Comparative Analysis of Functional Adaptations to Vivifrail Intervention Between Higher- and Lower-Fitness Healthy Older Adults
Abstract
1. Introduction
2. Materials and Methods
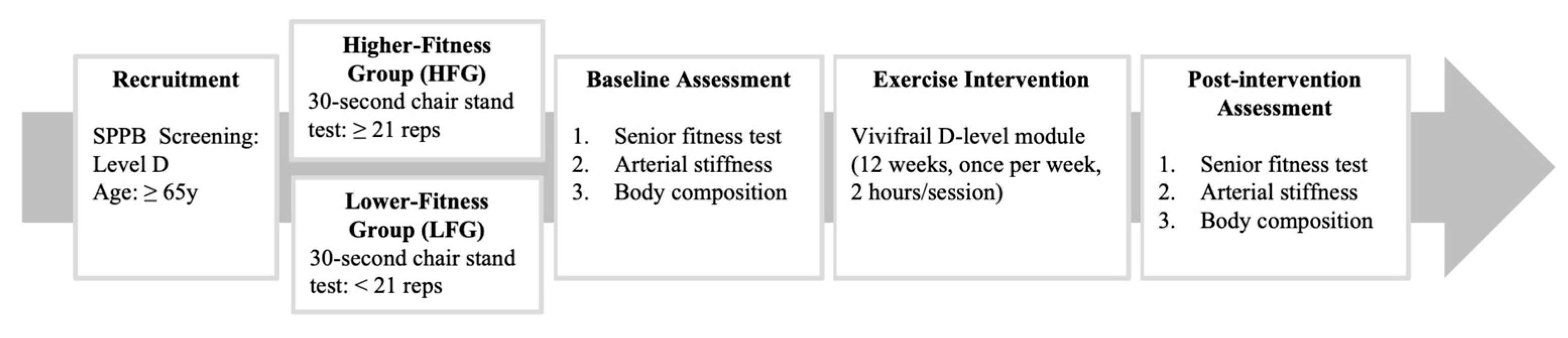
2.1. Study Design
2.2. Participants
2.3. Experimental Procedures
2.4. Exercise Intervention
2.5. Outcome Measures
2.5.1. Senior Fitness Test
2.5.2. Arterial Stiffness
2.5.3. Body Composition
2.5.4. Short Physical Performance Battery
2.5.5. Assessment Standardization and Rater Reliability
2.5.6. Vivifrail Classification
2.6. Data Availability
2.7. Statistical Analysis
3. Results
3.1. Intervention Effects on Vascular, Functional, and Muscular Health
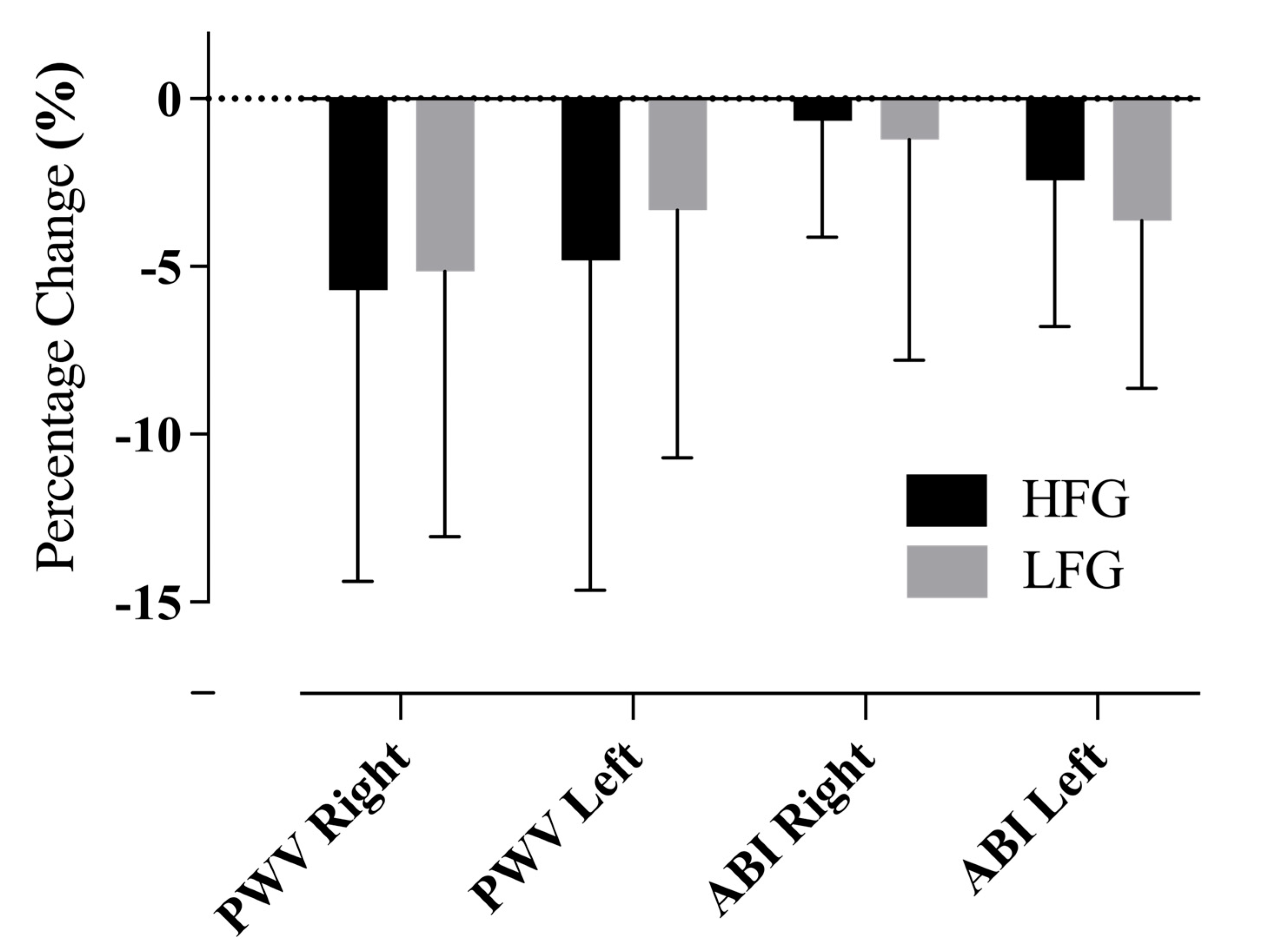
3.2. Improvements in Arterial Stiffness Indices
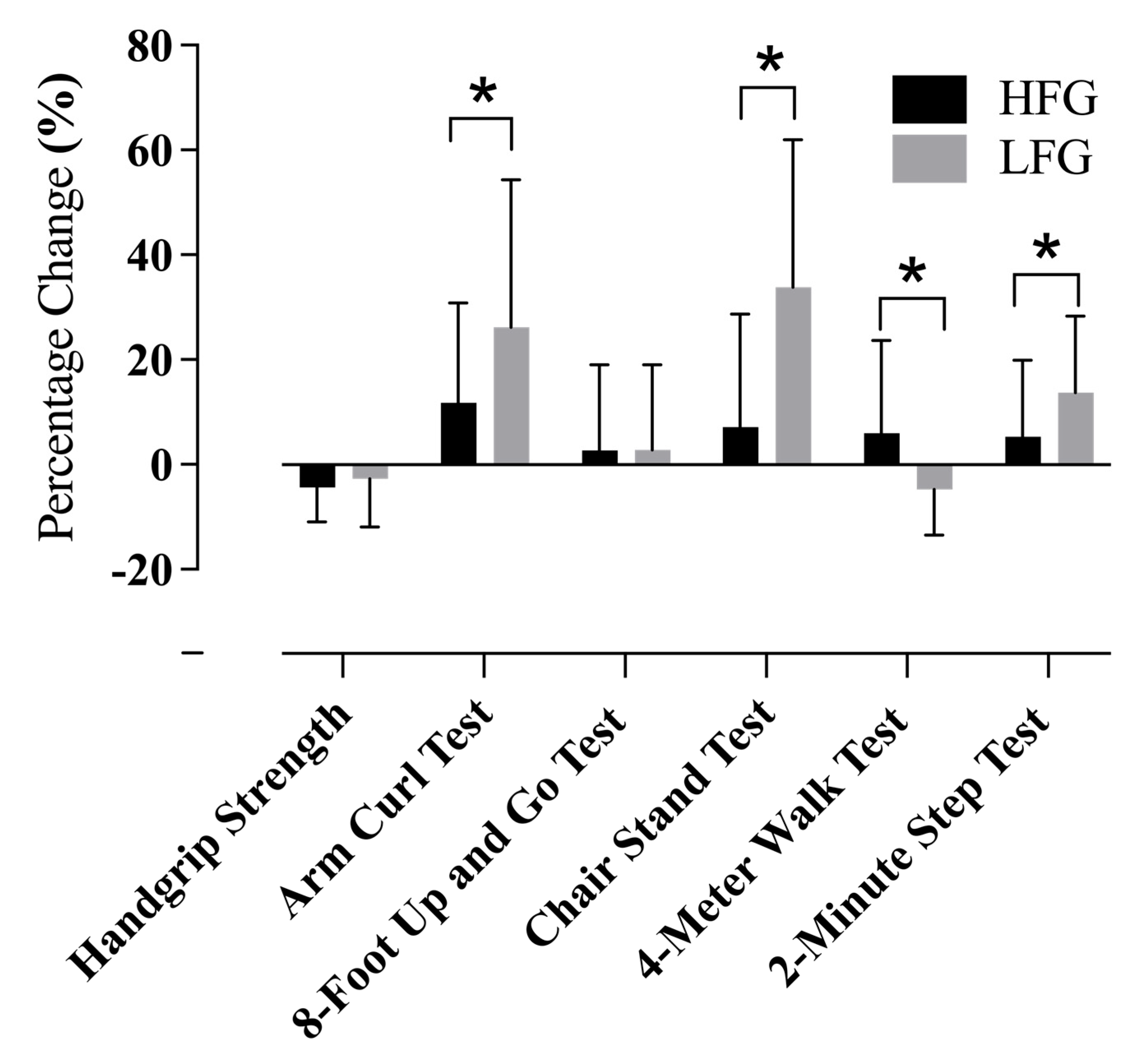
3.3. Functional Fitness Gains Across Groups
3.4. Changes in Body Composition Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Council, N.D. Population Projections for the Republic of China (Taiwan): 2024–2070; National Development Council: Taipei, Taiwan, 2024.
- Vermeiren, S.; Vella-Azzopardi, R.; Beckwee, D.; Habbig, A.K.; Scafoglieri, A.; Jansen, B.; Bautmans, I.; Gerontopole Brussels Study group. Frailty and the Prediction of Negative Health Outcomes: A Meta-Analysis. J. Am. Med. Dir. Assoc. 2016, 17, 1163.e1–1163.e17. [Google Scholar] [CrossRef]
- Ward, D.D.; Wallace, L.M.K.; Rockwood, K. Frailty and Risk of Dementia in Mild Cognitive Impairment Subtypes. Ann. Neurol. 2021, 89, 1221–1225. [Google Scholar] [CrossRef]
- Kim, T.N.; Choi, K.M. Sarcopenia: Definition, epidemiology, and pathophysiology. J. Bone Metab. 2013, 20, 1–10. [Google Scholar] [CrossRef]
- Izquierdo, M. Multicomponent physical exercise program: Vivifrail. Nutr. Hosp. 2019, 36, 50–56. [Google Scholar]
- Casas-Herrero, Á.; de Asteasu, M.L.S.; Antón-Rodrigo, I.; Sánchez-Sánchez, J.L.; Montero-Odasso, M.; Marín-Epelde, I.; Ramón-Espinoza, F.; Zambom-Ferraresi, F.; Petidier-Torregrosa, R.; Elexpuru-Estomba, J.; et al. Effects of Vivifrail multicomponent intervention on functional capacity: A multicentre, randomized controlled trial. J. Cachexia Sarcopenia Muscle 2022, 13, 884–893. [Google Scholar] [CrossRef]
- Monteiro, A.M.; Rodrigues, S.; Matos, S.; Encarnação, S.; Teixeira, J.E.; Barbosa, T.M.; Rodrigues, F.; Forte, P. The Impact of Multicomponent Exercise Protocols Order on the Maximum Voluntary Contraction of Older Women. Appl. Sci. 2023, 13, 8044. [Google Scholar] [CrossRef]
- Ferrari, R.; Fuchs, S.C.; Kruel, L.F.M.; Cadore, E.L.; Alberton, C.L.; Pinto, R.S.; Radaelli, R.; Schoenell, M.; Izquierdo, M.; Tanaka, H.; et al. Effects of Different Concurrent Resistance and Aerobic Training Frequencies on Muscle Power and Muscle Quality in Trained Elderly Men: A Randomized Clinical Trial. Aging Dis. 2016, 7, 697–704. [Google Scholar] [CrossRef] [PubMed]
- García-Hermoso, A.; Cavero-Redondo, I.; Ramírez-Vélez, R.; Ruiz, J.R.; Ortega, F.B.; Lee, D.-C.; Martínez-Vizcaíno, V. Muscular Strength as a Predictor of All-Cause Mortality in an Apparently Healthy Population: A Systematic Review and Meta-Analysis of Data From Approximately 2 Million Men and Women. Arch. Phys. Med. Rehabil. 2018, 99, 2100–2113.e5. [Google Scholar] [CrossRef] [PubMed]
- de Labra, C.; Guimaraes-Pinheiro, C.; Maseda, A.; Lorenzo, T.; Millán-Calenti, J.C. Effects of physical exercise interventions in frail older adults: A systematic review of randomized controlled trials. BMC Geriatr. 2015, 15, 154. [Google Scholar] [CrossRef]
- Cadore, E.L.; Casas-Herrero, A.; Zambom-Ferraresi, F.; Idoate, F.; Millor, N.; Gómez, M.; Rodríguez-Mañas, L.; Izquierdo, M. Multicomponent exercises including muscle power training enhance muscle mass, power output, and functional outcomes in institutionalized frail nonagenarians. Age 2014, 36, 773–785. [Google Scholar] [CrossRef]
- ACSM. Exercise and physical activity for older adults. Med. Sci. Sports Exerc. 1998, 30, 992–1008. [Google Scholar]
- Rikli, R.E.; Jones, C.J. Functional Fitness Normative Scores for Community-Residing Older Adults, Ages 60–94. J. Aging Phys. Act. 1999, 7, 162–181. [Google Scholar] [CrossRef]
- Langhammer, B.; Stanghelle, J. The Senior Fitness Test. J. Physiother. 2015, 61, 163. [Google Scholar] [CrossRef]
- Rikli, R.; Jones, J. Development and validation of a functional fitness test for a community-residing adults. J. Aging Phys. Act. 1999, 7, 129–161. [Google Scholar] [CrossRef]
- Chen, H.T.; Lin, C.H.; Yu, L.H. Normative physical fitness scores for community-dwelling older adults. J. Nurs. Res. 2009, 17, 30–41. [Google Scholar] [CrossRef]
- Chung, P.-K.; Zhao, Y.; Liu, J.-D.; Quach, B. Functional fitness norms for community-dwelling older adults in Hong Kong. Arch. Gerontol. Geriatr. 2016, 65, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Sang, T.; Lv, N.; Dang, A.; Cheng, N.; Zhang, W. Brachial-ankle pulse wave velocity and prognosis in patients with atherosclerotic cardiovascular disease: A systematic review and meta-analysis. Hypertens. Res. 2021, 44, 1175–1185. [Google Scholar] [CrossRef]
- Bilsborough, J.C.; Greenway, K.; Opar, D.; Livingstone, S.; Cordy, J.; Coutts, A.J. The accuracy and precision of DXA for assessing body composition in team sport athletes. J. Sports Sci. 2014, 32, 1821–1828. [Google Scholar] [CrossRef]
- Nana, A.; Slater, G.J.; Hopkins, W.G.; Burke, L.M. Effects of daily activities on dual-energy X-ray absorptiometry measurements of body composition in active people. Med. Sci. Sports Exerc. 2012, 44, 180–189. [Google Scholar] [CrossRef]
- Mangione, K.K.; Craik, R.L.; McCormick, A.A.; Blevins, H.L.; White, M.B.; Sullivan-Marx, E.M.; Tomlinson, J.D. Detectable changes in physical performance measures in elderly African Americans. Phys. Ther. 2010, 90, 921–927. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Ferrucci, L.; Pieper, C.F.; Leveille, S.G.; Markides, K.S.; Ostir, G.V.; Studenski, S.; Berkman, L.F.; Wallace, R.B. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55, M221–M231. [Google Scholar] [CrossRef] [PubMed]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Pavasini, R.; Guralnik, J.; Brown, J.C.; Di Bari, M.; Cesari, M.; Landi, F.; Vaes, B.; Legrand, D.; Verghese, J.; Wang, C.; et al. Short Physical Performance Battery and all-cause mortality: Systematic review and meta-analysis. BMC Med. 2016, 14, 215. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Dinenno, F.A.; Monahan, K.D.; Clevenger, C.M.; DeSouza, C.A.; Seals, D.R. Aging, habitual exercise, and dynamic arterial compliance. Circulation 2000, 102, 1270–1275. [Google Scholar] [CrossRef]
- Leitão, L.; Marocolo, M.; de Souza, H.L.R.; Arriel, R.A.; Vieira, J.G.; Mazini, M.; Figueiredo, T.; Louro, H.; Pereira, A. Multicomponent Exercise Program for Improvement of Functional Capacity and Lipidic Profile of Older Women with High Cholesterol and High Triglycerides. Int. J. Environ. Res. Public Health 2021, 18, 10731. [Google Scholar] [CrossRef]
- Cadore, E.L.; Izquierdo, M.; Teodoro, J.L.; Martínez-Velilla, N.; Zambom-Ferraresi, F.; Moriguchi, E.H.; de Asteasu, M.L.S. Effects of short-term multicomponent exercise intervention on muscle power in hospitalized older patients: A secondary analysis of a randomized clinical trial. J. Cachexia Sarcopenia Muscle 2023, 14, 2959–2968. [Google Scholar] [CrossRef]
- Brañas, F.; Díaz-Álvarez, J.; Fernández-Luna, J.; Vásquez-Brolen, B.D.; García-Molina, R.; Moreno, E.; Ryan, P.; Martínez-Sanz, J.; Luna, L.; Martínez, M.; et al. A 12-week multicomponent exercise program enhances frailty by increasing robustness, improves physical performance, and preserves muscle mass in older adults with HIV: MOVIhNG study. Front. Public Health 2024, 12, 1373910. [Google Scholar] [CrossRef] [PubMed]
- Mirelman, A.; Herman, T.; Brozgol, M.; Dorfman, M.; Sprecher, E.; Schweiger, A.; Giladi, N.; Hausdorff, J.M.; Laks, J. Executive function and falls in older adults: New findings from a five-year prospective study link fall risk to cognition. PLoS ONE 2012, 7, e40297. [Google Scholar] [CrossRef]
- Eggenberger, P.; Wolf, M.; Schumann, M.; de Bruin, E.D. Exergame and Balance Training Modulate Prefrontal Brain Activity during Walking and Enhance Executive Function in Older Adults. Front. Aging Neurosci. 2016, 8, 66. [Google Scholar] [CrossRef]
- Silsupadol, P.; Shumway-Cook, A.; Lugade, V.; van Donkelaar, P.; Chou, L.-S.; Mayr, U.; Woollacott, M.H. Effects of single-task versus dual-task training on balance performance in older adults: A double-blind, randomized controlled trial. Arch. Phys. Med. Rehabil. 2009, 90, 381–387. [Google Scholar] [CrossRef]
- Baek, J.-E.; Hyeon, S.-J.; Kim, M.; Cho, H.-Y.; Hahm, S.-C. Effects of dual-task resistance exercise on cognition, mood, depression, functional fitness, and activities of daily living in older adults with cognitive impairment: A single-blinded, randomized controlled trial. BMC Geriatr. 2024, 24, 369. [Google Scholar] [CrossRef] [PubMed]

| HFG—Pre (n = 22) | HFG—Post (n = 22) | LFG—Pre (n = 19) | LFG—Post (n = 19) | |
|---|---|---|---|---|
| Arterial Stiffness | ||||
| Pulse Wave Velocity—Right | 1824.8 ± 414.5 | 1709.5 ± 343.5 | 1731.8 ± 281 | 1651.2 ± 265.2 |
| Pulse Wave Velocity—Left | 1817.3 ± 360.8 | 1681.2 ± 323.6 | 1716.8 ± 309.4 | 1649.4 ± 264.1 |
| Ankle–Brachial Index—Right | 1.14 ± 0.06 | 1.16 ± 0.08 | 1.12 ± 0.07 | 1.11 ± 0.07 |
| Ankle–Brachial Index—Left | 1.16 ± 0.08 | 1.14 ± 0.07 | 1.14 ± 0.05 | 1.10 ± 0.06 * |
| Functional Fitness | ||||
| Handgrip Strength (kg) | 25.7 ± 6.3 | 23.9 ± 6.1 | 23.1 ± 7.6 | 22.2 ± 6.4 |
| Arm Curl Test (repetitions) | 21.2 ± 3.7 | 23.3 ± 4.1 * | 17.5 ± 3.8 | 21.6 ± 4.5 * |
| 8-Foot Up-and-Go Test (seconds) | 5.7 ± 1.1 | 5.7 ± 0.8 | 6.2 ± 1.2 | 6.3 ± 1.1 |
| Chair Stand Test (repetitions) | 21.7 ± 3.4 | 22.8 ± 4.7 | 14.6 ± 2.2 | 19.5 ± 4.6 * |
| 4-Meter Walk Test (seconds) | 2.7 ± 0.5 | 2.9 ± 0.7 | 3.0 ± 0.4 | 2.9 ± 0.6 |
| 2-Minute Step Test (steps) | 101.8 ± 16.2 | 105 ± 13.2 | 92.3 ± 10.6 | 103 ± 11.3 * |
| Body Composition | ||||
| Skeletal Muscle Index | 6.3 ± 0.7 | 6.3 ± 0.6 | 5.9 ± 0.6 | 6.1 ± 0.6 |
| Muscle Mass of Upper Limbs (g) | 3534.9 ± 677.0 | 3477.7 ± 584.1 | 3352.6 ± 565.2 | 3395.2 ± 553.2 |
| Muscle Mass of Lower Limbs (g) | 11,966 ± 1519.2 | 11,951.7 ± 1536.7 | 11,515.6 ± 1541.0 | 11,721.9 ± 1511.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, F.-R.; Li, Y.-J.; Tang, C.-Y.; Lai, C.-C.; Tai, H.-L.; Fu, S.-K. Comparative Analysis of Functional Adaptations to Vivifrail Intervention Between Higher- and Lower-Fitness Healthy Older Adults. Life 2025, 15, 988. https://doi.org/10.3390/life15070988
Lee F-R, Li Y-J, Tang C-Y, Lai C-C, Tai H-L, Fu S-K. Comparative Analysis of Functional Adaptations to Vivifrail Intervention Between Higher- and Lower-Fitness Healthy Older Adults. Life. 2025; 15(7):988. https://doi.org/10.3390/life15070988
Chicago/Turabian StyleLee, Fang-Ru, Yu-Jui Li, Chia-Yu Tang, Chang-Chi Lai, Hsia-Ling Tai, and Szu-Kai Fu. 2025. "Comparative Analysis of Functional Adaptations to Vivifrail Intervention Between Higher- and Lower-Fitness Healthy Older Adults" Life 15, no. 7: 988. https://doi.org/10.3390/life15070988
APA StyleLee, F.-R., Li, Y.-J., Tang, C.-Y., Lai, C.-C., Tai, H.-L., & Fu, S.-K. (2025). Comparative Analysis of Functional Adaptations to Vivifrail Intervention Between Higher- and Lower-Fitness Healthy Older Adults. Life, 15(7), 988. https://doi.org/10.3390/life15070988







