Watercress (Nasturtium officinale) as a Functional Food for Non-Communicable Diseases Prevention and Management: A Narrative Review
Abstract
1. Introduction
2. Phytochemical Composition of Watercress
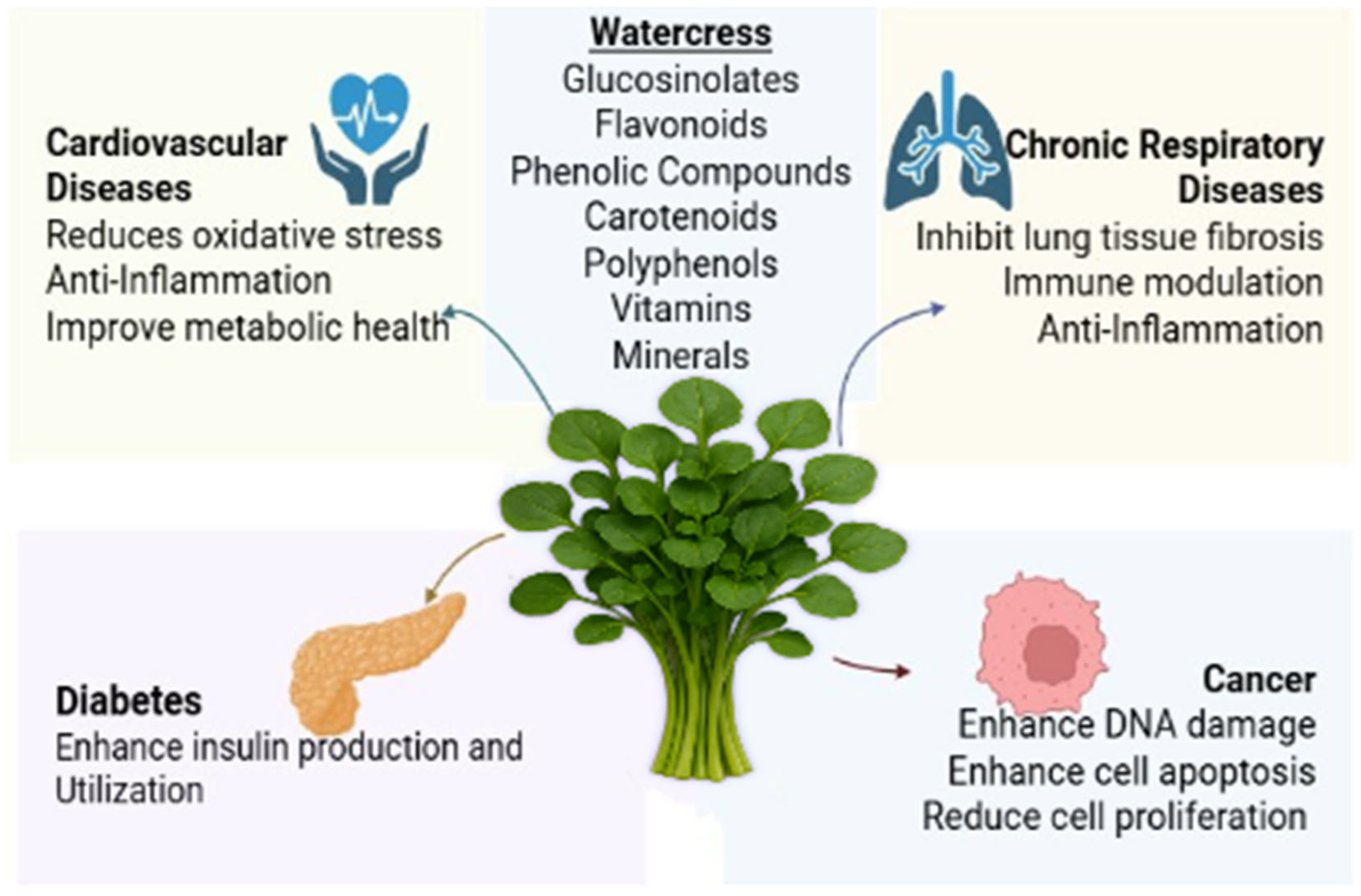
3. Effects of Watercress on Major NCDs
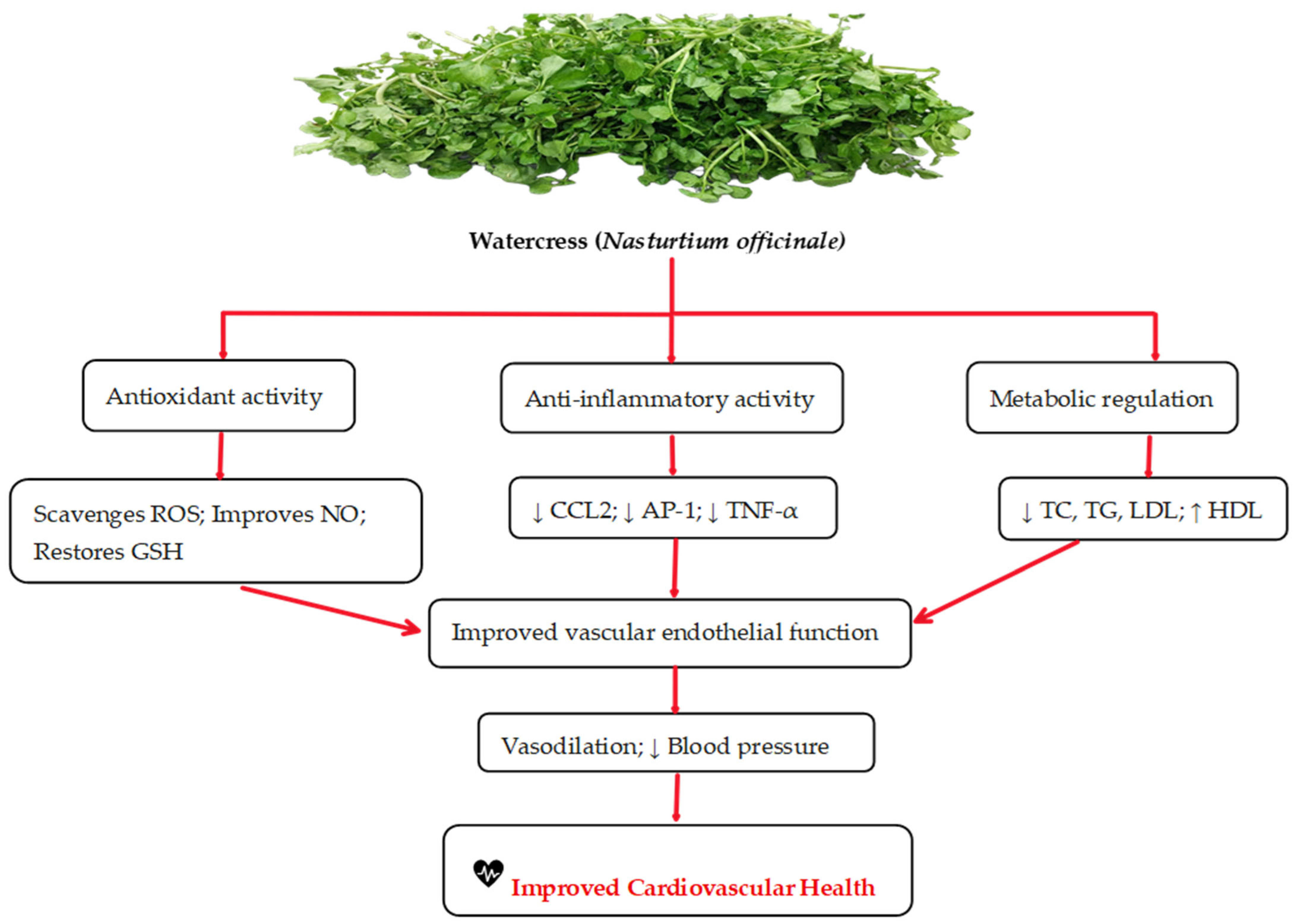
3.1. Effects of Watercress on Cardiovascular Diseases
3.2. Effects of Watercress on Cancer
3.3. Effects of Watercress on Diabetes
3.4. Effects of Watercress on Chronic Respiratory Diseases
4. Discussion
5. Materials and Methods
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NCDs | Non-Communicable Diseases |
| CVDs | Cardiovascular Diseases |
| CRDs | Chronic Respiratory Diseases |
| PEITC | Phenethyl Isothiocyanate |
| LDL | Low-Density Lipoprotein |
| RCT | Randomized Controlled Trial |
| SENO | Standardized Extract of Nasturtium officinale |
| CRP | C-Reactive Protein |
| EENO | Ethanolic Extract of Nasturtium officinale |
| MDA | Malondialdehyde |
| BUN | Blood Urea Nitrogen |
| SOD | Superoxide Dismutase |
| TGs | Triglycerides |
| T2D | Type 2 Diabetes |
| AGEs | Advanced Glycation End-Products |
| RAGEs | Receptor for Advanced Glycation End-Products |
| IRS1 | Insulin Receptor Substrate 1 |
| PI3K | Phosphoinositide 3-Kinase |
| AKT2 | Protein Kinase B (AKT2) pathway |
| BPA | Bisphenol A |
| DBP | Dibutyl Phthalate |
| CAT | Catalase |
| GSH | Glutathione |
| Nrf2 | Nuclear Factor Erythroid 2–Related Factor 2 |
| NF-κB | Nuclear Factor Kappa B |
| IL | Interleukin (e.g., IL-1β, IL-6) |
| TNF-α | Tumor Necrosis Factor-alpha |
| AP-1 | Activator Protein 1 |
| CCL2 | Chemokine (C-C motif) Ligand 2 |
| TMAO | Trimethylamine N-oxide |
| WNT/β-catenin | WNT Signaling Pathway/Beta-catenin |
| ER | Endoplasmic Reticulum |
| IC50 | Half Maximal Inhibitory Concentration |
| PRF | Phenolic-Rich Fraction |
| PhEF | Phenethyl Isothiocyanate-Enriched Extract |
| AuNPs | Gold Nanoparticles |
| PEG-PLGA | Polyethylene Glycol–Polylactic-co-Glycolic Acid |
| NOE-NPs | Nasturtium officinale-Extract Nanoparticles |
| HbA1c | Hemoglobin A1c |
| ALT | Alanine Aminotransferase |
| AST | Aspartate Aminotransferase |
| GLUT4 | Glucose Transporter Type 4 |
| AMPK | AMP-Activated Protein Kinase |
| COPD | Chronic Obstructive Pulmonary Disease |
| GPX | Glutathione Peroxidase |
| SMA-α | Alpha-Smooth Muscle Actin |
| NO | Nitric Oxide |
| FRAP | Ferric Reducing Antioxidant Power |
| Gata3 | Transcription Factor GATA-3 |
| TGF-β1 | Transforming Growth Factor Beta 1 |
| MEITCs | Methyl Isothiocyanates |
| PCOs | Protein Carbonyls |
References
- Upadhyay, R.K. Chronic non-communicable diseases: Risk factors, disease burden, mortalities and control. Acta Sci. Med. Sci. 2022, 6, 153–170. [Google Scholar] [CrossRef]
- WHO. Non Communicable Diseases. Non Communicable Diseases Fact Sheets. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 30 March 2025).
- Manderson, L.; Jewett, S. Risk, lifestyle and non-communicable diseases of poverty. Glob. Health 2023, 19, 13. [Google Scholar] [CrossRef] [PubMed]
- Al-Snafi, A.E. A review on Nasturtium officinale: A potential medicinal plant. IOSR J. Pharm. 2020, 10, 33–43. [Google Scholar]
- Buitrago-Villanueva, I.; Barbosa-Cornelio, R.; Coy-Barrera, E. Specialized Metabolite Profiling-Based Variations of Watercress Leaves (Nasturtium officinale R. Br.) from Hydroponic and Aquaponic Systems. Molecules 2025, 30, 406. [Google Scholar] [PubMed]
- Kyriakou, S.; Tragkola, V.; Alghol, H.; Anestopoulos, I.; Amery, T.; Stewart, K.; Winyard, P.G.; Trafalis, D.T.; Franco, R.; Pappa, A. Evaluation of bioactive properties of lipophilic fractions of edible and non-edible parts of Nasturtium officinale (Watercress) in a model of human malignant melanoma cells. Pharmaceuticals 2022, 15, 141. [Google Scholar] [CrossRef] [PubMed]
- Pinela, J.; Carvalho, A.M.; Ferreira, I.C. Watercress. In Nutritional Composition and Antioxidant Properties of Fruits and Vegetables; Elsevier: Amsterdam, The Netherlands, 2020; pp. 197–219. [Google Scholar]
- Hapsari, Y.; Rachman, F.; Septiana, E.; Simanjuntak, P. Identification of Antioxidant Active Compounds from Watercress (Nasturtium officinale R. Br). In Proceedings of the 1st International Conference for Health Research–BRIN (ICHR 2022), Jakarta, Indonesia, 23–24 November 2022; Atlantis Press: Paris, France, 2023; pp. 374–384. [Google Scholar]
- Kyriakou, S.; Michailidou, K.; Amery, T.; Stewart, K.; Winyard, P.G.; Trafalis, D.T.; Franco, R.; Pappa, A.; Panayiotidis, M.I. Polyphenolics, glucosinolates and isothiocyanates profiling of aerial parts of Nasturtium officinale (Watercress). Front. Plant Sci. 2022, 13, 998755. [Google Scholar] [CrossRef] [PubMed]
- Kijkuokool, P.; Stepanov, I.; Ounjaijean, S.; Koonyosying, P.; Rerkasem, K.; Chuljerm, H.; Parklak, W.; Kulprachakarn, K. Effects of Drying Methods on the Phytochemical Contents, Antioxidant Properties, and Anti-Diabetic Activity of Nasturtium officinale R. Br.(Betong Watercress) from Southern Thailand. Life 2024, 14, 1204. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Ding, Q.; Hou, X.; You, X. Analysis of flavonoid metabolites in watercress (Nasturtium officinale R. Br.) and the non-heading Chinese cabbage (Brassica rapa ssp. chinensis cv. Aijiaohuang) using UHPLC-ESI-MS/MS. Molecules 2021, 26, 5825. [Google Scholar] [CrossRef] [PubMed]
- Panahi Kokhdan, E.; Khodabandehloo, H.; Ghahremani, H.; Doustimotlagh, A.H. A narrative review on therapeutic potentials of watercress in human disorders. Evid. Based Complement. Altern. Med. 2021, 2021, 5516450. [Google Scholar] [CrossRef] [PubMed]
- Kyriakou, S.; Potamiti, L.; Demosthenous, N.; Amery, T.; Stewart, K.; Winyard, P.G.; Franco, R.; Pappa, A.; Panayiotidis, M.I. A naturally derived watercress flower-based phenethyl isothiocyanate-enriched extract induces the activation of intrinsic apoptosis via subcellular ultrastructural and Ca2+ efflux alterations in an in vitro model of human malignant melanoma. Nutrients 2023, 15, 4044. [Google Scholar] [CrossRef] [PubMed]
- Klimek-Szczykutowicz, M.; Szopa, A.; Blicharska, E.; Dziurka, M.; Komsta, Ł.; Ekiert, H. Bioaccumulation of selected macro-and microelements and their impact on antioxidant properties and accumulation of glucosinolates and phenolic acids in in vitro cultures of Nasturtium officinale (watercress) microshoots. Food Chem. 2019, 300, 125184. [Google Scholar] [CrossRef] [PubMed]
- Sathasivam, R.; Bong, S.J.; Park, C.H.; Kim, J.H.; Kim, J.K.; Park, S.U. Identification, characterization, and expression analysis of carotenoid biosynthesis genes and carotenoid accumulation in watercress (Nasturtium officinale R. Br.). ACS Omega 2021, 7, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Bulut, H.; Yılmaz, S.M. An alternative plant for sustainable future: Some biochemical properties of watercress (Nasturtium officinale R. Br.). Oceanol. Hydrobiol. Stud. 2025, 54, 1–9. [Google Scholar] [CrossRef]
- Baldelli, S.; Lombardo, M.; D’Amato, A.; Karav, S.; Tripodi, G.; Aiello, G. Glucosinolates in Human Health: Metabolic Pathways, Bioavailability, and Potential in Chronic Disease Prevention. Foods 2025, 14, 912. [Google Scholar] [CrossRef] [PubMed]
- Negi, N.; Upadhyay, S.; Rana, M. An Overview on Phytopharmacological Perspectives of a Potential plant Species: Nasturtium officinale. Syst. Rev. Pharm. 2024, 15, 257–262. [Google Scholar]
- Clemente, M.; Miguel, M.; Gribner, C.; Moura, P.F.; Angelica, A.; Rigoni, R.; Fernandes, L.; Miguel, O. Watercress, as a functional food, with protective effects on human health against oxidative stress: A review study. Int. J. Med. Plants Nat. Prod. 2019, 5, 12–16. [Google Scholar] [CrossRef]
- Tufail, T.; Fatima, S.; Bader Ul Ain, H.; Ikram, A.; Noreen, S.; Rebezov, M.; Al-Farga, A.; Saleh, R.; Shariati, M.A. Role of Phytonutrients in the Prevention and Treatment of Chronic Diseases: A Concrete Review. ACS Omega 2025, 10, 12724–12755. [Google Scholar] [CrossRef] [PubMed]
- Bhaswant, M.; Shanmugam, D.K.; Miyazawa, T.; Abe, C.; Miyazawa, T. Microgreens—A comprehensive review of bioactive molecules and health benefits. Molecules 2023, 28, 867. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torres, I.; Castrejón-Téllez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative stress, plant natural antioxidants, and obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef] [PubMed]
- Clemente, M.; Miguel, M.; Felipe, K.; Fujiwara, G.; Fernandes, L.; Dias, J.; Zenin, S.; Hirota, B.; Miguel, O. Can medicinal properties of watercress be relevant to human health? A systematic review based on preclinical study in vivo. Pharmacogn. Rev. 2019, 13, 10. [Google Scholar] [CrossRef]
- Schulze, H.; Hornbacher, J.; Wasserfurth, P.; Reichel, T.; Günther, T.; Krings, U.; Krüger, K.; Hahn, A.; Papenbrock, J.; Schuchardt, J.P. Immunomodulating effect of the consumption of watercress (Nasturtium officinale) on exercise-induced inflammation in humans. Foods 2021, 10, 1774. [Google Scholar] [CrossRef] [PubMed]
- Clemente, M.; Miguel, M.D.; Felipe, K.B.; Gribner, C.; Moura, P.F.; Rigoni, A.A.; Parisotto, E.B.; Henneberg, R.; Dias, J.d.F.G.; Piltz, M.T. Effect of watercress extract supplementation on lipid profile and oxidative stress markers in overweight people with physical disability: A randomized, double-blind, and placebo-controlled trial. Phytother. Res. 2021, 35, 2211–2219. [Google Scholar] [CrossRef] [PubMed]
- Nilash, A.B.; Jahanbani, J.; Jolehar, M. Effect of nasturtium extract on oral cancer. Adv. Biomed. Res. 2023, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Giallourou, N.S.; Rowland, I.R.; Rothwell, S.D.; Packham, G.; Commane, D.M.; Swann, J.R. Metabolic targets of watercress and PEITC in MCF-7 and MCF-10A cells explain differential sensitisation responses to ionising radiation. Eur. J. Nutr. 2019, 58, 2377–2391. [Google Scholar] [CrossRef] [PubMed]
- Tragkola, V.; Anestopoulos, I.; Kyriakou, S.; Amery, T.; Franco, R.; Pappa, A.; Panayiotidis, M.I. Naturally-derived phenethyl isothiocyanate modulates apoptotic induction through regulation of the intrinsic cascade and resulting apoptosome formation in human malignant melanoma cells. Toxicol. Mech. Methods 2024, 34, 985–999. [Google Scholar] [CrossRef] [PubMed]
- Voutsina, N.; Hancock, R.D.; Becerra-Sanchez, F.; Qian, Y.; Taylor, G. Characterization of a new dwarf watercress (Nasturtium officinale R Br.)‘Boldrewood’in commercial trials reveals a consistent increase in chemopreventive properties in a longer-grown crop. Euphytica 2024, 220, 106. [Google Scholar] [CrossRef]
- Thabet, S.R.; Ahmed, N.S.; Osman, A.S. Impact of watercress (nasturtium officinale) aqueous extract on biochemical markers of diabetic rats. Int. J. Compr. Vet. Res. 2023, 1, 14–26. [Google Scholar] [CrossRef]
- Yousef, R.S.; Thabet, S.R.; Ahmed, N.S.; Osman, A.S. Ameliorative effect of watercress (Nasturtium officinale) aqueous extract on gene expression of Glut4 and Ampk in diabetic rats. SVU Int. J. Med. Sci. 2024, 7, 686–697. [Google Scholar] [CrossRef]
- Elrayes, S. Exploring The Potential Biological Activities of Watercress (Nasturtium Officinale) Extract in Vitro. Adv. Environ. Life Sci. 2025, 6, 19–29. [Google Scholar] [CrossRef]
- Bayrami, A.; Ghorbani, E.; Pouran, S.R.; Habibi-Yangjeh, A.; Khataee, A.; Bayrami, M. Enriched zinc oxide nanoparticles by Nasturtium officinale leaf extract: Joint ultrasound-microwave-facilitated synthesis, characterization, and implementation for diabetes control and bacterial inhibition. Ultrason. Sonochemistry 2019, 58, 104613. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, S.; Javadi, I.; Kokhdan, E.P.; Omidifar, N.; Nikbakht, J.; Sadeghi, H.; Doustimotlagh, A.H.; Danaei, N.; Abbasi, R.; Sadeghi, H. Protective and therapeutic effects of ethanolic extract of Nasturtium officinale (watercress) and vitamin E against bleomycin-induced pulmonary fibrosis in rats. Res. Pharm. Sci. 2021, 16, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Kumar, S.; Malik, J.; Singh, G.; Siroliya, V.K. A Review of the Phytochemical and Pharmacological Potential of the Watercress Plant (Nasturitium Officinale): A Medicinal Plant. Int. J. Pharm. Biol. Sci. Arch. 2023, 11, 4. [Google Scholar]
- Shakerinasab, N.; Mottaghipisheh, J.; Eftekhari, M.; Sadeghi, H.; Bazarganipour, F.; Abbasi, R.; Doustimotlagh, A.H.; Iriti, M. The hydroalcoholic extract of Nasturtium officinale reduces oxidative stress markers and increases total antioxidant capacity in patients with asthma. J. Ethnopharmacol. 2024, 318, 116862. [Google Scholar] [CrossRef] [PubMed]
- Nicikowski, J.; Reguła, J. Selected bioactive compounds in food of plant origin as natural immunomodulators in asthma and chronic obstructive pulmonary disease. Acta Sci. Pol. Technol. Aliment. 2021, 20, 383–397. [Google Scholar] [PubMed]
- Lata, M. Nutritional, medicinal and indigenous use of Nasturtium officinale in tehsil Thunag of district Mandi, Himachal Pradesh, North Western Himalayas, India. Int. J. Chem. Stud. 2020, 8, 1648–1653. [Google Scholar] [CrossRef]
- Krisanits, B.A.; Kaur, B.; Fahey, J.W.; Turner, D.P. The Anti-AGEing and RAGEing Potential of Isothiocyanates. Molecules 2024, 29, 5986. [Google Scholar] [CrossRef] [PubMed]
- Clemente, M.; Miguel, M.D.; Felipe, K.B.; Gribner, C.; Moura, P.F.; Rigoni, A.A.R.; Parisotto, E.B.; Piltz, M.T.; Valdameri, G.; Henneberg, R.; et al. Biomarkers of oxidative stress and inflammation in people witha physical disability treated with a standardized extract of Nasturtium officinale: A randomized, double-blind, and placebo-controlled trial. Phytother. Res. 2020, 34, 2756–2765. [Google Scholar] [CrossRef] [PubMed]
- Sedaghattalab, M.; Razazan, M.; Sadeghi, H.; Doustimotlagh, A.H.; Toori, M.A.; Abbasi Larki, R.; Azarmehr, N.; Asfaram, A.; Panahi Kokhdan, E.; Taheri, T.; et al. Effects of Nasturtium officinale Extract on Antioxidant and Biochemical Parameters in Hemodialysis Patients: A Randomized Double-Blind Clinical Trial. Evid Based Complement. Altern. Med. 2021, 2021, 1632957. [Google Scholar] [CrossRef] [PubMed]
- Rashid, K.I.; Abdulrazak, D.I.; Hlail, Z.A. Detection of some active compounds in three herbal plants (Nasturtium officinale, Hibiscus sabdarriffa L. and Purtulaca oleracea) and their effect on reducing blood glucose level in diabetic patients. Nat. Volatiles Essent. Oils 2021, 8, 13827–13838. [Google Scholar]
- Schuchardt, J.P.; Hahn, A.; Greupner, T.; Wasserfurth, P.; Rosales-López, M.; Hornbacher, J.; Papenbrock, J. Watercress–cultivation methods and health effects. J. Appl. Bot. Food Qual. 2019, 92, 232–239. [Google Scholar]
- WHO. Cardiovascular Diseases (CVDs): Key Facts. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 8 April 2025).
- Qiao, J.; Zhang, M.; Wang, T.; Huang, S.; Zeng, P. Evaluating causal relationship between metabolites and six cardiovascular diseases based on GWAS summary statistics. Front. Genet. 2021, 12, 746677. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Yun, Z.; Fan, S.; Wang, H.; Xue, W.; Zhang, X.; Jia, B.; Hu, Y. Causal association between blood metabolites and risk of hypertension: A Mendelian randomization study. Front. Cardiovasc. Med. 2024, 11, 1373480. [Google Scholar] [CrossRef] [PubMed]
- Lind, L.; Fall, T.; Ärnlöv, J.; Elmståhl, S.; Sundström, J. Large-scale metabolomics and the incidence of cardiovascular disease. J. Am. Heart Assoc. 2023, 12, e026885. [Google Scholar] [CrossRef] [PubMed]
- Ottosson, F.; Engström, G.; Orho-Melander, M.; Melander, O.; Nilsson, P.M.; Johansson, M. Plasma Metabolome Predicts Aortic Stiffness and Future Risk of Coronary Artery Disease and Mortality After 23 Years of Follow-Up in the General Population. J. Am. Heart Assoc. 2024, 13, e033442. [Google Scholar] [CrossRef] [PubMed]
- Evans, B.R.; Yerly, A.; van der Vorst, E.P.; Baumgartner, I.; Bernhard, S.M.; Schindewolf, M.; Döring, Y. Inflammatory mediators in atherosclerotic vascular remodeling. Front. Cardiovasc. Med. 2022, 9, 868934. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhou, S.; He, H.; Shen, Z.; Liu, Y.; Hu, J.; Wang, J. Exploring the “gene–protein–metabolite” network of coronary heart disease with phlegm and blood stasis syndrome by integrated multi-omics strategy. Front. Pharmacol. 2022, 13, 1022627. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Xia, J.; Cheng, J.; Huang, H.; Zhou, Y.; Yang, X.; Su, X.; Ke, Y.; Ling, W. Inhibition of S-adenosylhomocysteine hydrolase induces endothelial dysfunction via epigenetic regulation of p66shc-mediated oxidative stress pathway. Circulation 2019, 139, 2260–2277. [Google Scholar] [CrossRef] [PubMed]
- Iliou, A.; Mikros, E.; Karaman, I.; Elliott, F.; Griffin, J.L.; Tzoulaki, I.; Elliott, P. Metabolic phenotyping and cardiovascular disease: An overview of evidence from epidemiological settings. Heart 2021, 107, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Du, J.; Deng, S.; Liu, B.; Jing, X.; Yan, Y.; Liu, Y.; Wang, J.; Zhou, X.; She, Q. The molecular mechanisms of cardiac development and related diseases. Signal Transduct. Target. Ther. 2024, 9, 368. [Google Scholar] [CrossRef] [PubMed]
- Oluranti, O.I.; Alabi, B.A.; Michael, O.S.; Ojo, A.O.; Fatokun, B.P. Rutin prevents cardiac oxidative stress and inflammation induced by bisphenol A and dibutyl phthalate exposure via NRF-2/NF-κB pathway. Life Sci. 2021, 284, 119878. [Google Scholar] [CrossRef] [PubMed]
- Bong, S.J.; Jeon, J.; Park, Y.J.; Kim, J.K.; Park, S.U. Identification and analysis of phenylpropanoid biosynthetic genes and phenylpropanoid accumulation in watercress (Nasturtium officinale R. Br.). 3 Biotech 2020, 10, 260. [Google Scholar] [CrossRef] [PubMed]
- Gianazza, E.; Brioschi, M.; Martinez Fernandez, A.; Casalnuovo, F.; Altomare, A.; Aldini, G.; Banfi, C. Lipid peroxidation in atherosclerotic cardiovascular diseases. Antioxid. Redox Signal. 2021, 34, 49–98. [Google Scholar] [CrossRef] [PubMed]
- Izzo, C.; Vitillo, P.; Di Pietro, P.; Visco, V.; Strianese, A.; Virtuoso, N.; Ciccarelli, M.; Galasso, G.; Carrizzo, A.; Vecchione, C. The role of oxidative stress in cardiovascular aging and cardiovascular diseases. Life 2021, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Senoner, T.; Dichtl, W. Oxidative stress in cardiovascular diseases: Still a therapeutic target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef] [PubMed]
- Henein, M.Y.; Vancheri, S.; Longo, G.; Vancheri, F. The role of inflammation in cardiovascular disease. Int. J. Mol. Sci. 2022, 23, 12906. [Google Scholar] [CrossRef] [PubMed]
- Batty, M.; Bennett, M.R.; Yu, E. The role of oxidative stress in atherosclerosis. Cells 2022, 11, 3843. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Aramouni, K.; Assaf, R.; Parenti, A.; Orekhov, A.; El Yazbi, A.; Pintus, G.; Eid, A.H. Oxidative stress-induced endothelial dysfunction in cardiovascular diseases. Front. Biosci. 2022, 27, 105. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Bayo Jimenez, M.T.; Vujacic-Mirski, K.; Helmstädter, J.; Kröller-Schön, S.; Münzel, T. Vascular inflammation and oxidative stress: Major triggers for cardiovascular disease. Oxidative Med. Cell. Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef] [PubMed]
- Tarin, D. Causes of cancer and mechanisms of carcinogenesis. In Understanding Cancer: The Molecular Mechanisms, Biology, Pathology and Clinical Implications of Malignant Neoplasia; Springer: Berlin/Heidelberg, Germany, 2023; pp. 229–279. [Google Scholar]
- Huang, Z.; Liu, Z.; Chen, L.; Liu, Y.; Yan, G.; Ni, Y.; Yan, Q.; He, W.; Liu, J.; Luo, S. Liquid-liquid phase separation in cell physiology and cancer biology: Recent advances and therapeutic implications. Front. Oncol. 2025, 15, 1540427. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, J. Mechanical tumor microenvironment and transduction: Cytoskeleton mediates cancer cell invasion and metastasis. Int. J. Biol. Sci. 2020, 16, 2014. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zheng, H.; Zhan, Y.; Fan, S. An emerging tumor invasion mechanism about the collective cell migration. Am. J. Transl. Res. 2019, 11, 5301. [Google Scholar] [PubMed]
- Li, Y.; Liu, F.; Cai, Q.; Deng, L.; Ouyang, Q.; Zhang, X.H.-F.; Zheng, J. Invasion and metastasis in cancer: Molecular insights and therapeutic targets. Signal Transduct. Target. Ther. 2025, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Adlravan, E.; Nejati, K.; Karimi, M.A.; Mousazadeh, H.; Abbasi, A.; Dadashpour, M. Potential activity of free and PLGA/PEG nanoencapsulated nasturtium officinale extract in inducing cytotoxicity and apoptosis in human lung carcinoma A549 cells. J. Drug Deliv. Sci. Technol. 2021, 61, 102256. [Google Scholar] [CrossRef]
- Arumugam, A.; Ibrahim, M.D.; Kntayya, S.B.; Mohd Ain, N.; Iori, R.; Galletti, S.; Ioannides, C.; Abdull Razis, A.F. Induction of Apoptosis by Gluconasturtiin-Isothiocyanate (GNST-ITC) in Human Hepatocarcinoma HepG2 Cells and Human Breast Adenocarcinoma MCF-7 Cells. Molecules 2020, 25, 1240. [Google Scholar] [CrossRef] [PubMed]
- Kyriakou, S.; Demosthenous, N.; Amery, T.; Stewart, K.J.; Winyard, P.G.; Franco, R.; Pappa, A.; Panayiotidis, M.I. Naturally Derived Phenethyl Isothiocyanate Modulates Induction of Oxidative Stress via Its N-Acetylated Cysteine Conjugated form in Malignant Melanoma. Antioxidants 2024, 13, 82. [Google Scholar] [CrossRef] [PubMed]
- Yayintas, O.T.; Demir, N.; Canbolat, F.; Ayna, T.K.; Pehlivan, M. Characterization, biological activity, and anticancer effect of green-synthesized gold nanoparticles using Nasturtium officinale L. BMC Complement. Med. Ther. 2024, 24, 346. [Google Scholar] [CrossRef]
- Taghavinia, F.; Teymouri, F.; Farokhrouz, F.; Bagherabad, E.H.; Farjami, S.; Karimi, E.; Oskoueian, E.; Le, H.H.; Shakeri, M. Nanoliposome-loaded phenolics from Nasturtium officinale improves health parameters in a colorectal cancer mouse model. Animals 2022, 12, 3492. [Google Scholar] [CrossRef] [PubMed]
- Forouhi, N.G.; Wareham, N.J. Epidemiology of diabetes. Medicine 2019, 47, 22–27. [Google Scholar] [CrossRef]
- Banday, M.Z.; Sameer, A.S.; Nissar, S. Pathophysiology of diabetes: An overview. Avicenna J. Med. 2020, 10, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.B.; Florez, J.C. Genetics of diabetes mellitus and diabetes complications. Nat. Rev. Nephrol. 2020, 16, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Balekundri, A.; Ahire, E.D.; Keservani, R.K. Plant Metabolites and Vegetables for Diabetes Prevention and Treatment. In Plant Metabolites and Vegetables as Nutraceuticals; Apple Academic Press: Waretown, NJ, USA, 2024; pp. 333–360. [Google Scholar]
- Chen, X.; Yuan, H.; Shi, F.; Zhu, Y. Effect of garden cress in reducing blood glucose, improving blood lipids, and reducing oxidative stress in a mouse model of diabetes induced by a high-fat diet and streptozotocin. J. Sci. Food Agric. 2020, 100, 2074–2081. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Agusti, A.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Criner, G.J.; Frith, P.; Halpin, D.M.; Han, M. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: The GOLD science committee report 2019. Eur. Respir. J. 2019, 53, 1900164. [Google Scholar] [CrossRef] [PubMed]
- Kahnert, K.; Jörres, R.A.; Behr, J.; Welte, T. The diagnosis and treatment of COPD and its comorbidities. Dtsch. Ärzteblatt Int. 2023, 120, 434. [Google Scholar] [CrossRef]
- Zaniku, H.R.; Connolly, E.; Aron, M.B.; Matanje, B.L.; Ndambo, M.K.; Complex Talama, G.; Munyaneza, F.; Ruderman, T.; Rylance, J.; Dullie, L.W. Prevalence and associated factors of chronic obstructive pulmonary disease among adults in Neno District, Malawi: A cross-sectional analytical study. Int. J. Chronic Obstr. Pulm. Dis. 2024, 19, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Habib, N.; Pasha, M.A.; Tang, D.D. Current understanding of asthma pathogenesis and biomarkers. Cells 2022, 11, 2764. [Google Scholar] [CrossRef] [PubMed]
- Shakerinasab, N.; Bejeshk, M.A.; Pourghadamyari, H.; Najafipour, H.; Eftekhari, M.; Mottaghipisheh, J.; Omidifar, N.; Azizi, M.; Rajizadeh, M.A.; Doustimotlagh, A.H. The Hydroalcoholic Extract of Nasturtium officinale Reduces Lung Inflammation and Oxidative Stress in an Ovalbumin-Induced Rat Model of Asthma. Evid. Based Complement. Altern. Med. 2022, 2022, 5319237. [Google Scholar] [CrossRef] [PubMed]
- Rajizadeh, M.A.; Bejeshk, M.A.; Doustimotlagh, A.H.; Najafipour, H.; Eftekhari, M.; Mahmoodi, M.; Azizi, M.; Rostamabadi, F.; Pourghadamyari, H. The alleviating impacts of quercetin on inflammation and oxidant-antioxidant imbalance in rats with allergic asthma. Iran. J. Allergy Asthma Immunol. 2023, 22, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Faizy, H.; Esmail, L.; Mahdi, H. Phytochemicals analysis in Watercress (Nasturtium officinale) plant extracts. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Banda Aceh, Indonesia, 21 September 2021; IOP Publishing: Bristol, UK, 2021; p. 012042. [Google Scholar]
- Fisher, A.; Villanueva, J.; Simington, L.; Jenkins, B.; Travis, K.; Roy, D.; Sengupta, B. Analysis of Phytochemicals in Watercress Leaves Using Chromatographic, Spectroscopic and In-Vitro Approaches. Results Chem. 2025. [Google Scholar] [CrossRef]
- Klimek-Szczykutowicz, M.; Dziurka, M.; Blažević, I.; Đulović, A.; Granica, S.; Korona-Glowniak, I.; Ekiert, H.; Szopa, A. Phytochemical and biological activity studies on Nasturtium officinale (watercress) microshoot cultures grown in RITA® temporary immersion systems. Molecules 2020, 25, 5257. [Google Scholar] [CrossRef] [PubMed]
- Rocha, D.C.; Kochi, L.Y.; Kitamura, R.S.A.; Brito, J.C.M.; da Silva Nogueira, K.; Gomes, M.P. Unveiling the impact of antimicrobial-infused water on hydroponic baby leafy vegetables (lettuce, rocket, and watercress): Physiological effects and food safety. J. Environ. Chem. Eng. 2024, 12, 112335. [Google Scholar] [CrossRef]
- Clemente, M.; Miguel, M.; Felipe, K.; Gribner, C.; Moura, P.; Rigoni, A.; Fernandes, L.; Carvalho, J.; Hartmann, I.; Piltz, M. Acute and sub-acute oral toxicity studies of standardized extract of Nasturtium officinale in Wistar rats. Regul. Toxicol. Pharmacol. 2019, 108, 104443. [Google Scholar] [CrossRef] [PubMed]
| Phytochemical Group | Key Compounds Identified | Reference |
|---|---|---|
| Glucosinolates | Gluconasturtiin, PEITC, and MEITCs | [4,5,6,7,8,9,10] |
| Phenolic acids | Coumaric acid, caffeic acid, ferulic acids, chlorogenic acids, protocatechuic acid, and dicaffeoyltartaric acid | [9,10,11,12,13,14,15] |
| Flavonoids | Quercetin, rutin, kaempferol, and isorhamnetin | [6,7,11] |
| Tannins | Gallo-tannins, ellagitannins, and proanthocyanidins | [10,11,12] |
| Carotenoids | Beta-carotene, lutein, and zeaxanthin | [16] |
| Vitamins and minerals | B3, E, K, C, A, calcium, iron, magnesium, phosphorus, and copper | [5,6] |
| Terpenes | α-terpinolene, limonene, caryophyllene oxide, and p-cymene-8-ol | [4] |
| Study Design | Population | Intervention | Key Findings | References |
|---|---|---|---|---|
| RCT, double-blind, placebo-controlled | 65 participants (disabled + controls) | 750 mg/kg/day watercress extract (SENO) from leaves for 5 weeks | ↓ Oxidative stress (lipid peroxidation and protein carbonyls) and ↓ CRP | [41] |
| RCT, double-blind, placebo-controlled | 34 overweight participants | 750 mg/kg/day (SENO) from leaves for 5 weeks | Improved LDL cholesterol, creatinine, and lipid peroxidation markers | [26] |
| RCT, double-blind | 46 hemodialysis patients | 500 mg/day ethanolic extract (EENO) from leaves for 4 weeks | ↓ MDA and BUN, ↑ SOD activity, and stabilized LDL/TG levels | [42] |
| Crossover intervention study | 19 healthy subjects (14 males and 5 females) | Fresh watercress leaves consumption | Immune modulation: ↓ IL-6/TNF-α and mild pro-inflammatory response (↑ IL-1β/IL-6) | [25] |
| Crossover acute intervention study | 4 healthy adults (1 male and 3 females) | Single dose (85 g fresh watercress leaves) | Acute antioxidant and anti-inflammatory effects | [44] |
| Non-randomized clinical trial | 60 diabetic patients | Watercress leaf extract (20 mL/day orally) for 6 weeks | Reduced blood glucose levels | [43] |
| RCT, double-blind, placebo-controlled | Asthma patients | 500 mg watercress leaf extract twice daily for 4 weeks | ↓ Oxidative stress (MDA and PCO) and ↑ total antioxidant capacity (FRAP) | [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maluwa, C.; Zinan’dala, B.; Chuljerm, H.; Parklak, W.; Kulprachakarn, K. Watercress (Nasturtium officinale) as a Functional Food for Non-Communicable Diseases Prevention and Management: A Narrative Review. Life 2025, 15, 1104. https://doi.org/10.3390/life15071104
Maluwa C, Zinan’dala B, Chuljerm H, Parklak W, Kulprachakarn K. Watercress (Nasturtium officinale) as a Functional Food for Non-Communicable Diseases Prevention and Management: A Narrative Review. Life. 2025; 15(7):1104. https://doi.org/10.3390/life15071104
Chicago/Turabian StyleMaluwa, Chikondi, Blecious Zinan’dala, Hataichanok Chuljerm, Wason Parklak, and Kanokwan Kulprachakarn. 2025. "Watercress (Nasturtium officinale) as a Functional Food for Non-Communicable Diseases Prevention and Management: A Narrative Review" Life 15, no. 7: 1104. https://doi.org/10.3390/life15071104
APA StyleMaluwa, C., Zinan’dala, B., Chuljerm, H., Parklak, W., & Kulprachakarn, K. (2025). Watercress (Nasturtium officinale) as a Functional Food for Non-Communicable Diseases Prevention and Management: A Narrative Review. Life, 15(7), 1104. https://doi.org/10.3390/life15071104






