 13, 365. [Google Scholar] [CrossRef] [PubMed]
- Royal Botanical Gardens KEW, Plants of the World Online. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:457813-1 (accessed on 7 May 2025).
- Jafari Ghoshchi, M.; Abbaszadeh, B.; Oraei, M.; Azimi, R.; Faramarzi, A. Effects of different drying methods on phytochemical quality and microbial load of Satureja spicigera. J. Essent. Oil-Bear. Plants 2024, 27, 1347–1361. [Google Scholar] [CrossRef]
- Sefidkon, F.; Jamzad, Z. Essential oil composition of Satureja spicigera (C. Koch) Boiss. from Iran. Flavour Frag. J. 2004, 19, 571–573. [Google Scholar] [CrossRef]
- Gohari, A.R.; Hadjiakhoondi, A.; Sadat-Ebrahimi, E.; Saeidnia, S.; Shafiee, A. Composition of the volatile oils of Satureja spicigera C. Koch Boiss. and S. macrantha C. A. Mey from Iran. Flavour Fragr. J. 2006, 21, 510–512. [Google Scholar] [CrossRef]
- Farzaneh, M.; Kiani, H.; Sharifi, R.; Reisi, M.; Hadian, J. Chemical composition and antifungal effects of three species of Satureja (S. hortensis, S. spicigera, and S. khuzistanica) essential oils on the main pathogens of strawberry fruit. J. Postharvest Biol. Technol. 2015, 109, 145–151. [Google Scholar] [CrossRef]
- Eftekhar, F.; Raei, F.; Yousefzadi, M.; Nejad Ebrahimi, S.; Hadian, J. Antibacterialactivity and essential oil composition of Satureja spicigera from Iran. Z. Naturforschung C 2009, 64, 20–24. [Google Scholar] [CrossRef]
- Hasanvandi, S.; Neisi, E.; Meshkat, M.H. Comparative analysis of essential oils from two Satureja species; extraction methods, chemical composition, and antimicrobial activities. Biocatal. Agric. Biotechnol. 2023, 50, 102731. [Google Scholar] [CrossRef]
- Kordali, S.; Usanmaz Bozhuyuk, A.; Komaki, A.; Ilhan, G.; Ercisli, S. Biological control of Penicillium on lemon fruits by essential oils of Satureja species. Erwerbs-Obstbau 2022, 64, 703–715. [Google Scholar] [CrossRef]
- Gokturk, T. Chemical composition of Satureja spicigera essential oil and its insecticidal effectiveness on Halyomorpha halys nymphs and adults. Z. Naturforschung C 2021, 76, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Karan, T.; Belguzar, S.; Selvi, B. Antibacterial activity of essential oils of Origanum bilgeri, Origanum onites, Satureja spicigera leaves against agricultural plant pathogenic bacteria. J. Essent. Oil Bear. Plants 2021, 24, 1159–1168. [Google Scholar] [CrossRef]
- Kotan, R.; Cakir, A.; Dadasoglu, F.; Aydin, T.; Cakmakci, R.; Ozer, H.; Kordali, S.; Mete, E.; Dikbas, N. Antibacterial activities of essential oils and extracts of Turkish Achillea, Satureja and Thymus species against plant pathogenic bacteria. J. Sci. Food Agric. 2010, 90, 145–160. [Google Scholar] [CrossRef]
- Bektaş, E.; Sahin, H.; Beldüz, A.O.; Güler, H.İ. HIV-1-RT inhibition activity of Satureja spicigera (C. KOCH) BOISS. Aqueous extract and docking studies of phenolic compounds identified by RP-HPLC-DAD. J. Food Biochem. 2022, 46, e13921. [Google Scholar] [CrossRef]
- Jafari, S.A.; Khorshidi, J.; Morshedloo, M.R.; Houshidari, F. Comparative study on the quantity and chemical composition of essential oil, antioxidant activity and total phenol content of some Iranian native Satureja species under the same conditions. J. Med. Plants By-Prod. 2023, 12, 259–266. [Google Scholar] [CrossRef]
- Bozhuyuk, A.; Kordali, S.; Güneş, A.; Beyzi, E.; Turan, M.; Ersoy, N. Variation in phenolic, antioxidant and vitamin amounts among some medicinal plants and investigation by PCA analysis: Lamiaceae family. Bol. Latinoam. Caribe Plantas Med. Aromat. 2022, 21, 446–454. [Google Scholar] [CrossRef]
- Eminagaoglu, O.; Tepe, B.; Yumrutas, O.; Akpulat, H.A.; Daferera, D.; Polissiou, M.; Sokmen, A. The in vitro antioxidative properties of the essential oils and methanol extracts of Satureja spicigera (K. Koch.) Boiss. and Satureja cuneifolia ten. Food Chem. 2007, 100, 339–343. [Google Scholar] [CrossRef]
- Gohari, A.R.; Ostad, S.N.; Moradi-Afrapoli, F.; Malmir, M.; Tavajohi, S.; Akbari, H.; Saeidnia, S. Evaluation of the cytotoxicity of Satureja spicigera and its main compounds. Sci. World J. 2012, 2012, 203861. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Abramov, A.Y. Mechanism of oxidative stress in neurodegeneration. Oxidative Med. Cell. Longev. 2012, 2012, 428010. [Google Scholar] [CrossRef]
- Kınoğlu, B.K.; Gülçin, İ.; Gören, A.C. Quantification of secondary metabolites of Satureja pilosa (Lamiaceae) by LC-HRMS and evaluation of antioxidant and cholinergic activities. Rec. Nat. Prod. 2024, 18, 674–686. [Google Scholar] [CrossRef]
- Taslimi, P.; Köksal, E.; Gören, A.C.; Bursal, E.; Aras, A.; Kılıç, Ö.; Alwasel, S.; Gülçin, İ. Anti-Alzheimer, antidiabetic and antioxidant potential of Satureja cuneifolia and analysis of its phenolic contents by LC-MS/MS. Arab. J. Chem. 2020, 13, 4528–4537. [Google Scholar] [CrossRef]
- Atukeren, P.; Cengiz, M.; Yavuzer, H.; Gelisgen, R.; Altunoglu, E.; Oner, S.; Uzun, H. The efficacy of donepezil administration on acetylcholinesterase activity and altered redox homeostasis in Alzheimer’s disease. Biomed. Pharmacother. 2017, 90, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.; Kartal, M.; Kan, Y.; Şener, B. Activity of essential oils and individual components against acetyl and butyrylcholinesterase. Z. Naturforschung C 2008, 63, 547–553. [Google Scholar] [CrossRef]
- Zaidun, N.H.; Thent, Z.C.; Abd Latiff, A. Combating oxidative stress disorders with citrus flavonoid: Naringenin. Life Sci. 2018, 208, 111–122. [Google Scholar] [CrossRef]
- Dikici, E.; Altın, S.; Alp, C.; Işık, M.; Köksal, E.; Gülçin, İ. Determination of secondary metabolites of Cydonia oblonga (Quince) by LC-MS/MS method together with evaluation of its antioxidant and cholinergic potentials. J. Chem. Metrol. 2024, 18, 146–164. [Google Scholar] [CrossRef]
- Tuzimski, T.; Petruczynik, A. Determination of anti-Alzheimer’s disease activity of selected plant ingredients. Molecules 2022, 27, 3222. [Google Scholar] [CrossRef]
- Karagecili, H.; İzol, E.; Kirecci, E.; Gulcin, İ. Determination of antioxidant, anti-Alzheimer, antidiabetic, antiglaucoma and antimicrobial effects of Zivzik pomegranate (Punica granatum)—A chemical profiling by LC-MS/MS. Life 2023, 13, 735. [Google Scholar] [CrossRef]
- Saeedi, M.; Vahedi-Mazdabadi, Y.; Rastegari, A.; Soleimani, M.; Eftekhari, M.; Akbarzadeh, T. Evaluation of Asarum europaeum L. rhizome for the biological activities related to Alzheimer’s disease. Res. J. Pharmacogn. 2020, 7, 25–33. [Google Scholar] [CrossRef]
- Çarıkçı, S.; Kılıç, T.; Gören, A.C.; Dirmenci, T.; Alim Toraman, G.Ö.; Topçu, G. Chemical profile of the Anatolian Sideritis species with bioactivity studies. Pharm. Biol. 2023, 61, 1484–1511. [Google Scholar] [CrossRef]
- Lai, P.; Li, X.; Qiu, S.; Song, S. Chemical composition and evaluation of the antibacterial, synergistic antibacterial, antioxidant and cytotoxic activities of the essential oil of Macrothelypteris torresiana (gaudich.) Ching. Rec. Nat. Prod. 2024, 18, 538–543. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Özer, Z.; Çarıkçı, S.; Kılıç, T.; Selvi, S.; Gören, A.C. Determination of the effect of different drying methods on secondary metabolites of Lavandula pedunculata (Mill.) Cav. subsp. cariensis (Boiss.) Upson & S. Andrews by LC-HRMS. J. Chem. Metrol. 2024, 18, 124–133. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, I. Antioxidants-A comprehensive review. Arch. Toxicol. 2025, 99, 1893–1997. [Google Scholar] [CrossRef] [PubMed]
- Akaslan, T.; Yalçın, Ş.; Akyıldız, A.G.; Gören, A.C. Determination of adulteration in hair serums by LC-HRMS. J. Chem. Metrol. 2023, 17, 93–99. [Google Scholar] [CrossRef]
- Çarıkçı, S.; Kılıç, T.; Dirmenci, T.; Gören, A.C. Phenolic compounds from section Majorana (Mill.) Benth of Origanum L. species extracts via validated LC-MS/MS method. J. Chem. Metrol. 2022, 16, 147–151. [Google Scholar] [CrossRef]
- Mutlu, M.; Bingöl, Z.; Uç, E.M.; Köksal, E.; Gören, A.C.; Alwasel, S.H.; Gülçin, İ. Comprehensive metabolite profiling of cinnamon (Cinnamomum zeylanicum) leaf oil using LC-HR/MS, GC/MS, and GC-FID: Determination of antiglaucoma, antioxidant, anticholinergic, and antidiabetic profiles. Life 2023, 13, 136. [Google Scholar] [CrossRef]
- Kızıltaş, H.; Bingöl, Z.; Gören, A.; Alwasel, S.; Gülçin, İ. Analysis of phenolic compounds by LC-HRMS and determination of antioxidant and enzyme inhibitory properties of Verbascum speciosum Schrad. Rec. Nat. Prod. 2023, 17, 485–500. [Google Scholar] [CrossRef]
- EURACHEM/CITAC Guide CG4. Quantifying Uncertainty in Analytical Measurements, 2nd ed.; EURACHEM: London, UK, 2000.
- Çarıkçı, S.; Gören, A.C.; Kılıç, T. Diterpenoid and phenolic contents of Sideritis hololeuca Boiss & Heldr. Apud Bentham with antioxidant and anticholinesterase activity. Z. Naturforschung C 2020, 75, 161–169. [Google Scholar] [CrossRef]
- Shah, K.; Chokshi, A.; Vyas, N. Development of RP-HPLC-DAD method for quantitative analysis of quercetin and piperine in botanical extracts. J. Chem. Metrol. 2024, 18, 114–123. [Google Scholar] [CrossRef]
- Karageçili, H.; Polat, T.; Yılmaz, M.A.; Fidan, M.; Karaismailoğlu, M.C.; Gülçin, İ. Evaluation of the antioxidant, Antidiabetic and Anti-Alzheimer Effects of Capsella bursa-pastoris-Polyphenolic profiling by LC-MS/MS. Rec. Nat. Prod. 2024, 18, 643–662. [Google Scholar] [CrossRef]
- Blois, M. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Gulcin, İ. Measurement of antioxidant ability of melatonin and serotonin by the DMPD and CUPRAC methods as Trolox equivalent. J. Enzyme Inhib. Med. Chem. 2008, 23, 871–876. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reactions: Antioxidative activities of product of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–90. [Google Scholar] [CrossRef]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phytother. Res. 2015, 29, 323–331. [Google Scholar] [CrossRef]
- Garg, A.; Garg, S.; Zaneveld, L.J.D.; Singla, A. Chemistry and pharmacology of the citrus bioflavonoid hesperidin. Phytother. Res. 2001, 15, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Fahad, F.I.; Barua, N.; Islam, M.S.; Sayem, S.A.J.; Barua, K.; Uddin, M.J.; Chy, M.N.U.; Adnan, M.; Islam, M.N.; Sayeed, M.A.; et al. Investigation of the pharmacological properties of Lepidagathis hyalina Nees through experimental approaches. Life 2021, 11, 180. [Google Scholar] [CrossRef]
- Wróbel-Biedrawa, D.; Grabowska, K.; Galanty, A.; Sobolewska, D.; Podolak, I. A Flavonoid on the Brain: Quercetin as a potential therapeutic agent in central nervous system disorders. Life 2022, 12, 591. [Google Scholar] [CrossRef]
- Singh, S.; Singh, R.K. Recent advancements in the understanding of the alterations in mitochondrial biogenesis in Alzheimer’s disease. Mol. Biol. Rep. 2025, 52, 173. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.A.; Maduakolam-Aniobi, T.C.; Gyebi, G.A.; Soyinka, T.O.; Ejiogu, O.F.; Ojo, A.B.; Alruwaili, M.; Ali, N.H.; Alnaaim, S.A.; Alsfouk, B.A.; et al. Experimental and computational analyses of the anti-Alzheimer and antidiabetic effects of flavonoid-rich extract of avocado seeds (Persea americana Mill.). Nutrire 2025, 50, 32. [Google Scholar] [CrossRef]
- Abramov, A.Y.; Potapova, E.V.; Dremin, V.V.; Dunaev, A.V. Interaction of oxidative stress and misfolded proteins in the mechanism of neurodegeneration. Life 2020, 10, 101. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Z.; Zhu, J.; Zhu, X.; Zhao, J.; Liu, X. Chemical composition, antioxidant, acetylcholinesterase and β-Lactamase inhibitory activities of essential oils from Clerodendrum cyrtophyllum Turcz. and Clerodendrum fortunatum L. Rec. Nat. Prod. 2025, 19, 263–277. [Google Scholar] [CrossRef]
- Bayrak, Ç.; Taslimi, P.; Gülçin, İ.; Menzek, A. The first synthesis of 4-phenylbutenone derivative bromophenols including natural products and their inhibition profiles for carbonic anhydrase, acetylcholinesterase and butyrylcholinesterase enzymes. Bioorganic Chem. 2017, 72, 359–366. [Google Scholar] [CrossRef]
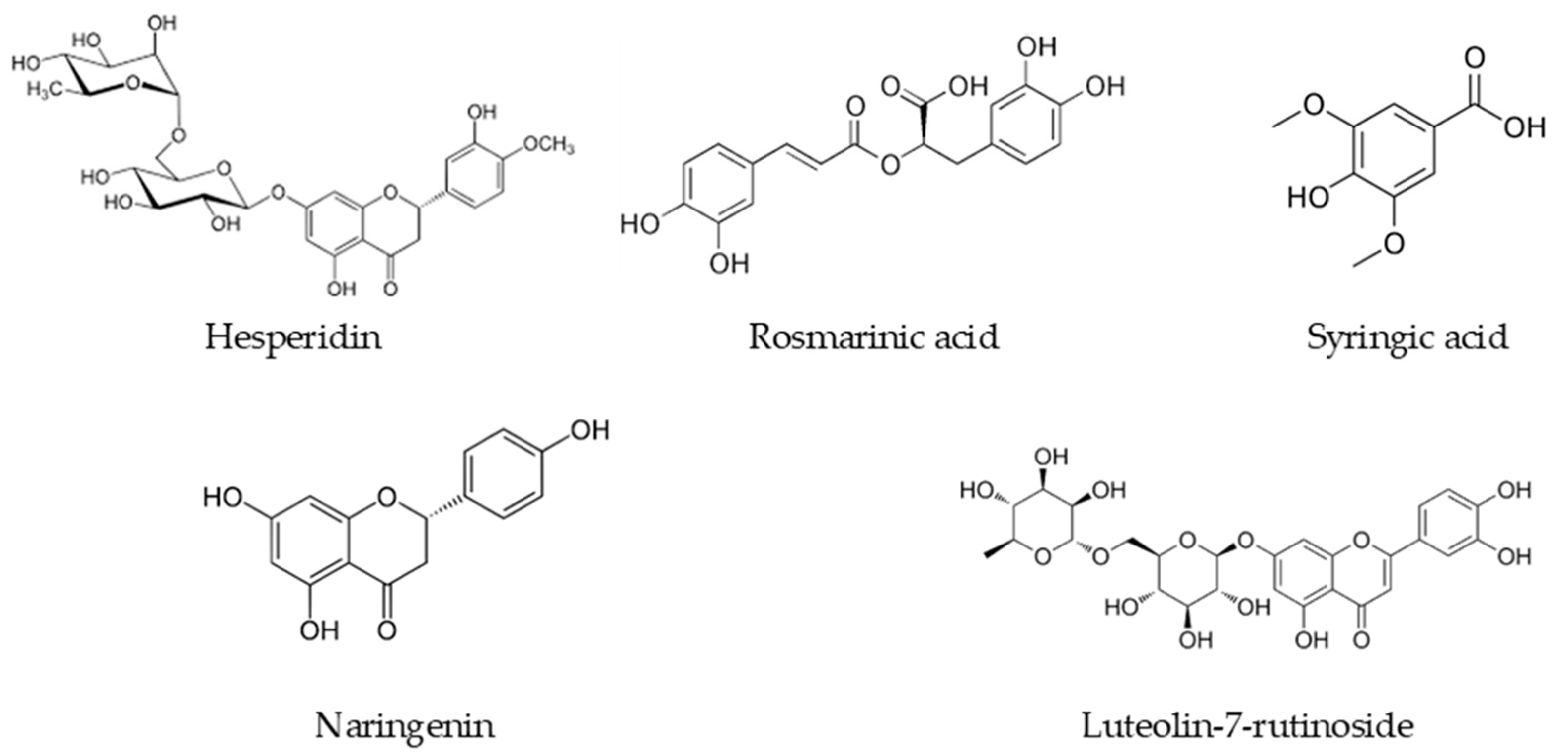
| Compounds | Satureja aintabensis | Satureja spicigera | U% (k = 2) * |
|---|---|---|---|
| Flavonoids and Derivatives | |||
| Apigenin | 0.040 | 0.006 | 11.54 |
| Chrysin | <LOD ** | <LOD ** | 11.09 |
| Luteolin | 0.110 | 0.026 | 12.41 |
| Luteolin-7-rutinoside | 0.065 | 1.682 | 11.45 |
| Luteolin-7-glucoside | 0.009 | 0.126 | 11.29 |
| Apigenin-7-glucoside | 0.003 | 0.004 | 11.9 |
| Orientin | 0.015 | 0.007 | 11.47 |
| Acacetin | 0.108 | 0.018 | 11.36 |
| Hispidulin | 0.048 | 0.005 | 11.23 |
| Nepetin | 0.014 | <LOD ** | 11.24 |
| Penduletin | 0.006 | 0.002 | 11.81 |
| Quercetin | 0.026 | 0.005 | 11.42 |
| Hyperoside | 0.163 | 0.044 | 11.5 |
| Quercitrin | 0.010 | 0.010 | 11.69 |
| (−)-Epicatechin | <LOD ** | <LOD ** | 11.91 |
| (−)-Epicatechin gallate | <LOD ** | <LOD ** | 11.21 |
| (+)-trans Taxifolin | 0.162 | 0.031 | 11.19 |
| Dihydrokaempferol | 0.125 | 0.032 | 11.35 |
| Naringenin | 0.395 | 0.034 | 11.04 |
| Isosakuranetin | 0.021 | 0.001 | 11.48 |
| Naringin | 0.077 | 0.674 | 12 |
| Hesperidin | 6.465 | 1.723 | 11.15 |
| Coumaric acids and Derivatives | |||
| Caffeic acid | 0.028 | 0.037 | 11.07 |
| Chlorogenic acid | 0.110 | 0.020 | 11.14 |
| Rosmarinic acid | 5.248 | 2.757 | 11.63 |
| Caffeic acid phenethyl ester | <LOD ** | <LOD ** | 11.38 |
| Simple Phenolics and Others *** | |||
| Syringic acid | 5.964 | 3.081 | 12.37 |
| Salicylic acid | 0.022 | 0.034 | 11.4 |
| Vanilic acid | 0.157 | 0.172 | 11.61 |
| Verbascoside | <LOD ** | 0.045 | 12.08 |
| Ascorbic acid | 0.064 | 0.047 | 11.07 |
| Fumaric acid | 0.243 | 0.661 | 11.14 |
| Antioxidants | Fe3+ Reducing | Cu2+ Reducing | ||
|---|---|---|---|---|
| λ (593 nm) | r2 | λ (450 nm) | r2 | |
| BHA | 2.347 | 0.9086 | 1.649 | 0.9584 |
| BHT | 0.952 | 0.9154 | 0.998 | 0.9834 |
| Trolox | 2.119 | 0.9586 | 1.108 | 0.9910 |
| α-Tocopherol | 0.957 | 0.9863 | 0.693 | 0.9934 |
| S. aintabensis | 0.597 | 0.9618 | 1.016 | 0.9954 |
| S. spicigera | 0.421 | 0.9236 | 0.757 | 0.9999 |
| Antioxidants | DPPH·Scavenging * | ABTS+ Scavenging * | DMPD+ Scavenging * | |||
|---|---|---|---|---|---|---|
| IC50 | r2 | IC50 | r2 | IC50 | r2 | |
| BHA | 10.10 | 0.9015 | 5.07 | 0.9356 | 0.070 | 0.9465 |
| BHT | 25.95 | 0.9221 | 6.99 | 0.9350 | 0.070 | 0.9390 |
| Trolox | 7.05 | 0.9614 | 6.16 | 0.9692 | 0.072 | 0.9382 |
| α-Tocopherol | 11.31 | 0.9642 | 8.37 | 0.9015 | - | - |
| S. aintabensis | 13.07 | 0.9426 | 8.77 | 0.9478 | 30.13 | 0.9804 |
| S. spicigera | 12.37 | 0.9996 | 9.49 | 0.9343 | 33.00 | 0.9254 |
| Inhibitors | AChE Inhibition (%) * | BChE Inhibition (%) * |
|---|---|---|
| Satureja aintabensis | 31.8 ± 2.2 | 9.4 ± 1.4 |
| Satureja spicigera | 39.7 ± 1.6 | 1.3 ± 0.7 |
| Galantamine | 96.8 ± 1.3 | 83.3 ± 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yıldız, A.N.; Çarıkçı, S.; Dirmenci, T.; Kartal, M.; Gülcin, İ.; Gören, A.C. Secondary Metabolite Profiling of Satureja aintabensis P.H. Davis and Satureja spicigera (K. Koch) Boiss. by LC-HRMS and Evaluation of Antioxidant and Anticholinergic Activities. Life 2025, 15, 1272. https://doi.org/10.3390/life15081272
Yıldız AN, Çarıkçı S, Dirmenci T, Kartal M, Gülcin İ, Gören AC. Secondary Metabolite Profiling of Satureja aintabensis P.H. Davis and Satureja spicigera (K. Koch) Boiss. by LC-HRMS and Evaluation of Antioxidant and Anticholinergic Activities. Life. 2025; 15(8):1272. https://doi.org/10.3390/life15081272
Chicago/Turabian StyleYıldız, Ayşe Nur, Sema Çarıkçı, Tuncay Dirmenci, Murat Kartal, İlhami Gülcin, and Ahmet C. Gören. 2025. "Secondary Metabolite Profiling of Satureja aintabensis P.H. Davis and Satureja spicigera (K. Koch) Boiss. by LC-HRMS and Evaluation of Antioxidant and Anticholinergic Activities" Life 15, no. 8: 1272. https://doi.org/10.3390/life15081272
APA StyleYıldız, A. N., Çarıkçı, S., Dirmenci, T., Kartal, M., Gülcin, İ., & Gören, A. C. (2025). Secondary Metabolite Profiling of Satureja aintabensis P.H. Davis and Satureja spicigera (K. Koch) Boiss. by LC-HRMS and Evaluation of Antioxidant and Anticholinergic Activities. Life, 15(8), 1272. https://doi.org/10.3390/life15081272









