Upcycled Cocoa Pod Husk: A Sustainable Source of Phenol and Polyphenol Ingredients for Skin Hydration, Whitening, and Anti-Aging
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Plant Material Collection and Preparation
2.3. Cocoa Pod Husk Extract Preparation
2.4. Total Phenolic Content
2.5. Total Flavonoid Content
2.6. LC-MS/MS Analysis
2.7. Antioxidant Activities
2.7.1. DPPH Free-Radical Scavenging Assay
2.7.2. Ferric Acid Reducing Power
2.8. Anti-Glycation Activity
2.9. Tyrosinase Inhibitory Activity
2.10. Anti-Collagenase Activity
2.11. Cytotoxicity Using MTT Assay
2.12. Nitric Oxide (NO) Production Inhibition Activity
2.13. Formulation and Stability Evaluation
2.14. Clinical Evaluation
2.14.1. Ethical Conduction
2.14.2. Volunteer Recruitment
2.14.3. Skin Irritation Test
2.14.4. Clinical Skin Efficacy Evaluation
2.15. Statistical Analysis
3. Results and Discussion
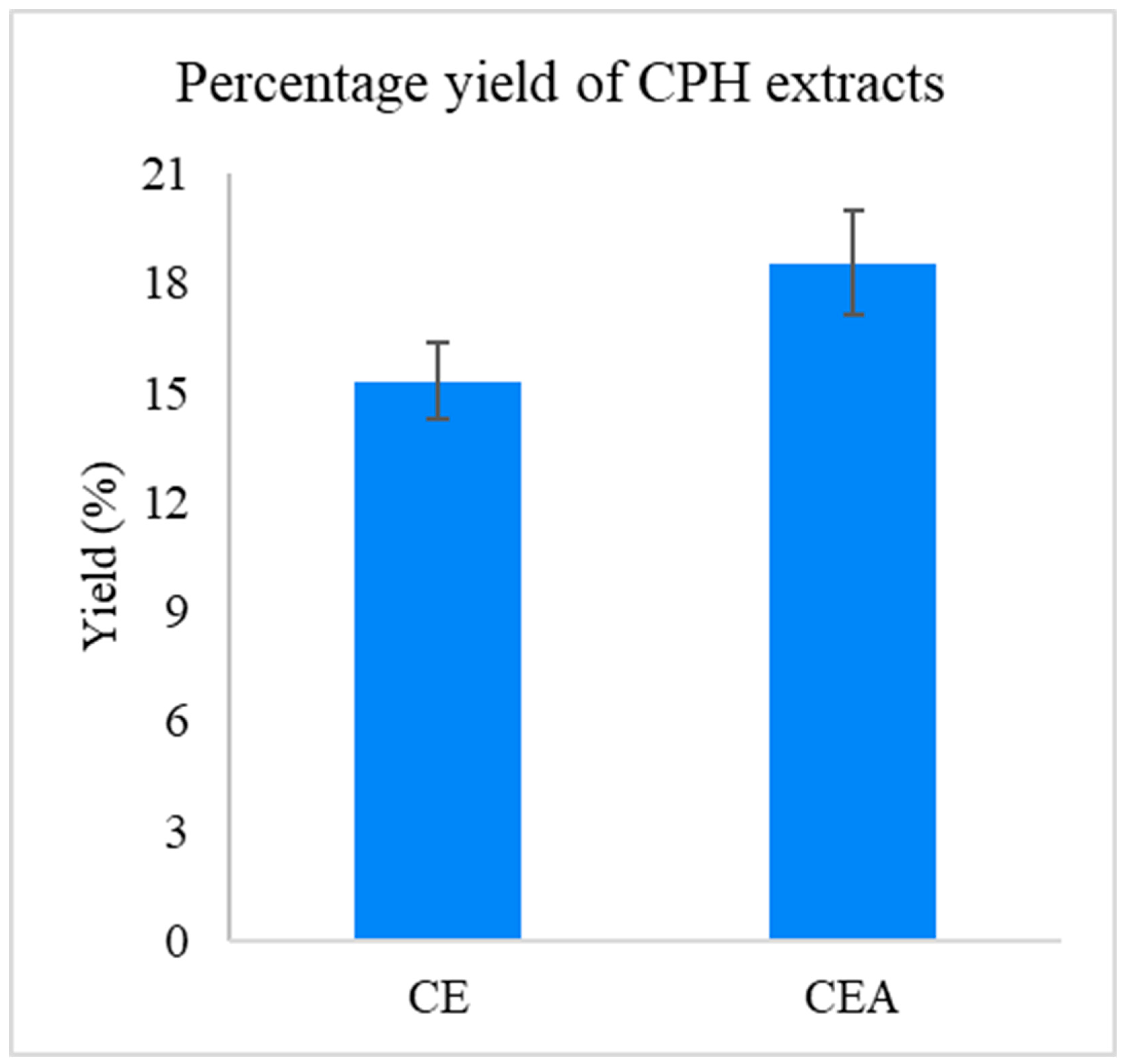
3.1. Yield
3.2. Total Phenolic Content
3.3. Total Flavonoid Content
3.4. Identification of Chemical Composition of CPH Extracts by Using LC-MS/MS
| Compound | Peak No. | Chemical Formula | RT(min) | Major MS-MS Fragments | a | b | References |
|---|---|---|---|---|---|---|---|
| Ferulic acid | 1 | C10H10O4 | 14.14 | 176, 152, 144, 116, 105 | [28,30] | ||
| 14.15 | Yes | ||||||
| 15.74 | Yes | ||||||
| Chlorogenic acid | 2 | C16H18O9 | 17.20 | 337, 181, 163 | [28,30] | ||
| 17.22 | Yes | ||||||
| 20.59 | Yes | ||||||
| Naringenin | 3 | C15H12O5 | 17.66 | 231, 179, 152, 147 | [28,30] | ||
| 17.67 | Yes | ||||||
| 20.97 | Yes | ||||||
| Apigenin-7-O-glucoside | 4 | C21H22O10 | 19.26 | 409, 367, 323, 247 | [28,30] | ||
| 19.97 | Yes | ||||||
| 22.57 | Yes | ||||||
| Procyanidin B1 | 5 | C30H26O12 | 22.83 | 407, 339, 289, 248 | Yes | [28,30] | |
| 24.60 | Yes | ||||||
| Procyanidin B2 | 6 | C30H26O12 | 23.14 | 425, 407, 289, 161, 125 | [28,29,31] | ||
| 23.15 | Yes | ||||||
| 24.89 | Yes | ||||||
| 2-O-caffeoltartaric acid | 7 | C13H12O9 | 23.70 | 312, 296,252 | [28] | ||
| 25.20 | Yes | ||||||
| Protocatechuic acid | 8 | C7H6O4 | 26.51 | 137, 109, 108 | Yes | [28,30] | |
| 26.52 | |||||||
| 27.70 | Yes | ||||||
| N-[3’,4’-dihydroxy-(E)cinnamoyl]-L-aspartic acid | 9 | C13H13NO6 | 26.76 | 163 | Yes | [28] | |
| 26.79 | |||||||
| 26.80 | |||||||
| 27.88 | Yes | ||||||
| N-[4’-dihydroxy-(E)cinnamoyl]-L-aspartic acid | 10 | C13H13NO6 | 27.14 | 178, 163 | Yes | [28] | |
| 28.13 | Yes | ||||||
| Kaempferol | 11 | C15H10O6 | 29.50 | 257, 229, 149 | Yes | [28,30] | |
| Procyanidin C1 | 12 | C45H38O18 | 28.96 | 712, 695, 575, 425, 407, 287, 125 | Yes | [28,30] | |
| 29.95 | Yes |
3.5. Antioxidant Activities
3.6. Anti-Glycation Activity
3.7. Tyrosinase Inhibitory Activity
3.8. Anti-Collagenase Activity
3.9. Cytotoxicity and Nitric Oxide (NO) Production Inhibition Activity
3.10. Formulation and Stability Evaluation
3.11. Clinical Evaluation
3.11.1. Skin Irritation Test
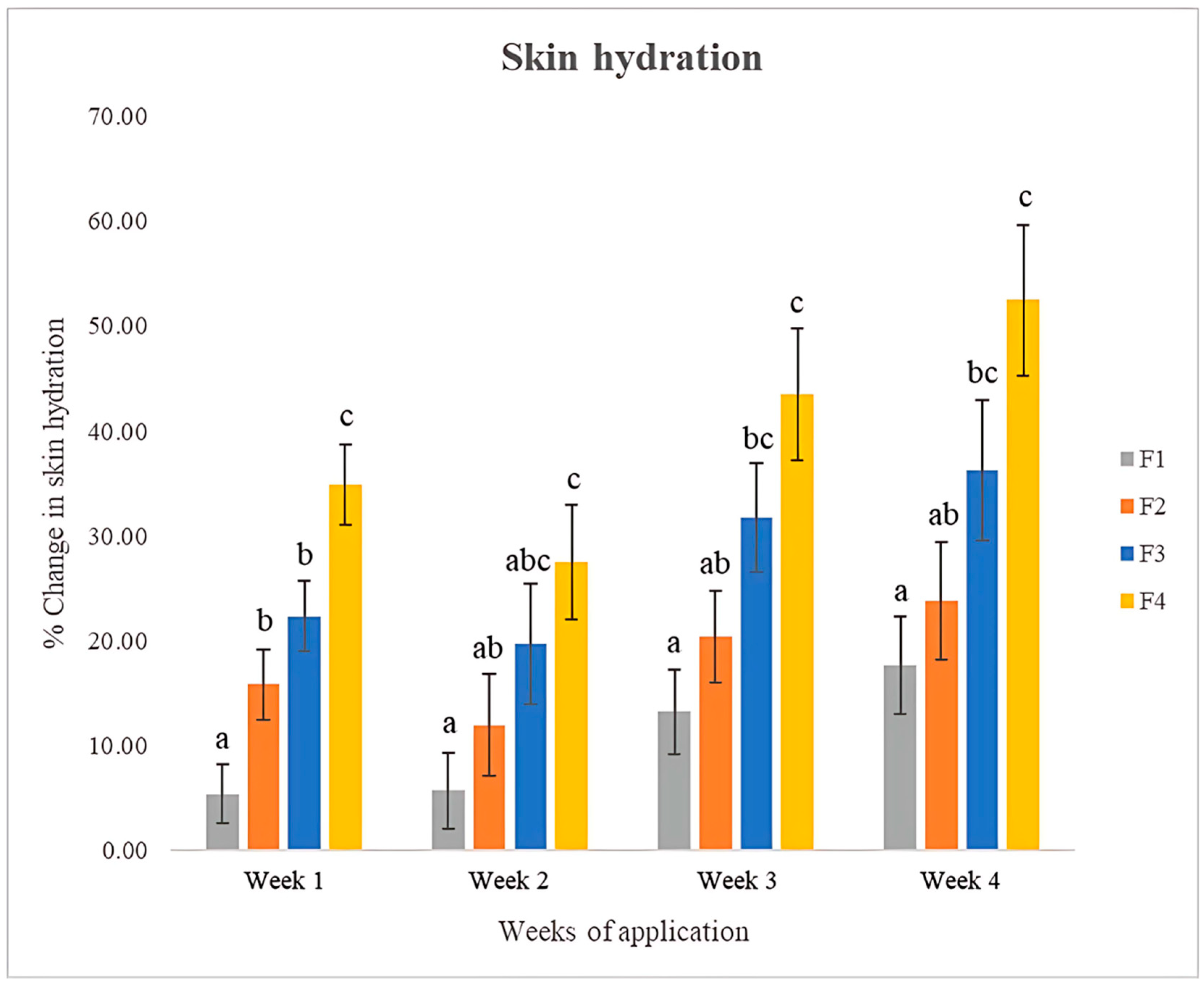
3.11.2. Skin Hydration
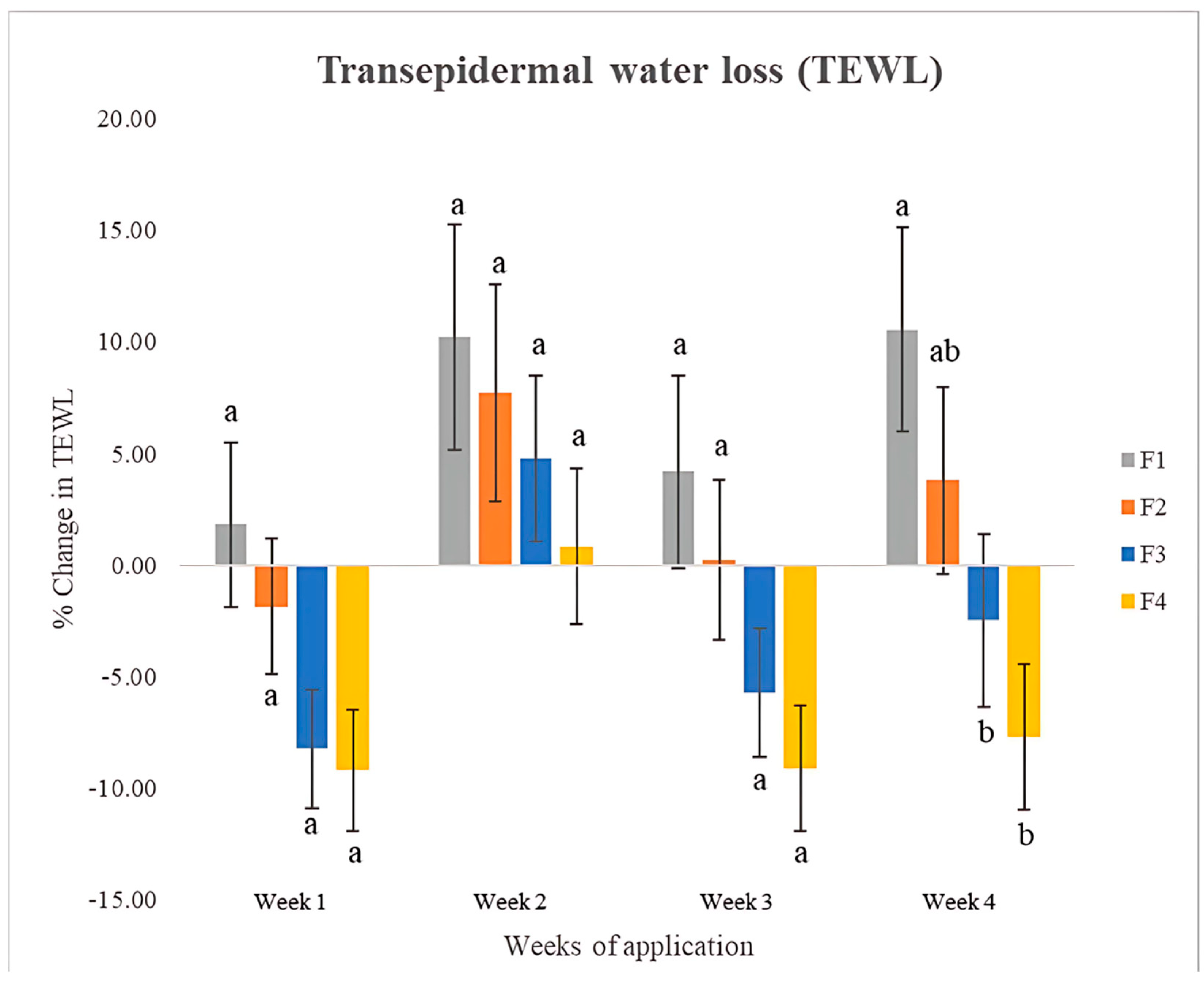
3.11.3. Transepidermal Water Loss (TEWL)
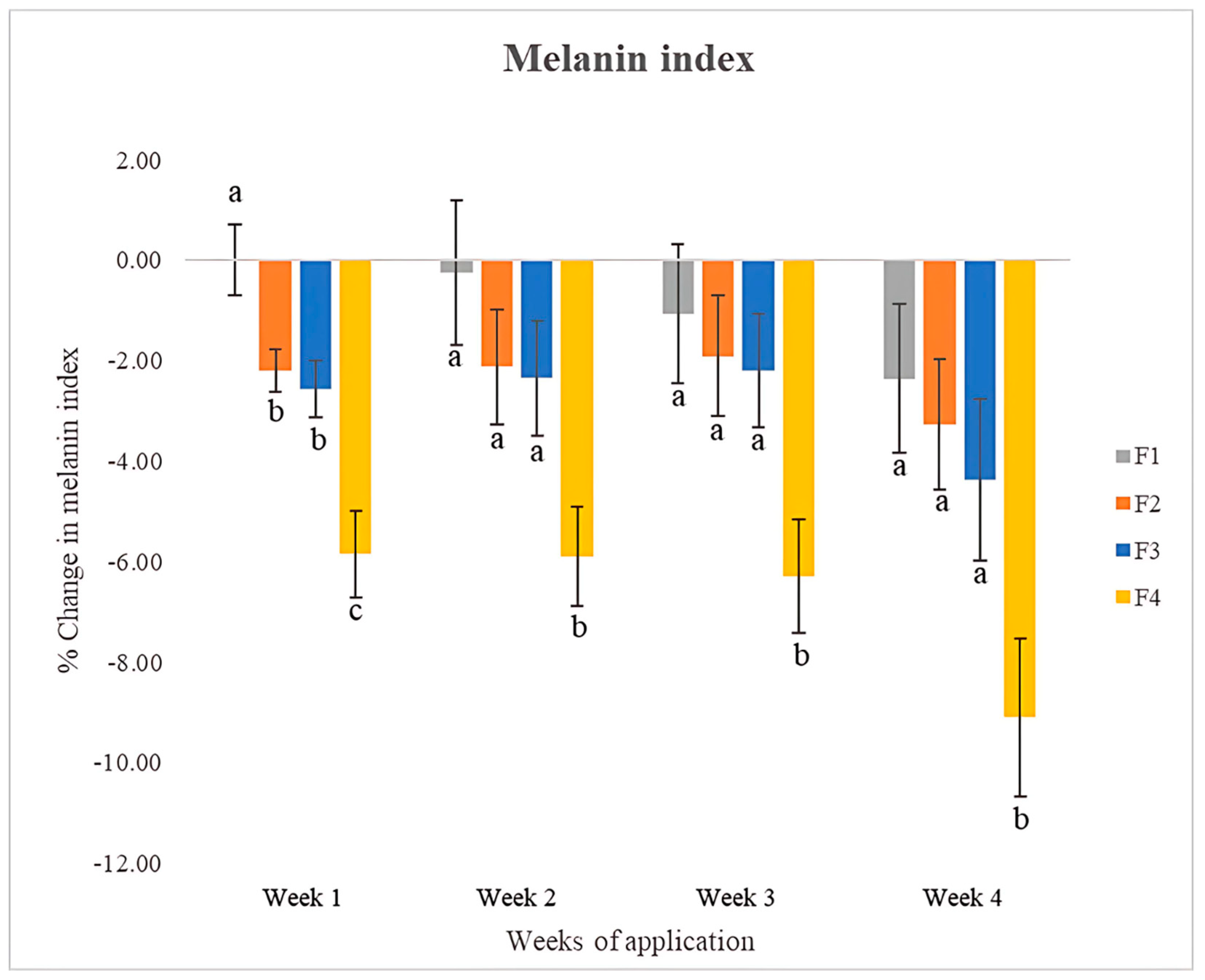
3.11.4. Skin Pigmentation
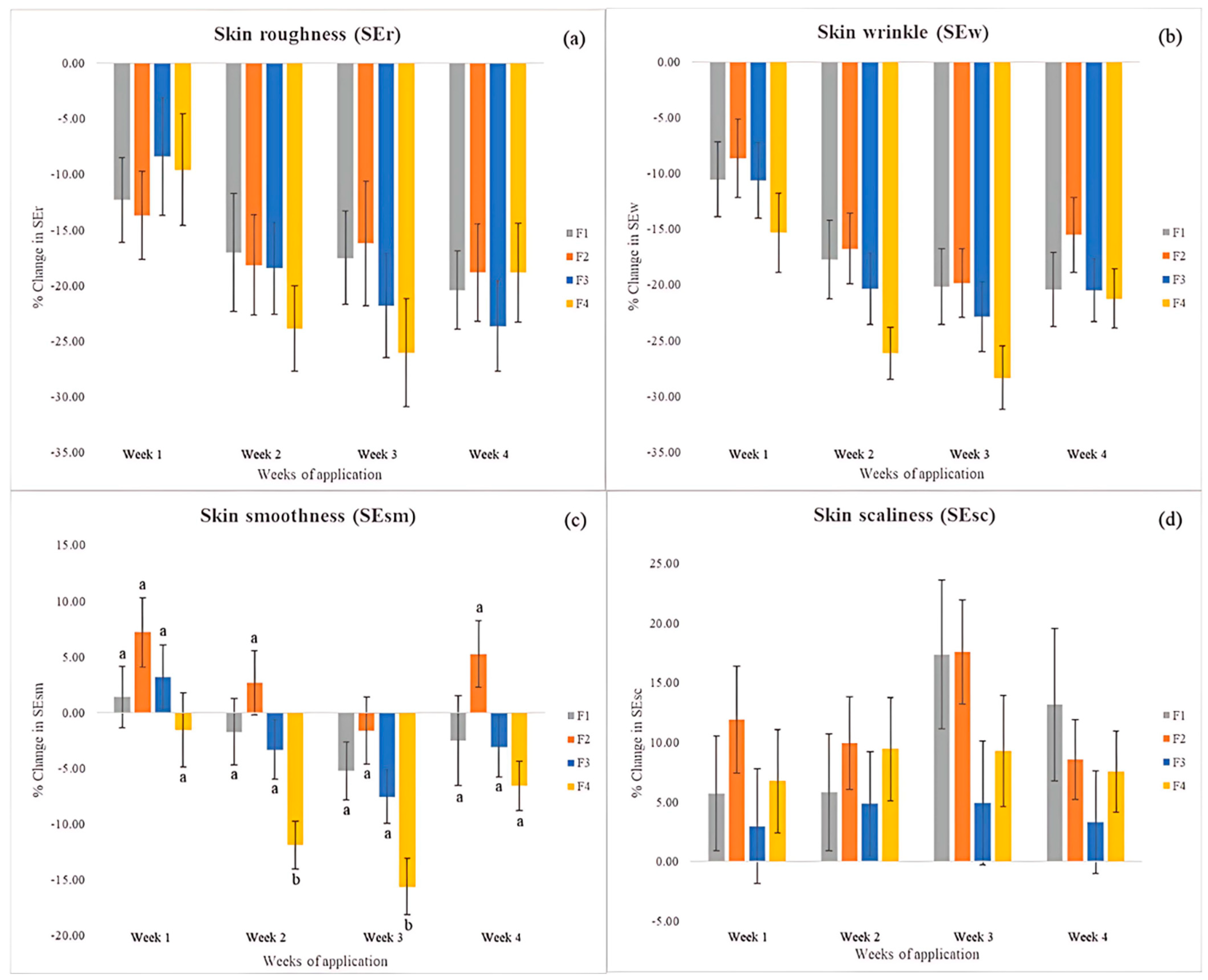
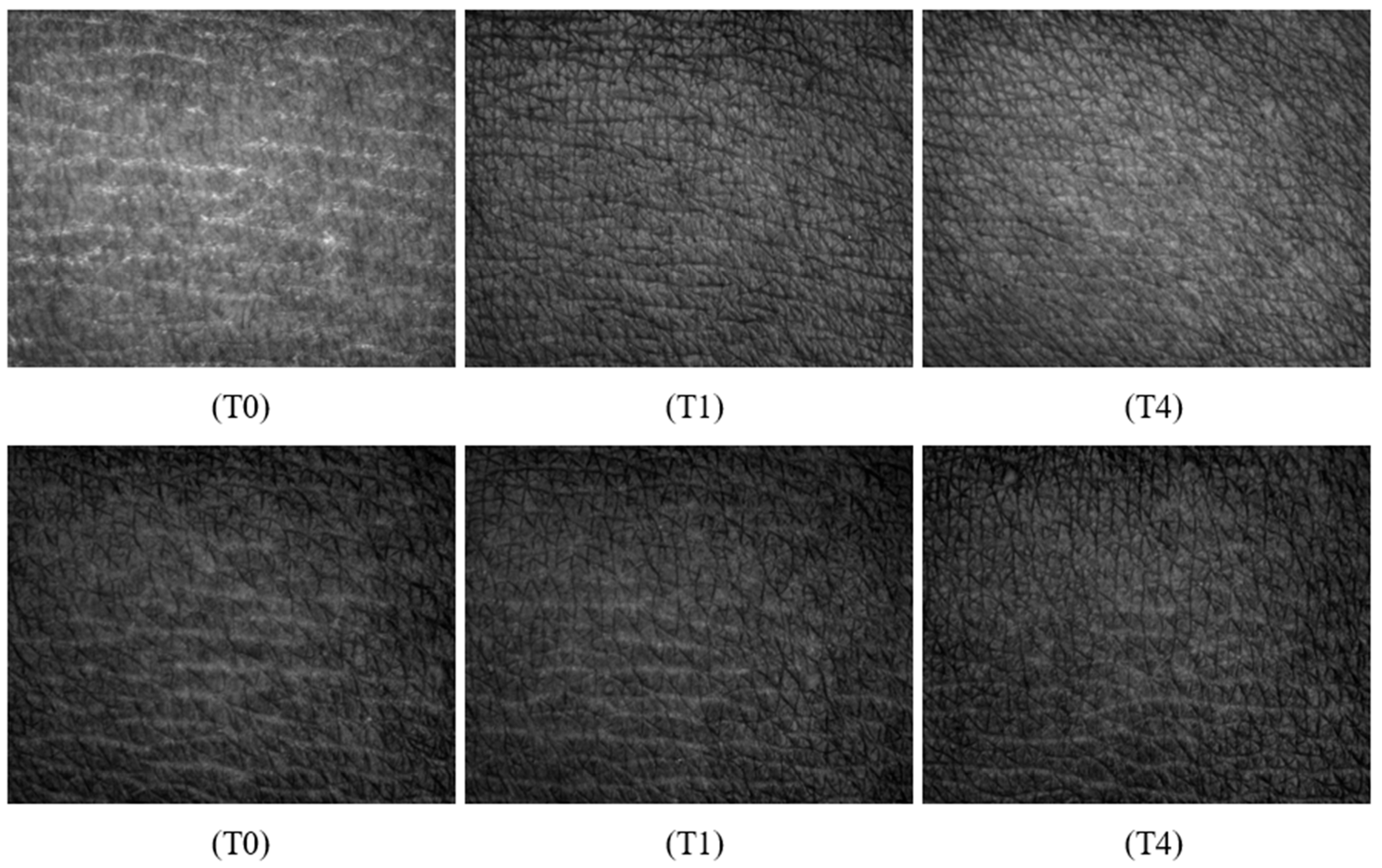
3.11.5. Skin Surface Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garg, N. Technology for the production of agricultural wines. In Science and Technology of Fruit Wine Production; Academic Press: Cambridge, MA, USA, 2017; pp. 463–486. [Google Scholar]
- Follana, C. Quarterly Bulletin of Cocoa Statistics. International Cocoa Organization. Available online: https://www.icco.org/may-2021-quarterly-bulletin-of-cocoa-statistics/ (accessed on 3 June 2021).
- 360 Market Updates. Cocoa Products Market Size 2022 with a CAGR of 1.7%, Top Companies Data Report Covers, Market-Specific Challenges, Brief Analysis and Application, Growth by 2025. Marketwatch. Available online: https://www.marketwatch.com/press-release/cocoa-products-market-size-2022-with-a-cagr-of-17-top-companies-data-report-covers-market-specific-challenges-brief-analysis-and-application-growth-by-2025-2022-05-20 (accessed on 20 May 2022).
- Chocolate Market Size & Share Analysis-Growth Trends & Forecasts up to 2030. Available online: https://www.mordorintelligence.com/industry-reports/chocolate-market (accessed on 20 June 2025).
- Abdul Karim, A.; Azlan, A.; Ismail, A.; Hashim, P.; Abd Gani, S.S.; Zainudin, B.H.; Abdullah, N.A. Phenolic composition, antioxidant, anti-wrinkles and tyrosinase inhibitory activities of cocoa pod extract. BMC Complement. Altern. Med. 2014, 14, 381. [Google Scholar] [CrossRef] [PubMed]
- Martínez, R.; Torres, P.; Meneses, M.A.; Figueroa, J.G.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, technological and in vitro antioxidant properties of cocoa (Theobroma cacao L.) co-products. Food Res. Int. 2012, 49, 39–45. [Google Scholar] [CrossRef]
- Agudelo, C.; Bravo, K.; Ramírez-Atehortúa, A.; Torres, D.; Carrillo-Hormaza, L.; Osorio, E. Chemical and skincare property characterization of the main cocoa byproducts: Extraction optimization by RSM approach for development of sustainable ingredients. Molecules 2021, 26, 7429. [Google Scholar] [CrossRef] [PubMed]
- Priani, S.E.; Aprilia, S.; Aryani, R.; Purwanti, L. Antioxidant and tyrosinase inhibitory activity of face serum containing cocoa pod husk phytosome (Theobroma cacao L.). J. Appl. Pharm. Sci. 2019, 9, 110–115. [Google Scholar] [CrossRef][Green Version]
- Anoraga, S.B.; Shamsudin, R.; Hamzah, M.H.; Sharif, S.; Saputro, A.D. Cocoa by-products: A comprehensive review on potential uses, waste management, and emerging green technologies for cocoa pod husk utilization. Heliyon 2024, 10, e35537. [Google Scholar] [CrossRef] [PubMed]
- Soares, T.F.; Oliveira, M.B.P.P. Cocoa by-products: Characterization of bioactive compounds and beneficial health effects. Molecules 2022, 27, 1625. [Google Scholar] [CrossRef] [PubMed]
- Rojo-Povede, O.; Barbosa-Pereira, L.; Zeppa, G.; Stévigny, C. Cocoa bean shell—A by-product with nutritional properties and biofunctional potential. Nutrients 2020, 12, 1123. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Inda, B.; Jiménez-Moreno, N.; Esparza, I.; Ancín-Azpilicueta, C. Coffee and cocoa by-products as valuable sources of bioactive compounds: The influence of ethanol on extraction. Antioxidants 2025, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Ramos, L.H.; Cisneros-Yupanqui, M.; Soto, D.V.; Lante, A.; Favaro, L.; Casella, S.; Basaglia, M. Exploitation of cocoa pod residues for the production of antioxidants, polyhydroxyalkanoates, and ethanol. Fermentation 2023, 9, 843. [Google Scholar] [CrossRef]
- Švarc-Gajić, J.; Brezo-Borjan, T.; Dzedik, V.; Rodrigues, F.; Morais, S.; Delerue-Matos, C. ESG approach in the valorization of cocoa (Theobroma cacao) by-products by subcritical water: Application in the cosmetic industry. Sustain. Chem. Pharm. 2023, 31, 100908. [Google Scholar] [CrossRef]
- Cvetanović, A.; Švarc-Gajić, J.; Zeković, Z.; Mašković, P.; Đurović, S.; Zengin, G.; Delerue-Matos, C.; Lozano-Sánchez, J.; Jakišić, A. Chemical and biological insights on aronia stems extracts obtained by different extraction techniques: From wastes to functional products. J. Supercrit. Fluids 2017, 128, 173–181. [Google Scholar] [CrossRef]
- Sitthichai, P.; Chanpirom, S.; Maneerat, T.; Charoensup, R.; Tree-Udom, T.; Pintathong, P.; Laphookhieo, S.; Sripisut, T. Kaempferia parviflora rhizome extract as potential anti-acne ingredient. Molecules 2022, 27, 4401. [Google Scholar] [CrossRef] [PubMed]
- Chanpirom, S.; Khat-udomkiri, N.; Tree-Udom, T.; Ditthawutthikul, N.; Saewan, N.; Vinardell, M.; Sripisut, T. Potential of Cissampelos pareira L. Pectin as a Bioactive compound in moisturizing and anti-aging applications. Cosmetics 2025, 12, 5. [Google Scholar] [CrossRef]
- Venkatesan, T.; Choi, Y.W.; Kim, Y.K. Impact of different extraction solvents on phenolic content and antioxidant potential of Pinus densiflora bark extract. Biomed Res. Int. 2019, 1, 3520675. [Google Scholar] [CrossRef] [PubMed]
- Tantapakul, C.; Khat-udomkiri, N.; Sitthichai, P.; Chomsak, A.; Thananusak, N.; Phukhatmuen, P.; Vinardell, M.P.; Sripisut, T. Exploring the physicochemical properties of polysaccharides extracted from cocoa pod husk waste and their efficacy in skin hydration. Ind. Crops Prod. 2024, 222, 119940. [Google Scholar] [CrossRef]
- Kanlayavattanakul, M.; Lourith, N. Spent arabica coffee as a rich source of antioxidant appraisal for cosmetic applications. Adv. Sci. Eng. Med. 2013, 5, 173–176. [Google Scholar] [CrossRef]
- Loypimai, P.; Moongngarm, A.; Chottanom, P. Extraction solvents affecting phytochemicals in food colorant prepared from purple glutinous rice bran. Appl. Biol. Chem. 2017, 60, 181–189. [Google Scholar] [CrossRef]
- Jiamphun, S.; Chaiyana, W. Enhanced antioxidant, hyaluronidase, and collagenase inhibitory activities of glutinous rice husk extract by aqueous enzymatic extraction. Molecules 2022, 27, 3317. [Google Scholar] [CrossRef] [PubMed]
- Suthiphasilp, V.; Raksat, A.; Maneerat, T.; Hadsadee, S.; Jungsuttiwong, S.; Pyne, S.G.; Chomnunti, P.; Jaidee, W.; Charoensup, R.; Laphookhieo, S. Cytotoxicity and nitric oxide production inhibitory activities of compounds isolated from the plant pathogenic fungus Curvularia sp. J. Fungi 2021, 7, 408. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.F.S.; Hadi, H.A.; Hanif, N.M.; Ahmad, H.; Ng, S.F. Lightening effect of skin lightening cream containing Piper betle L. extract in human volunteers. Cosmetics 2021, 8, 32. [Google Scholar] [CrossRef]
- Stanoeva, J.P.; Balshikevska, E.; Stefova, M.; Tusevski, O.; Simic, S.G. Comparison of the effect of acids in solvent mixtures for extraction of phenolic compounds from Aronia melanocarpa. Nat. Prod. Commun. 2020, 15, 10. [Google Scholar] [CrossRef]
- Mokrani, A.; Madani, K. Effect of solvent, time and temperature on the extraction of phenolic compounds and antioxidant capacity of peach (Prunus persica L.) fruit. Sep. Purif. Technol. 2016, 162, 68–76. [Google Scholar] [CrossRef]
- Hernández-Hernández, C.; Viera-Alcaide, I.; Morales-Sillero, A.M.; Fernández-Bolaños, J.; Rodríguez-Gutiérrez, G. Bioactive compounds in Mexican genotypes of cocoa cotyledon and husk. Food Chem. 2018, 240, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; O’Maille, G.; Want, E.J.; Qin, C.; Trauger, S.A.; Brandon, T.R.; Custodio, D.E.; Abagyan, R.; Siuzdak, G. METLIN: A metabolite mass spectral database. Ther. Drug Monit. 2005, 27, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Magi, E.; Bono, L.; Di Carro, M. Characterization of cocoa liquors by GC–MS and LC–MS/MS: Focus on alkylpyrazines and flavanols. J. Mass Spectrom. 2012, 47, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rabaneda, F.; Jáuregui, O.; Casals, I.; Andrés-Lacueva, C.; Izquierdo-Pulido, M.; Lamuela-Raventós, R.M. Liquid chromatographic/electrospray ionization tandem mass spectrometric study of the phenolic composition of cocoa (Theobroma cacao). J. Mass Spectrom. 2003, 38, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Calderón, A.I.; Wright, B.J.; Hurst, W.J.; Van Breemen, R.B. Screening antioxidants using LC-MS: Case study with cocoa. J. Agric. Food Chem. 2009, 57, 5693–5699. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.R.; Hossain, M.M.; Akter, R.; Jamila, M.; Mazumder, M.E.H.; Rahman, S. DPPH free radical scavenging activity of some Bangladeshi medicinal plants. J. Med. Plant Res. 2009, 3, 875–879. [Google Scholar]
- Chen, C.; Zhang, J.-Q.; Li, L.; Guo, M.; He, Y.; Dong, Y.; Meng, H.; Yi, F. Advanced glycation end products in the skin: Molecular mechanisms, methods of measurement, and inhibitory pathways. Front. Med. 2022, 9, 837222. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Lee, P. Inhibitory effect of procyanidin b2 on melanin biosynthesis in melanocytes. J. Korean Soc. Food Sci. Nutr. 2019, 48, 1039–1043. [Google Scholar] [CrossRef]
- Shoji, T.; Miura, T. Apple polyphenols in cancer prevention. Polyphen. Hum. Health Dis. 2014, 2, 1373–1383. [Google Scholar]
- Qu, Y.; Zhan, Q.; Du, S.; Ding, Y.; Fang, B.; Du, W.; Wu, Q.; Yu, H.; Li, L.; Huang, W. Catalysis-based specific detection and inhibition of tyrosinase and their application. J. Pharm. Anal. 2020, 10, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, F.T.; Garcia-Blanco, Y.; Germer, E.M.; Franco, A.T. The pH influence on the Carbopol® solutions stabilization by addition of NaOH. In Proceedings of the 26th International Congress of Mechanical Engineering (COBEM 2021), Virtual Conference, Brazil, 22–26 November 2021. [Google Scholar]
- Varges, P.R.; Costa, C.R.; Fonseca, B.M.; Naccache, M.F.; De Souza Mendes, P.R. Rheological characterization of carbopol® dispersions in water and in water/glycerol solutions. Fluids 2019, 4, 3. [Google Scholar] [CrossRef]
- Warda, J.; Brückle, I.; Bezúr, A.; Kushel, D.A. Analysis of agarose, carbopol, and laponite gel poultices in paper conservation. J. Am. Inst. Conserv. 2007, 46, 263–279. [Google Scholar] [CrossRef]
- Mamari, H.H.A. Phenolic compounds: Classification, chemistry, and updated techniques of analysis and synthesis. In Biochemistry, 1st ed.; Badria, F.A., Blumenberg, M., Eds.; IntechOpen: London, UK, 2021; Volume 1, pp. 73–94. [Google Scholar]
- Girsang, E.; Lister, I.N.E.; Ginting, C.N.; Bethasari, M.; Amalia, A.; Widowati, W. Comparison of antiaging and antioxidant activities of protocatechuic and ferulic acids. Mol. Cell. Biomed. Sci. 2020, 4, 68. [Google Scholar] [CrossRef]
- Maruyama, H.; Kawakami, F.; Lwin, T.T.; Imai, M.; Shamsa, F. Biochemical characterization of ferulic acid and caffeic acid which effectively inhibit melanin synthesis via different mechanisms in B16 melanoma cells. Biol. Pharm. Bull. 2018, 41, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Taherkhani, N.; Gheibi, N. Inhibitory effects of quercetin and kaempferol as two propolis derived flavonoids on tyrosinase enzyme. Biotech Health Sci. 2014, 1, e22242. [Google Scholar] [CrossRef]
- Hecker, A.; Schellnegger, M.; Hofmann, E.; Luze, H.; Nischwitz, S.P.; Kamolz, L.; Kotzbeck, P. The impact of resveratrol on skin wound healing, scarring, and aging. Int. Wound J. 2021, 19, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Y.; Lin, Y.; Hu, H.; Liao, H. Resveratrol inhibits alpha-melanocyte-stimulating hormone signaling, viability, and invasiveness in melanoma cells. Evid. Based Complement. Altern. Med. 2013, 2013, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Thuy, P.T.; Quan, P.M.; Duc, D.X.; Son, N.T. The antioxidative potential of procyanidin B1: DFT (density functional theory) and docking approaches. J. Mol. Model. 2022, 28, 356. [Google Scholar] [CrossRef] [PubMed]
- Truong, X.; Park, S.; Lee, Y.S.; Jeong, H.Y.; Moon, J.; Jeon, T. Protocatechuic acid from pear inhibits melanogenesis in melanoma cells. Int. J. Mol. Sci. 2017, 18, 1809. [Google Scholar] [CrossRef] [PubMed]
- Khalid, K.A.; Nawi, A.F.M.; Zulkifli, N.; Barkat, M.A.; Hadi, H.A. Aging and wound healing of the skin: A review of clinical and pathophysiological hallmarks. Life 2022, 12, 2142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Duan, E. Fighting against skin aging: The way from bench to bedside. Cell Transpl. 2018, 27, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Roux, J.; Horton, L.; Babadjouni, A.; Kincaid, C.M.; Mesinkovska, N.A. Ferulic acid use for skin applications: A systematic review. J Clin Aesthet Dermatol. 2025, 1, 38–42. [Google Scholar]
- Xu, Q.; Fu, Q.; Li, Z.; Liu, H.; Wang, Y.; Lin, X.; He, R.; Zhang, X.; Ju, Z.; Campisi, J.; et al. The flavonoid procyanidin C1 has senotherapeutic activity and increases lifespan in mice. Nat. Metab. 2021, 3, 1706–1726. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Kim, H.P. Inhibition of mammalian collagenase, matrix metalloproteinase-1, by naturally-occurring flavonoids. Planta Med. 2007, 12, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Chaikul, P.; Kanlayavattanakul, M.; Somkumnerd, J.; Lourith, N. Phyllanthus emblica L. (amla) branch: A safe and effective ingredient against skin aging. J. Tradit. Complement. Med. 2021, 11, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Lee, J.P.; Son, D.; Park, D.; Jung, E. Anti-skin-aging activity of a standardized extract from panax ginseng leaves in vitro and in human volunteer. Cosmetics 2017, 4, 18. [Google Scholar] [CrossRef]



| Phase | Ingredient | INCI Name | Amount (% w/w) | Function | |||
|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | ||||
| A | Deionized Water | Water | 81.7 | 81.64 | 81.5 | 80.3 | Solvent |
| Disodium EDTA | Disodium EDTA | 0.1 | 0.1 | 0.1 | 0.1 | Chelating Agent | |
| Carbopol Ultrez 21 | Acrylates/C10-30 Alkyl Acrylate Crosspolymer | 0.5 | 0.5 | 0.5 | 0.5 | Thickening Agent | |
| Glycerin | Glycerin | 1.0 | 1.0 | 1.0 | 1.0 | Humectant | |
| B | PEG-40 Hydrogenated Castor Oil | PEG-40 Hydrogenated Castor Oil | 2.5 | 2.5 | 2.5 | 2.5 | Emulsifier |
| Dimethicone | Dimethicone | 1.0 | 1.0 | 1.0 | 1.0 | Sensory Modifier | |
| Isononyl Isononanoate | Isononyl Isononanoate | 4.0 | 4.0 | 4.0 | 4.0 | Emollient | |
| Caprylic/Capric Triglyceride | Caprylic/Capric Triglyceride | 4.0 | 4.0 | 4.0 | 4.0 | Emollient | |
| Silk Lotion Maker | Bis-PEG/PPG-16/16 PEG/PPG16/16 Dimethicone | 1.0 | 1.0 | 1.0 | 1.0 | Sensory Modifier, Co-emulsifier | |
| C | Propylene Glycol | Propylene Glycol | 3.0 | 3.0 | 3.0 | 3.0 | Solvent, Humectant |
| Cocoa Pod Husk Extract | Theobroma Cacao Husk Extract | - | 0.01 | 0.1 | 1.0 | Active | |
| D | Phenoxyethanol (and) Ethyhexylglycerin | Phenoxyethanol (and) Ethyhexylglycerin | 1.0 | 1.0 | 1.0 | 1.0 | Preservative |
| E | Triethanolamine | Triethanolamine | 0.2 | 0.25 | 0.3 | 0.6 | pH Adjuster |
| Samples | TPC (mg GAE/g Extract) | TFC (mg QE/g Extract) |
|---|---|---|
| CE | 157.491 ± 7.941 a | 2.22 ± 0.20 a |
| CEA | 170.977 ± 7.412 b | 3.91 ± 0.27 b |
| Sample | DPPH Assay (IC50, μg/mL) | FRAP Assay (mg AAE/g Extract) | Anti-Glycation Activity (IC50, µg/mL) | Tyrosinase Inhibitory Activity (IC50, mg/mL) | Anti-Collagenase Activity (IC50, mg/mL) |
|---|---|---|---|---|---|
| CE | 6.91 ± 0.14 a | 173.44 ± 4.06 b | ND | ND | ND |
| CEA | 5.83 ± 0.11 b | 234.17 ± 4.01 a | 66.96 ± 0.57 a | 9.51 ± 0.01 a | 0.43 ± 0.01 a |
| Ascorbic acid | 1.48 ± 0.06 c | ND | ND | ND | ND |
| Quercetin | ND | ND | 3.87 ± 0.19 b | ND | ND |
| Kojic acid | ND | ND | ND | 0.01 ± 0.00 b | ND |
| EGCG | ND | ND | ND | ND | 0.07 ± 0.01 b |
| Sample | Concentration (µg/mL) | % of NO Inhibition | Sample (IC50, µg/mL) |
|---|---|---|---|
| CEA | 6.25 | 20.42 ± 1.34 | 62.68 |
| 12.5 | 31.55 ± 3.41 | ||
| 25 | 39.47 ± 2.78 | ||
| 50 | 51.43 ± 3.81 | ||
| 100 | 88.41 ± 0.05 | ||
| 200 | 98.28 ± 1.03 | ||
| Indomethacin | 13.23 |
| Formulation | Parameter | T0 | T1 | T3 | T6 |
|---|---|---|---|---|---|
| F1 | Color | White | White | White | White |
| pH | 5.26 ± 0.01 | 5.28 ± 0.01 | 5.29 ± 0.01 | 5.29 ± 0.01 | |
| Viscosity (cP) | 6564.33 ± 88.95 | 6521.33 ± 37.61 | 6512.00 ± 25.06 | 6507.33 ± 21.50 | |
| F2 | Color | White | White | White | White |
| pH | 5.30 ± 0.01 | 5.28 ± 0.01 | 5.27 ± 0.01 | 5.25 ± 0.00 | |
| Viscosity (cP) | 6530.67 ± 51.79 | 6452.67 ± 25.11 | 6378.33 ± 19.09 * | 6321.33 ± 28.29 * | |
| F3 | Color | Ivory | Ivory | Ivory | Ivory |
| pH | 5.30 ± 0.01 | 5.28 ± 0.01 | 5.27 ± 0.01 | 5.24 ± 0.01 | |
| Viscosity (cP) | 6450.00 ± 43.00 | 6373.67 ± 41.59 * | 6276.00 ± 35.76 * | 6131.33 ± 50.16 * | |
| F4 | Color | Cream | Cream | Cream | Cream |
| pH | 5.33 ± 0.01 | 5.30 ± 0.01 | 5.26 ± 0.01 | 5.24 ± 0.01 | |
| Viscosity (cP) | 6285.05 ± 22.00 | 6252.00 ± 45.20 | 6012.00 ± 15.04 * | 5976.31 ± 25.23 * |
| Formulation | Skin Roughness (SEr) Value | ||||
|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | T4 | |
| F1 | 2.48 ± 0.09 | 2.15 ± 0.10 * | 2.03 ± 0.13 * | 2.00 ± 0.09 * | 1.96 ± 0.10 * |
| F2 | 2.71 ± 0.12 | 2.27 ± 0.10 * | 2.16 ± 0.12 * | 2.22 ± 0.15 * | 2.22 ± 0.18 * |
| F3 | 2.41 ± 0.11 | 2.12 ± 0.09 * | 1.94 ± 0.11 * | 1.85 ± 0.12 * | 1.85 ± 0.14 * |
| F4 | 2.04 ± 0.08 | 1.81 ± 0.10 * | 1.55 ± 0.09 * | 1.50 ± 0.10 * | 1.65 ± 0.11 * |
| Formulation | Skin Wrinkle (SEw) Value | ||||
|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | T4 | |
| F1 | 62.79 ± 3.40 | 54.03 ± 1.84 * | 49.69 ± 2.17 * | 48.31 ± 2.16 * | 48.07 ± 2.06 * |
| F2 | 59.38 ± 2.97 | 52.80 ± 2.44 * | 47.95 ± 2.05 * | 46.18 ± 2.14 * | 49.02 ± 2.46 * |
| F3 | 58.05 ± 2.42 | 51.11 ± 2.39 * | 45.46 ± 2.14 * | 44.04 ± 2.20 * | 45.68 ± 2.15 * |
| F4 | 59.16 ± 2.31 | 49.00 ± 2.09 * | 43.20 ± 1.98 * | 41.83 ± 2.12 * | 46.38 ± 2.35 * |
| Formulation | Skin Smoothness (SEsm) Value | ||||
|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | T4 | |
| F1 | 147.26 ± 6.89 | 145.61 ± 4.46 | 141.77 ± 5.57 | 137.00 ± 5.43 * | 140.67 ± 6.98 |
| F2 | 156.28 ± 6.95 | 165.58 ± 7.58 | 157.71 ± 6.44 | 150.26 ± 5.25 | 162.18 ± 6.96 |
| F3 | 170.35 ± 6.78 | 174.73 ± 7.89 | 163.02 ± 6.80 | 155.92 ± 6.38 * | 163.46 ± 6.47 |
| F4 | 185.54 ± 7.23 | 179.95 ± 7.29 | 162.36 ± 7.18 * | 155.17 ± 6.92 * | 172.40 ± 7.33 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anatachodwanit, A.; Chanpirom, S.; Tree-Udom, T.; Kitthaweesinpoon, S.; Jiamphun, S.; Aryuwat, O.; Tantapakul, C.; Vinardell, M.P.; Sripisut, T. Upcycled Cocoa Pod Husk: A Sustainable Source of Phenol and Polyphenol Ingredients for Skin Hydration, Whitening, and Anti-Aging. Life 2025, 15, 1126. https://doi.org/10.3390/life15071126
Anatachodwanit A, Chanpirom S, Tree-Udom T, Kitthaweesinpoon S, Jiamphun S, Aryuwat O, Tantapakul C, Vinardell MP, Sripisut T. Upcycled Cocoa Pod Husk: A Sustainable Source of Phenol and Polyphenol Ingredients for Skin Hydration, Whitening, and Anti-Aging. Life. 2025; 15(7):1126. https://doi.org/10.3390/life15071126
Chicago/Turabian StyleAnatachodwanit, Aknarin, Setinee Chanpirom, Thapakorn Tree-Udom, Sunsiri Kitthaweesinpoon, Sudarat Jiamphun, Ongon Aryuwat, Cholpisut Tantapakul, Maria Pilar Vinardell, and Tawanun Sripisut. 2025. "Upcycled Cocoa Pod Husk: A Sustainable Source of Phenol and Polyphenol Ingredients for Skin Hydration, Whitening, and Anti-Aging" Life 15, no. 7: 1126. https://doi.org/10.3390/life15071126
APA StyleAnatachodwanit, A., Chanpirom, S., Tree-Udom, T., Kitthaweesinpoon, S., Jiamphun, S., Aryuwat, O., Tantapakul, C., Vinardell, M. P., & Sripisut, T. (2025). Upcycled Cocoa Pod Husk: A Sustainable Source of Phenol and Polyphenol Ingredients for Skin Hydration, Whitening, and Anti-Aging. Life, 15(7), 1126. https://doi.org/10.3390/life15071126










