Exercise as Modulator of Brain-Derived Neurotrophic Factor (BDNF) in Children: A Systematic Review of Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Approach to the Problem
2.2. Information Sources
2.3. Search Strategy
(preschool* OR kindergarten OR child* OR young OR childhood OR school) AND (exercise OR movement OR activity OR sport OR fitness OR aerobic OR training OR performance) AND (BDNF OR “brain-derived neurotrophic factor”) AND (“randomized controlled trial”)
2.4. Eligibility Criteria
2.5. Data Extraction
2.6. Assessment of Study Methodology
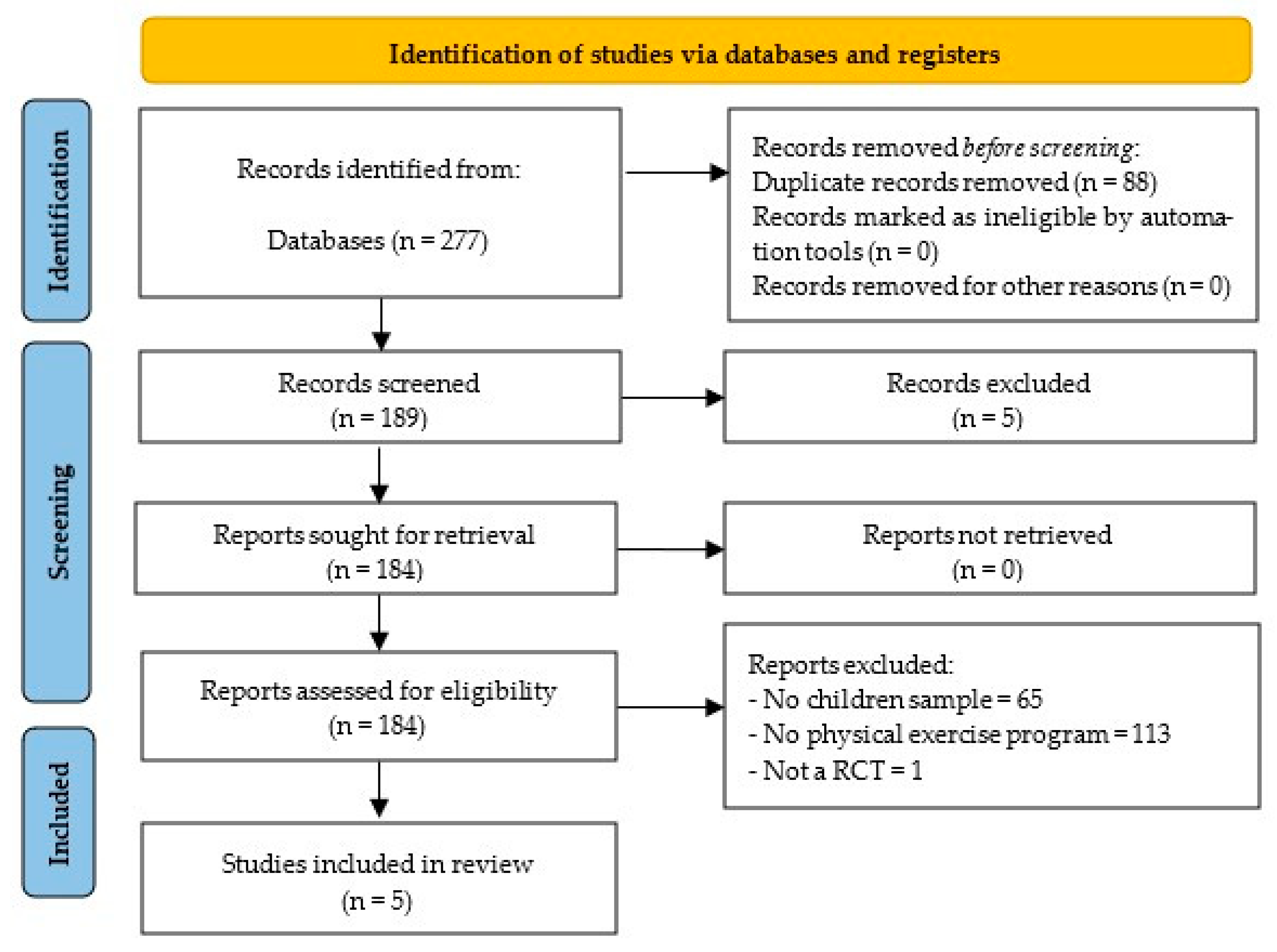
3. Results
3.1. Methodological Quality
3.2. Study Characteristics
3.3. Main Results
4. Discussion
4.1. Exercise Modality and BDNF Response
4.2. Population-Specific Responses
4.3. Frequency and Duration of Intervention
4.4. Biological Matrix and Measurement Considerations
4.5. Mechanism Considerations
4.6. Practical Applications and Clinical Implications
5. Limitations and Future Research Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Numakawa, T.; Kajihara, R. Involvement of Brain-Derived Neurotrophic Factor Signaling in the Pathogenesis of Stress-Related Brain Diseases. Front. Mol. Neurosci. 2023, 16, 1247422. [Google Scholar] [CrossRef] [PubMed]
- Neeper, S.A.; Gómez-Pinilla, F.; Choi, J.; Cotman, C. Exercise and Brain Neurotrophins. Nature 1995, 373, 109. [Google Scholar] [CrossRef] [PubMed]
- Iughetti, L.; Casarosa, E.; Predieri, B.; Patianna, V.; Luisi, S. Plasma Brain-Derived Neurotrophic Factor Concentrations in Children and Adolescents. Neuropeptides 2011, 45, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Mizuno, M.; Nabeshima, T. Role for Brain-Derived Neurotrophic Factor in Learning and Memory. Life Sci. 2002, 70, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.G.; Pratesi, R.; Paz, G.S.C.; Dos Santos, M.A.A.L.; Uenishi, R.H.; Nakano, E.Y.; Gandolfi, L.; Pratesi, C.B. Assessment of BDNF Serum Levels as a Diagnostic Marker in Children with Autism Spectrum Disorder. Sci. Rep. 2020, 10, 17348. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-J. Attention-Deficit Hyperactivity Disorder May Be Associated with Decreased Central Brain-Derived Neurotrophic Factor Activity: Clinical and Therapeutic Implications. Med. Hypotheses 2007, 68, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Autry, A.E. Function of Brain-Derived Neurotrophic Factor in the Hypothalamus: Implications for Depression Pathology. Front. Mol. Neurosci. 2022, 15, 1028223. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Dhapola, R.; Reddy, D.H. Apoptosis in Alzheimer’s Disease: Insight into the Signaling Pathways and Therapeutic Avenues. Apoptosis 2023, 28, 943–957. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.J.; Tschakovsky, M.E. Exercise and Circulating BDNF: Mechanisms of Release and Implications for the Design of Exercise Interventions. Appl. Physiol. Nutr. Metab. 2018, 43, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Gravesteijn, E.; Mensink, R.P.; Plat, J. Effects of Nutritional Interventions on BDNF Concentrations in Humans: A Systematic Review. Nutr. Neurosci. 2022, 25, 1425–1436. [Google Scholar] [CrossRef] [PubMed]
- Giese, M.; Unternaehrer, E.; Brand, S.; Calabrese, P.; Holsboer-Trachsler, E.; Eckert, A. The Interplay of Stress and Sleep Impacts BDNF Level. PLoS ONE 2013, 8, e76050. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization WHO Guidelines on Physical Activity and Sedentary Behaviour; World Health Organization: Geneva, Switzerland, 2020; ISBN 978-92-4-001512-8.
- Fernández-Rodríguez, R.; Álvarez-Bueno, C.; Martínez-Ortega, I.A.; Martínez-Vizcaíno, V.; Mesas, A.E.; Notario-Pacheco, B. Immediate Effect of High-Intensity Exercise on Brain-Derived Neurotrophic Factor in Healthy Young Adults: A Systematic Review and Meta-Analysis. J. Sport Health Sci. 2022, 11, 367–375. [Google Scholar] [CrossRef] [PubMed]
- García-Suárez, P.C.; Rentería, I.; Plaisance, E.P.; Moncada-Jiménez, J.; Jiménez-Maldonado, A. The Effects of Interval Training on Peripheral Brain Derived Neurotrophic Factor (BDNF) in Young Adults: A Systematic Review and Meta-Analysis. Sci. Rep. 2021, 11, 8937. [Google Scholar] [CrossRef] [PubMed]
- de Azevedo, K.P.M.; de Oliveira, V.H.; de Medeiros, G.C.B.S.; de Sousa Mata, Á.N.; García, D.Á.; Martínez, D.G.; Leitão, J.C.; Knackfuss, M.I.; Piuvezam, G. The Effects of Exercise on BDNF Levels in Adolescents: A Systematic Review with Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 6056. [Google Scholar] [CrossRef] [PubMed]
- de Menezes-Junior, F.J.; Jesus, Í.C.; Brand, C.; Mota, J.; Leite, N. Physical Exercise and Brain-Derived Neurotrophic Factor Concentration in Children and Adolescents: A Systematic Review With Meta-Analysis. Pediatr. Exerc. Sci. 2022, 34, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Rico-González, M.; Pino-Ortega, J.; Clemente, F.M.; Los Arcos, A. Guidelines for Performing Systematic Reviews in Sports Science. Biol. Sport 2022, 39, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Cashin, A.G.; McAuley, J.H. Clinimetrics: Physiotherapy Evidence Database (PEDro) Scale. J. Physiother. 2020, 66, 59. [Google Scholar] [CrossRef] [PubMed]
- Ghafori, R.; Heirani, A.; Aghadsi, M.T. Effect of Motor Exercises on Serum Level of Brain-Derived Neurotrophic Factor and Executive Function in Children with Dysgraphia. J. Kermanshah Univ. Med. Sci. 2018, 22, e79187. [Google Scholar] [CrossRef]
- Plaza-Florido, A.; Esteban-Cornejo, I.; Mora-Gonzalez, J.; Torres-Lopez, L.V.; Osuna-Prieto, F.J.; Gil-Cosano, J.J.; Radom-Aizik, S.; Labayen, I.; Ruiz, J.R.; Altmäe, S.; et al. Gene–Exercise Interaction on Brain Health in Children with Overweight/Obesity: The ActiveBrains Randomized Controlled Trial. J. Appl. Physiol. 2023, 135, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ayllon, M.; Plaza-Florido, A.; Mendez-Gutierrez, A.; Altmäe, S.; Solis-Urra, P.; Aguilera, C.M.; Catena, A.; Ortega, F.B.; Esteban-Cornejo, I. The Effects of a 20-Week Exercise Program on Blood-Circulating Biomarkers Related to Brain Health in Overweight or Obese Children: The ActiveBrains Project. J. Sport Health Sci. 2023, 12, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-T.; Song, Y.-E.; Kang, E.-B.; Cho, J.-Y.; Kim, B.-W.; Kim, C.-H.; Kim, H.-T.; Song, Y.-E.; Kang, E.-B.; Cho, J.-Y.; et al. The Effects of Combined Exercise on Basic Physical Fitness, Neurotrophic Factors and Working Memory of Elementary Students. Exerc. Sci. 2015, 24, 243–251. [Google Scholar] [CrossRef]
- Cho, S.-Y.; So, W.-Y.; Roh, H.-T. The Effects of Taekwondo Training on Peripheral Neuroplasticity-Related Growth Factors, Cerebral Blood Flow Velocity, and Cognitive Functions in Healthy Children: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2017, 14, 454. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Wang, Z.; Zhu, L.; Huang, G.; Li, B.; Chen, C.; Huang, J.; Ma, F.; Liu, T.C. Effects of Different Physical Activities on Brain-Derived Neurotrophic Factor: A Systematic Review and Bayesian Network Meta-Analysis. Front. Aging Neurosci. 2022, 14, 981002. [Google Scholar] [CrossRef] [PubMed]
- Roh, H.-T.; Cho, S.-Y.; So, W.-Y. Effects of Regular Taekwondo Intervention on Oxidative Stress Biomarkers and Myokines in Overweight and Obese Adolescents. Int. J. Environ. Res. Public Health 2020, 17, 2505. [Google Scholar] [CrossRef] [PubMed]
- Estébanez, B.; Huang, C.-J.; Rivera-Viloria, M.; González-Gallego, J.; Cuevas, M.J. Exercise Outcomes in Childhood Obesity-Related Inflammation and Oxidative Status. Front. Nutr. 2022, 9, 886291. [Google Scholar] [CrossRef] [PubMed]
- Khalafi, M.; Symonds, M.E.; Faramarzi, M.; Sharifmoradi, K.; Maleki, A.H.; Rosenkranz, S.K. The Effects of Exercise Training on Inflammatory Markers in Children and Adolescents: A Systematic Review and Meta-Analysis. Physiol. Behav. 2024, 278, 114524. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, H.İ.; Silva, A.F.; Ramirez-Campillo, R.; Murawska-Ciałowicz, E. Exploring the Effect of Acute and Regular Physical Exercise on Circulating Brain-Derived Neurotrophic Factor Levels in Individuals with Obesity: A Comprehensive Systematic Review and Meta-Analysis. Biology 2024, 13, 323. [Google Scholar] [CrossRef] [PubMed]
- Dinoff, A.; Herrmann, N.; Swardfager, W.; Lanctôt, K.L. The Effect of Acute Exercise on Blood Concentrations of Brain-Derived Neurotrophic Factor in Healthy Adults: A Meta-Analysis. Eur. J. Neurosci. 2017, 46, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-H.; Zhou, H.-H.; Luo, Q.; Cui, S. The Effect of Physical Exercise on Circulating Brain-Derived Neurotrophic Factor in Healthy Subjects: A Meta-Analysis of Randomized Controlled Trials. Brain Behav. 2022, 12, e2544. [Google Scholar] [CrossRef] [PubMed]
- Andreu-Caravaca, L.; Ramos-Campo, D.J.; Moncada-Jiménez, J.; Abellán-Aynés, O.; Rubio-Arias, J.Á. Immediate and Short-Term Effect of Physical Exercise on BDNF in Multiple Sclerosis Patients: A Systematic Review and Meta-Analysis. Sci. Rep. 2025, 15, 19696. [Google Scholar] [CrossRef] [PubMed]
- Gejl, A.K.; Enevold, C.; Bugge, A.; Andersen, M.S.; Nielsen, C.H.; Andersen, L.B. Associations between Serum and Plasma Brain-Derived Neurotrophic Factor and Influence of Storage Time and Centrifugation Strategy. Sci. Rep. 2019, 9, 9655. [Google Scholar] [CrossRef] [PubMed]
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; El Hayek, L.; Abou Haidar, E.; Stringer, T.; Ulja, D.; Karuppagounder, S.S.; Holson, E.B.; Ratan, R.R.; et al. Exercise Promotes the Expression of Brain Derived Neurotrophic Factor (BDNF) through the Action of the Ketone Body β-Hydroxybutyrate. eLife 2016, 5, e15092. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.J.; Scribbans, T.D.; Bentley, R.F.; Kellawan, J.M.; Gurd, B.; Tschakovsky, M.E. Neurotrophic Growth Factor Responses to Lower Body Resistance Training in Older Adults. Appl. Physiol. Nutr. Metab. 2016, 41, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Yarrow, J.F.; White, L.J.; McCoy, S.C.; Borst, S.E. Training Augments Resistance Exercise Induced Elevation of Circulating Brain Derived Neurotrophic Factor (BDNF). Neurosci. Lett. 2010, 479, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Ferris, L.T.; Williams, J.S.; Shen, C.-L. The Effect of Acute Exercise on Serum Brain-Derived Neurotrophic Factor Levels and Cognitive Function. Med. Sci. Sports Exerc. 2007, 39, 728–734. [Google Scholar] [CrossRef] [PubMed]
| Item | Inclusion | Exclusion | Search Coherence |
|---|---|---|---|
| Population | School-aged children | Children out of school age Children under medical treatment | preschool* OR kindergarten OR child* OR young OR childhood OR school |
| Intervention or Exposure | Children doing exercise or physical activity | Population from other age range. Children not doing exercise or physical activity. Interventions where other factor is implemented (e.g., supplementation, transcranial stimulation) Study protocols Adolescents receiving pharmacological treatments | exercise OR movement OR activity OR sport OR fitness OR aerobic OR training OR performance |
| Comparation | - | - | |
| Outcome[s] | Outcomes related to brain-derived neurotrophic factor | Outcomes not related to brain-derived neurotrophic factor | BDNF OR “brain-derived neurotrophic factor” |
| Design | Randomized controlled trial | Non-randomized controlled trials | “randomized controlled trial” |
| Other criteria | Peer-reviewed full-text studies published in original journal articles | Non-peer reviewed journal articles. Non-original full-text studies (conference papers…). |
| Ghafori et al. [20] | Plaza-Florido et al. [21] | Rodriguez-Ayllon et al. [22] | Kim et al. [23] | Cho et al. [24] | |
|---|---|---|---|---|---|
| Subjects were randomly allocated to groups. | 1 | 1 | 1 | 1 | 1 |
| Allocation was concealed. | 0 | 0 | 1 | 0 | 0 |
| The groups were similar at baseline regarding the most important prognostic indicators. | 1 | 1 | 1 | 1 | 1 |
| There was blinding of all subjects. | 0 | 0 | 0 | 0 | 0 |
| There was blinding of all therapists who administered the therapy. | 0 | 0 | 0 | 0 | 0 |
| There was blinding of all assessors who measured at least one key outcome. | 0 | 0 | 0 | 0 | 0 |
| Measures of at least one key outcome were obtained from more than 85% of the subjects initially allocated to groups. | 0 | 0 | 0 | 1 | 1 |
| All subjects for whom outcome measures were available received the treatment or control condition as allocated or, where this was not the case, data for at least one key outcome was analyzed by “intention to treat”. | 0 | 0 | 0 | 1 | 1 |
| The results of between-group statistical comparisons are reported for at least one key outcome. | 1 | 1 | 1 | 1 | 1 |
| The study provides both point measures and measures of variability for at least one key outcome. | 1 | 1 | 1 | 1 | 1 |
| SCORE | 4 | 4 | 5 | 5 | 6 |
| Fair | Fair | Fair | Fair | Good |
| Ref. | Participants | BDNF Registration | Other Criteria to Consider | Exercise Information | Results | Conclusions |
|---|---|---|---|---|---|---|
| Ghafori et al. [20] | N = 40 males (10.4 ± 3.5 years) Country: Iran With diagnosed dysgraphia Mean intelligence quotient: 78.81 ± 3.78 | Biological matrix: Serum ELISA method (Quantikine® R&D Systems, kit #DBD00). Fasting sample collection, 48 h before and after the procedure. Venous extraction (5 cc), with processing by centrifugation at 3000 rpm for 10 min at 4 °C. | Pre-screening by interview, child symptom inventory (parent/teacher version), RCPM and apraxia index by block test. Exclusion of other relevant psychological or motor disorders. | IG: 12 weeks (3 sessions/week of 45 min each session) Three types of exercise (each for 10–15 min). Exercises comprising fine (e.g., cutting and sticking colored paper, Frostig exercises, targeted exercises) and gross motor exercises (e.g., throwing various types of balls; catching; dribbling; passing; walking on a spiral path while bouncing the ball off the ground) Intensity: Exercises were arranged from easy to difficult CG: NR | IG showed a significant increase in BDNF *** (from 542.47 ± 5.08 to 642.80 ± 21.41 pg/mL). Significant increase in BDNF serum levels in IG compared with CG after intervention. Significant reduction in perseverative errors (18.81 → 15.50) and total errors (35.56 → 27.13) in IG. Negative correlation between BDNF and errors (r = −0.445 for perseverative errors; r = −0.461 for total errors). Fifty-two percent of the variance in perseverative errors and 39% in total errors were explained by BDNF level. | Twelve weeks of motor exercise significantly improve both serum BDNF level and executive function in children with dysgraphia. The increase in BDNF could be a neurophysiological mechanism associated with cognitive improvements. The usefulness of structured motor interventions in clinical and school contexts for this population with specific educational needs is highlighted. |
| Plaza-Florido et al. [21] | N = 99 (57 males and 42 females; 10.03 ± 1.51 years) Country: Spain ActiveBrains clinical trial participants Overweight or obese | Biological matrix: Serum ELISA method (kit Human BDNF Quantikine®, R&D Systems, Cat# DBD00). | The BDNF Val66Met (rs6265) polymorphism was analyzed using DNA extracted from peripheral blood (Puregene Kit, QIAGEN). Genotype × intervention interactions on BDNF levels were analyzed. | IG: 20 weeks (3–5 sessions/week of 90 min each session) Concurrent exercise: - Aerobic: 60 min - Strength: 30 min Average intensity of 38 min per session at >80% HRmax. CG: Continued their usual routines | No significant differences in serum BDNF levels in the IG (1552.26 ± 387.87 → 1552.31 ± 472.26 pg/mL). No significant interactions between Val66Met genotype and exercise response were observed in BDNF (p = 0.281) No effects of exercise on BDNF were reported when subgroups were analyzed by sex, age, or pubertal maturation. | The 20-week multicomponent exercise program did not produce changes in serum BDNF levels in overweight/obese children. These results suggest that, in this pediatric population, BDNF may not be sensitive to interventions of this type or that higher doses or longer durations are required to generate measurable effects. |
| Rodriguez-Ayllon et al. [22] | N = 81 (48 males and 33 females; 10.12 ± 1.11 years) Country: Spain ActiveBrains clinical trial participants Overweight or obese | Biological matrix: Plasma. XMap method (Luminex) with EMD Milliplex Map Kit panel (Millipore). | Other candidate biomarkers: β-hydroxybutyrate (BHB) by colorimetry; Cathepsin B (CTSB), FGF21, and Kynurenine by ELISA; sVCAM-1 by XMap. Exploratory biomarkers: 92 neurological proteins by proximity extension assay (Olink Bioscience). | IG: 20 weeks (3–5 sessions/week of 90 min each session) Concurrent exercise: - Aerobic: 60 min - Strength: 30 min Average intensity of 38 min per session at >80% HRmax. CG: Continued their usual routines | There was no significant effect (p > 0.05) on BDNF or BHB, CTSB, kynurenine, FGF21, or sVCAM-1. Significant reduction in 6 neurological proteins (CPA2, KYNU, LAIR2, MSR1, PLXNB3, SCARB2), although only MSR1 maintained significance after correction for FDR ***. There was no protein mediator between exercise and brain health outcomes. | No chronic effects of exercise on candidate biomarkers related to brain health were found. However, a consistent reduction in MSR1 was observed, potentially relevant as a new biomarker in future research. Further investigation of the effects of chronic exercise on MSR1 and other markers, as well as its relationship to brain health in overweight or obese pediatric populations is recommended. |
| Cho et al. [24] | N = 30 (18 males and 12 females; 11.20 ± 0.77 years) Country: South Korea Without pathologies | Biological matrix: Serum ELISA method (BDNF Quantikine Kit, Cat# DBD00, R&D Systems). Other biochemical variables: VEGF and IGF-1 also analyzed by ELISA. | Cognitive function parameters (Stroop test) and cerebral blood flow velocity (CBF) were evaluated by transcranial Doppler. VO2max was measured with modified Balke protocol. | IG: 16 weeks (5 sessions/week of 60 min each session) Content of the sessions: - General physical training (shuttle run, Burpee, vertical jump, etc.). - Basic Taekwondo movements - Poomsae (Taegeuk forms 1–8) - Kicking and displacement techniques - Taekwondo-based gymnastics Average intensity of 11–15 RPE CG: NR | Significant increase * in BDNF values only in the IG (Pre: 24.03 ± 6.16 ng/mL → Post: 27.62 ± 7.58 ng/mL). Significant increase in BDNF serum levels in IG compared with CG after intervention. VEGF and IGF-1 values were also significantly increased in IG alone. Significant increase in VEGF and IGF-1 in IG compared with CG after intervention. No significant changes were observed in MCAs, MCAd, MCAm, or PI (p > 0.05). Cognitive function: Significant improvement *** in cognitive function, specifically in color–word Stroop subtest score in the IG compared to pre-intervention and CG. | Taekwondo training for 16 weeks caused a significant increase in serum levels of BDNF, VEGF, and IGF-1 in healthy children, suggesting an activation of neuroplastic mechanisms similar to those of aerobic exercise. Although no changes in cerebral blood flow were observed, exercise could indirectly modulate brain health through increased growth factors. |
| Kim et al. [23] | N = 30 males (10.93 ± 0.26 years) Country: South Korea Without pathologies | Biological matrix: Serum ELISA method (Human BDNF Quantikine®, R&D Systems, Cat# DBD00). Additional comparison: NGF also analyzed with ELISA (Abnova, Cat# KA0399). | Working memory was measured using the K-WISC-III test (digits forward and digits backward subtests). Complete physical fitness assessment: cardiovascular endurance, strength, flexibility, speed and agility. | IG: 12 weeks (5 sessions/week of 60 min each session) Content of the sessions: Progressive exercises per week, including jumping, hexagonal ladder, pull-ups, sit-ups, kickboxing, balance board exercises, plank, Taekwondo-type musical circuits. Average intensity of 50–80% HRmax CG: NR | Increase in BDNF values in IG post-intervention (31.74 ± 5.46 to 34.32 ± 3.21 ng/mg; p > 0.005). Significant *** increase in NGF values in IG (33.12 ± 3.32 → 39.15 ± 2.70 pg/mL). Significant increase in NGF in IG compared with CG after intervention. No significant changes (p > 0.05) were observed in parameters related to working memory. Significant improvements * in cardiovascular endurance, strength and agility in the exercise group. | Although the 12-week Taekwondo-based intervention significantly improved NGF and several physical abilities in male schoolchildren, no statistically significant changes in BDNF or working memory were observed. Programs that integrate cognitive tasks or learning during the session could be more effective in inducing brain functional improvements. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rico-González, M.; González-Devesa, D.; Gómez-Carmona, C.D.; Moreno-Villanueva, A. Exercise as Modulator of Brain-Derived Neurotrophic Factor (BDNF) in Children: A Systematic Review of Randomized Controlled Trials. Life 2025, 15, 1147. https://doi.org/10.3390/life15071147
Rico-González M, González-Devesa D, Gómez-Carmona CD, Moreno-Villanueva A. Exercise as Modulator of Brain-Derived Neurotrophic Factor (BDNF) in Children: A Systematic Review of Randomized Controlled Trials. Life. 2025; 15(7):1147. https://doi.org/10.3390/life15071147
Chicago/Turabian StyleRico-González, Markel, Daniel González-Devesa, Carlos D. Gómez-Carmona, and Adrián Moreno-Villanueva. 2025. "Exercise as Modulator of Brain-Derived Neurotrophic Factor (BDNF) in Children: A Systematic Review of Randomized Controlled Trials" Life 15, no. 7: 1147. https://doi.org/10.3390/life15071147
APA StyleRico-González, M., González-Devesa, D., Gómez-Carmona, C. D., & Moreno-Villanueva, A. (2025). Exercise as Modulator of Brain-Derived Neurotrophic Factor (BDNF) in Children: A Systematic Review of Randomized Controlled Trials. Life, 15(7), 1147. https://doi.org/10.3390/life15071147








