Abstract
The assessment of hearing in children is important, as hearing deficits can impair child development. The Auditory Steady-State Response (ASSR) is an electrophysiological technique that is able to simultaneously evaluate both ears at four frequencies, making it advantageous for testing children where the test time needs to be as short as possible. The objective of this work was to perform a literature review on the effectiveness of ASSR to gauge hearing thresholds in babies, infants, and children, examining its ability to distinguish mild hearing loss from normal cases. This review used PubMed, Web of Science, and Scopus databases from 2014 to 2024. A total of 1226 articles were identified, although only 16 met the previously established inclusion criteria. It was found that ASSR is a reliable diagnostic tool for babies, infants, and children. Recent work appears better able to distinguish mild hearing loss from normal hearing. One unresolved aspect that needs additional attention is the effectiveness of using bone-conducted stimuli.
1. Introduction
Hearing loss that occurs while a child is developing can have a profound effect on the brain. The loss not only disrupts the formation of new synapses and the maturation of central auditory pathways but also accelerates unwanted synaptic pruning [1,2,3]. These neural alterations directly compromise central auditory function, which in turn can significantly impair the child’s communication ability and their quality of social interaction [4]. The integrity and effective functioning of the peripheral and central auditory systems are essential for speech development. Early diagnosis of any impairment is important for ongoing management [5,6].
An auditory diagnosis is usually made first through neonatal hearing screening conducted before discharge in a hospital’s maternity ward. However, in certain cases where the baby, for some reason, is not evaluated at the hospital, auditory function needs to be investigated in an outpatient setting. If poor responses in one or both ears are found, further diagnostic work is then needed [7,8].
To evaluate the auditory function of small babies and infants, electrophysiological methods are called for, since such instruments do not require the active collaboration of the child [9,10]. Currently, the Specific Frequency Auditory Brainstem Response (SF-ABR) and the Auditory Steady-State Response (ASSR) both allow for auditory thresholds to be objectively determined [9].
In SF-ABR, an evaluator identifies the presence of wave V to estimate the auditory threshold. However, the detection of a response is based on identifying peaks and troughs in the waveform and, therefore, introduces an element of subjectivity in interpretation. Testing is usually conducted at four separate frequencies (500, 1000, 2000, and 4000 Hz), with each ear being investigated individually, factors that increase the test time required. The stimuli used can be tone bursts or chirps [11,12,13,14].
By contrast, ASSR investigates auditory thresholds based on statistical tests that allow four frequencies (500, 1000, 2000, and 4000 Hz) to be analyzed simultaneously in both ears [15,16]. Again, tone bursts or chirps are used. This approach reduces the time taken to conduct an exam, and this is especially beneficial for testing children, who find difficulty remaining still for long periods. In addition, ASSR eliminates possible subjective bias in interpreting results [17,18,19].
Early diagnosis of hearing loss is essential for a child’s ongoing development. ASSR allows for a swift and accurate diagnosis, allowing interventions to be initiated as early as possible, ideally before 6 months of age, to maximize treatment outcomes. However, there are still some controversies regarding the use of ASSRs in cases where individuals have hearing close to normal or have only mild hearing loss [17].
With technological advances, hearing assessment equipment now offers greater precision, especially in cases of mild hearing loss [20]. At the same time, given the importance of early detection in infants, it is essential that all the methods used are effective and appropriate [21]. In this context, the present study aims to conduct a systematic review of the literature surrounding the use of the Auditory Steady-State Response (ASSR) in babies, infants, and children who have normal hearing or mild hearing loss. Our goal is to assess the clinical applicability and limitations of the method in early diagnosis in the pediatric population.
2. Materials and Methods
2.1. Ethics Considerations
In accordance with Resolution No. 510/2016 of the Brazilian National Health Council, this study was exempt from approval by a Research Ethics Committee because it was based exclusively on a review of previously published scientific material.
2.2. Study Design
This systematic literature review aimed to evaluate the applicability of the Auditory Steady-State Response (ASSR) in detecting mild hearing loss or normal auditory thresholds in the pediatric population (children aged 0–12 years). The review followed PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.
2.3. Search Strategy
A comprehensive search was conducted between August and September 2024 across the PubMed, Web of Science, and Scopus databases. Search terms were derived from Medical Subject Headings (MeSH) and included combinations of the following descriptors: auditory steady-state response, objective audiometry, hearing normative, infant, neonatal, diagnostic, auditory evoked potential, and frequency-specific hearing threshold. The Boolean operators “AND” and “OR” were used to combine terms. Table 1 sets out the search strategy.

Table 1.
Search strategy used in databases based on MeSH descriptors and Boolean operators.
2.4. Eligibility Criteria
Studies were included if they met the following inclusion criteria:
- (i)
- Original research articles;
- (ii)
- Participants aged 0–12 years (covering newborns, infants, and children);
- (iii)
- Evaluation of steady-state auditory evoked potential (ASSR) via air conduction;
- (iv)
- Published in English between 2014 and 2024;
- (v)
- Peer-reviewed publication.
Additionally, the following exclusion criteria were applied:
- (i)
- Studies involving only adults;
- (ii)
- Animal studies;
- (iii)
- Gray literature (dissertations, theses, conference abstracts, or non-peer-reviewed publications;
- (iv)
- Studies that evaluated ASSR exclusively via bone conduction.
2.5. Study Selection Process
Two independent reviewers screened the titles and abstracts retrieved from the databases. Articles that met the inclusion criteria underwent full-text review. Discrepancies were resolved through discussion and consensus. The reference lists of the included studies were not screened for additional articles.
2.6. Data Extraction and Analysis
A structured spreadsheet was used to include data from the selected studies. The information collected included (i) study title and year of publication; (ii) participant characteristics (age, sex, hearing status); (iii) type and configuration of ASSR stimuli (e.g., CE-Chirp, modulation rate, intensity, frequency range); (iv) equipment used; (v) presence of complementary audiological assessments (e.g., ABR, otoacoustic emissions, tympanometry); and (vi) main results and conclusions. The results are shown in Table 2. No meta-analysis was performed due to heterogeneity in study designs, populations, and outcome measures.

Table 2.
Characteristics and results achieved of studies included in the present review.
3. Results
3.1. Study Selection
A total of 1266 articles were retrieved through database searches. After screening titles and abstracts and applying inclusion and exclusion criteria, 16 studies were included in the final review. The process for selecting papers is set out in the PRISMA flowchart (Figure 1).
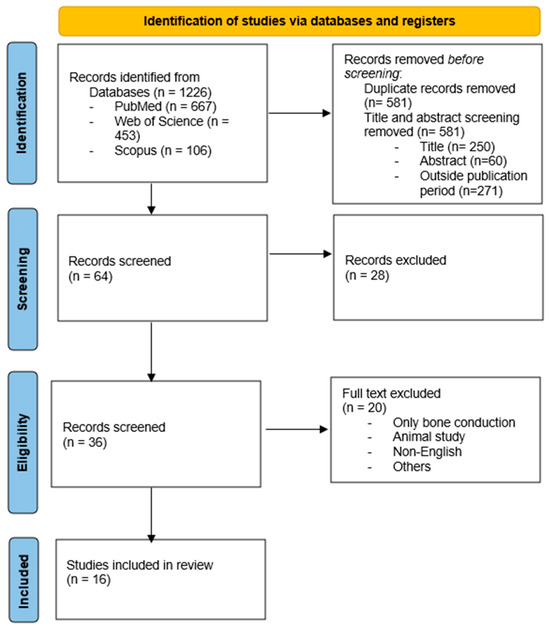
Figure 1.
Flowchart for selecting studies.
3.2. Sample Characteristics
The studies included samples of babies, infants, and children ranging from 24 h [25] to 11 years of age [30]. Most studies focused on infants younger than 2 years [20,22,23,24,25,26,27,28,29,31,32,33,34,35,36], with a predominance of females [20,23,28,30,33]. Table 2 summarizes the main features of the studies.
3.3. Use of Control Groups
Of the 16 studies reviewed, 9 (56%) included a control group in their methodology [23,24,28,29,31,32,34,35,36]. This allowed comparisons to be made between normal hearing individuals and those with hearing impairments [23,28,29,35] and between preterm and full-term infants [24,31,34], as well as to see the effects of age [32,36]. The remaining 6 studies (38%) performed intra-group analyses or focused on just one population group [20,22,25,26,27,33].
3.4. Complementary Evaluations
In terms of complementary audiological evaluations, the auditory brainstem response (ABR) was the most frequently used procedure, appearing in 14 (88%) of the studies [20,22,23,25,26,27,28,29,30,31,32,33,34,36]. Evaluation with a click-type stimulus was used in 8 of the studies [20,22,23,25,26,27,32,33], whereas a tone burst was used in just 1 of the studies [34], while a chirp stimulus was also mentioned in 1 other study [29]. In 4 articles (25%), the type of auditory stimulus used in the ABR evaluation was not mentioned [28,30,31,36].
Otoacoustic emissions were used in 10 studies (63%) [20,22,23,24,25,28,30,31,34,35]. Transient evoked otoacoustic emissions were used in 6 of them (38%) [22,23,24,25,28,29,30,35], while distortion product otoacoustic emissions were applied in just 1 [20]. In 2 studies [31,34], use of emissions was mentioned, but the type was not specified.
Immittance testing was reported in 6 studies [20,22,24,26,28,35], most often using a 1000 Hz probe in infants younger than 9 months of age [20,22,24]. In 1 of the studies [20], a combination of 226 and 1000 Hz probes was used. In 3 studies, tympanometry was used, but the probe frequency was not given [26,28,35].
Behavioral hearing assessments were used in 1 study [35], and visual reinforcement audiometry (VRA) was employed in 5 [23,26,27,28,30]. Only 2 study used the cochleopalpebral reflex [34], and another used behavioral audiometry [35].
4. ASSR Assessments
4.1. Equipment
For ASSR, the Eclipse system by Interacoustics [20,26,27,29,30] was the most commonly reported equipment utilized, followed by the SmartEP from Intelligent Hearing Systems [22,34,35,36] and the Navigator Pro from Biologic-Natus [31,32]. Some 5 studies [23,24,25,28,33] did not specify the device used, presenting a limitation for replication.
4.2. Sound Stimuli
As for the characteristics of the stimuli, the CE-Chirp stimulus was the most frequently used (25%) [20,26,27,29], while the traditional tone burst was reported in only 1 study [34]. Regrettably, most of the studies [22,23,24,25,28,30,31,32,33,35,36] failed to report the type of stimulus used (n = 11). Only 1 study clearly stated the use of a second-generation CE-Chirp stimulus [20]. Modulation rates ranged from 67 Hz [23] to 115 Hz [25], with a 90 Hz modulation rate being the most prevalent among the studies.
Although reported stimulus intensity varied from 20 dBnHL [20] to 100 dBnHL [35], 6 studies did not specify the intensity used [23,24,25,27,28,31].
Frequency-specific and ear-specific analyses were conducted in most studies [20,22,26,27,28,29,30,31,34,36]. Usually, studies assessed thresholds at 500, 1000, 2000, and 4000 Hz [20,22,24,25,26,27,28,29,30,31,32,34,36]. All the studies applied air conduction stimulation, in line with the eligibility criteria. However, 25% of the studies also included bone conduction assessments [22,24,25,28], although these were not the primary focus.
4.3. Clinical Applicability of ASSR
Regarding the clinical applicability and diagnostic accuracy of ASSR, most of the studies [24,25,26,27,28,30,32,34,35,36,37] supported its use as a reliable method in pediatric populations, especially when estimating auditory thresholds. A smaller portion [23,24,34] recommended its use as a complementary technique alongside other electrophysiological or behavioral methods, while another 3 did not reach definitive conclusions about its effectiveness [29,31,33]. Only 1 study explored the feasibility of using ASSR as a tool for hearing screening [33]. Four studies found high sensitivity and specificity for 500 and 2000 Hz in healthy newborns [33,34,35,36], and some assert that there is commendable sensitivity and specificity; however, Nodarse et al. [33] reported values of 100% for both metrics.
In studies involving preterm infants, some authors reported lower precision and greater variability in ASSR responses compared with full-term peers, particularly at lower frequencies [26,33,36]. However, other studies found no significant differences between groups, suggesting that age-related maturation effects may stabilize responses over time [29,31,34,35]. In children with hearing loss, ASSR was shown to be a consistent method for estimating auditory thresholds and provided results comparable to behavioral tests in many cases [23,26,27,30,35].
5. Discussion
In cases of significant hearing loss, the ASSR test is recognized as having a high correlation with behavioral responses [37,38]. However, its applicability in cases where there is a normal hearing threshold, or just mild hearing loss, is still the subject of debate [39]. The present research aimed to focus on ASSR’s abilities in such cases.
The literature review highlighted that one brand (Interacoustics, Middelfart, Dinamarca) was highly represented, justifying its place as a pioneer in the development of the technology. However, the expiry of the original patent has opened up the introduction of alternative models in the market, leading to a proliferation of available systems. Unfortunately, several studies did not mention what sort of equipment they used, and this creates difficulties in trying to replicate them.
In terms of the sound stimuli used, it is pertinent to note that there have been changes and improvements over time. Sound stimuli for assessing ASSR can be classified as first-generation stimuli (stimuli used in the first years after launch of the technology) or second-generation stimuli (the modified stimuli currently available). The CE-Chirp stimulus was introduced as an alternative to the traditional tone burst, and initial studies on its use date back to mid-2014 [40]. When the first studies [41,42] based on the second-generation CE-Chirp appeared, it was discovered that this new sound stimulus allowed responses to be optimized by adjusting the duration and intensity. The low-frequency values, particularly at 500 Hz, exhibited substantial enhancement due to modifications in the second-generation stimulus, resulting in a 20-decibel increase in sensitivity [43]. This improvement was facilitated by adjustments, including the alteration of the standard amplitude modulation stimulus, a phase correction that optimized cochlear delay time, and a frequency shift that permitted the overlap of harmonic responses, thereby significantly enhancing the overall responses [43,44]. Venail et al. [27] observed that second-generation stimuli in ASSR can enhance auditory diagnostics, since the average test time with four frequencies in both ears concurrently is 22.90 min, whereas Sininger [20] reported a test duration of 19.93 min, which is valuable for pediatric diagnosis. The more advanced stimulus model also yielded auditory thresholds that correlated better with Specific Frequency ABR. However, children should be evaluated under natural sleep or sedation to ensure a satisfactory signal-to-noise ratio [44].
Although our literature review covered the years 2014 to 2024, only one study mentioned the use of the second-generation CE-Chirp stimulus [20]. Sininger and colleagues note how this stimulus allows ASSR to identify auditory thresholds at a lower intensity and in a shorter time, with greater consistency with behavioral responses. It is likely that more new studies will be conducted with this stimulus, potentially making ASSR more effective.
Additionally, it is important to consider the auditory stimuli that ASRR values for complex stimuli can be used to estimate how much acoustic speech information is available to the listener. According to Cone and Garinis (2009) [45], electrophoretic measures have the potential to measure children’s perceptual abilities of speech characteristics. Cortical auditory evoked potentials (CAEPs) can be employed in the pediatric population to study the physiological processes and neurological substrates underpinning speech-feature perception in neonates, and they have demonstrated effectiveness in predicting language outcomes [46,47,48].
Another aspect of the sound stimulus is that most researchers have opted to use a 90 Hz modulation rate [20,26,28,30,32,33,34,35,36]. This is appropriate when dealing with children, since such a high rate provides a good correlation with behavioral hearing thresholds—both in children with normal hearing thresholds and in those with some degree of sensorineural hearing loss. However, some researchers warn about limitations of the 90 Hz rate. For example, 40 Hz modulation performs better when hearing at the lower end of the scale (500 and 1000 Hz) is being tested [37,49,50].
This is important because, in general, such frequencies are more difficult to test using other methods, whether behavioral or electrophysiological [51,52]. Furthermore, it is not uncommon for children in a neonatal ICU to have hearing loss at high frequencies and a residual response at lower frequencies, possibly due to the use of ototoxic medications that mainly affect high frequencies [53]. Therefore, an accurate diagnosis at 500 and 1000 Hz is important, since assistance devices need to be set to positively contribute to patient acceptance and rehabilitation [52]. However, if one intends to examine hearing beyond 2000 Hz, using 90 Hz modulation will be more effective [50]. When using high-level intensity stimuli, the use of masking might be considered, since the contralateral ear might be stimulated, discounting the interaural attenuation, which varies from 0 (bone conduction) to 65 dB (air conduction with insert earphones). The use of masking in evoked auditory responses is challenging but needs to be addressed especially in cases of conductive and/or mixed hearing losses [54,55].
Although ASSR responses have been widely investigated in different age groups, there is still disagreement regarding the effect of age on test responses. Thus, Porto et al. [34] and Sousa et al. [24] disagree on whether there are differences between the ASSR responses of premature infants and those of their full-term peers. Porto et al. [34] found no differences between premature and full-term infants, while Sousa et al. [24] reported that the responses of premature infants decreased with time/maturation, and at about 18 months of age, the differences between preterm and at-term babies disappeared. It is important to register that differences between preterm and at-term babies were statistically different but clinically similar. Finally, it is worth mentioning that both studies differed in methodology; the first used only one frequency, and the last used multiple frequency ASSR.
Another work has found no significant differences in the responses of younger babies (5 months) and infants (up to 2 years) [8]. These findings corroborate the work of Van Maanen et al. [36], who found that, from 5 months of age, the responses were broadly the same as those from 2-year-old children. Similarly, when comparisons were made between the responses of babies and adults, it was found that babies had higher thresholds than adults [35]. On the other hand, one study [28] reported that the difference between adults and children is largely restricted to bone conduction stimulation, a finding compatible with those of Alerts et al. [35].
This brings us to the bone conduction issue. One of the most striking aspects of the use of ASSR is different bone conduction responses. According to Casey and Small [28], the assessment of ASSR by bone stimulation is not as accurate as that obtained by means of VRA, since the thresholds obtained by ASSR were, on average, worse. However, there is still much controversy about this. Some authors say that bone conduction stimulation does not seem to be as specific, since they find differences between the values obtained by air conduction and bone conduction. In general, bone conduction values appear to be higher than those obtained by air conduction, but this problem only tends to occur in adults. On the other hand, some researchers have reported that there is no difference between auditory thresholds obtained by air or bone stimulation, either in infants or in adults [22,28]. Most researchers find that, for infants at least, ASSR responses are similar for both stimulation pathways (air and bone) at all frequencies analyzed [20,23,24,25,26,31,34].
Our conclusion is that ASSR assessments via bone are effective in the pediatric population, although in the adult population, they should be used sparingly, since conductive impairments may not be adequately identified. Cases with mild conductive hearing loss may not be identified using ASSR with bone conduction [29].
The differences between air and bone conduction results are important when investigating the type of hearing loss a patient has, especially in the case of babies, infants, and young children [22], whereas conduction stimuli in infants seem to be more intense than the same stimuli in adults, likely due to infant skull maturation [56]. However, most ASSR studies have used air conduction, and so there is a need for new studies exploring whether bone conduction stimulation can contribute to an effective diagnosis.
In their research on ASSR by bone conduction, Small and Stapells [56] found thresholds within normal limits, with an increase for 500 Hz stimuli. Building on this, Hatton et al. [57] proposed a correction factor for 2000 Hz bone conduction ABR in relation to bone conduction ASSR. Further guidance, such as the BSA Early Assessment Guidance [19], offers provisional corrections for calculating dB eHL. These corrections are based on current evidence, accounting for the median difference between ASSR and behavioral thresholds, as well as age-related adjustments.
Similarly, Hulecki and Small [58] observed that behavioral bone conduction minimal response levels using VRA were better in low frequencies compared with high frequencies for infants, like bone conduction ASSRs. This finding suggests the potential for using bone conduction to assess specific frequencies. However, it also highlights the importance of establishing validated reference thresholds to ensure accurate interpretation [29].
To overcome possible deficiencies in the use of bone-conducted ASSR, some researchers have used additional objective assessments such as acoustic immittance measurements [20,22,24,26,28,35]. It is important to mention that, in the pediatric population, particular attention should be given to the probe frequency, since 1000 Hz probes are more effective for younger babies (up to 6 months of age) and 226 Hz probes for older ones. A probe frequency of 1000 Hz is employed for infants due to the mass-dominated admittance of their ears, as a higher-frequency tone is more effective in differentiating diseases from normal middle ear conditions [59,60]. In this review, three research groups used a 1000 Hz probe [20,22,24], but it should be mentioned that broadband tympanometry can also be valuable in assessing whether there are dysfunctions in the tympanic–ossicular system [61,62,63]. Here, the evaluation of pressurized otoacoustic emissions can help in making a differential diagnosis of conductive impairment in children [64] and might be an important diagnostic aid when using ASSR in suspected cases of conductive impairment. However, combined wideband tympanometry and pressurized otoacoustic emissions were not used in the studies we examined.
The main aim of complementary assessments in testing pediatric hearing is to increase diagnostic accuracy and reduce false positives or negatives [65]. They can help differentiate between types and degrees of hearing loss, contributing to a more reliable diagnosis and allowing better monitoring of auditory maturation. A commonly used principle is cross-checking, which refers to the need to use different hearing assessment methods to confirm each finding, reducing the chance of a diagnostic error. This approach ensures that the results obtained by one test are corroborated by another, increasing reliability. In audiology, this is essential, especially for infants and young children, since possible hearing loss needs to be confirmed electrophysiologically and behaviorally.
Cross-checking was observed in all selected articles, where the findings of an ASSR assessment were complemented with other tests. Auditory brainstem response and otoacoustic emissions were the most used tests [20,22,23,25,26,27,28,29,30,31,32,33,34,35,36], probably because they are widely available and can identify hearing deficits in babies, infants, and small children. The assessment of ASSR has been demonstrated by researchers to exhibit a strong correlation with the findings of OAE, as well as with TEOAE [66] and DPOAE [67]. Nevertheless, the responses were more compatible at high frequencies in both cases; thus, the analysis of the responses should be carried out with caution. Porto et al. [34] documented that both ASSR and tone burst ABR at 2000 Hz had good applicability and had the same measurement time (i.e., 20 min).
Furthermore, the integration of methodologies is exceedingly beneficial in detecting patients predisposed to hearing loss, particularly those affected by Congenital Cytomegalovirus (cCMV). In these patients, sensorineural hearing loss is the predominant and debilitating consequence, which may be gradual and late onset; hence, assessment and surveillance are essential. The correlation of ASSR with click and Specific Frequency ABR can yield accurate data, enabling early detection of auditory impairment, hence facilitating the timely administration of suitable drugs to alleviate the advancement of hearing loss [21,68,69].
According to Sininger et al. [20], the ASSR assessment provides lower thresholds and greater diagnostic accuracy than Frequency-Specific ABR. A study by Valeriote et al. [22] investigated how both ASSR and Specific Frequency ABR, applied via air and bone conduction, could detect mild conductive hearing losses. They found that the size of the air–bone gap was insufficient for ASSR to reliably separate normal hearing from mild conductive hearing loss.
François et al. [26] found that the thresholds obtained by ASSR were better by 8–15 dB compared with those obtained through behavioral methods, making it a reliable method for estimating hearing thresholds in children under 6 years of age. However, Panahi et al. [30] observed no differences between the responses obtained by ASSR and those obtained behaviorally. Interestingly, Casey and Small [28] found that ASSR thresholds obtained by bone stimulation were, on average, worse and not as reliable as those obtained through visual reinforcement audiometry (VRA).
In contrast, Panahi et al. [30] found no differences between the responses obtained by ASSR and those obtained by behavioral methods. However, in children with hearing loss, there was no statistically significant difference between the ASSR and VRA thresholds. At the same time, Lu et al. [23] also evaluated this relationship, and found that, in children with normal hearing, mean ASSR thresholds were significantly higher than VRA thresholds, especially at low frequencies.
A pertinent issue to emphasize is the limitation of ASSR in instances of children with auditory neuropathy spectrum disorder (ANSD). Lightfoot et al. [70] indicated that it is not unusual to observe thresholds close to the boundaries of normality in ASSR, even when frequency-specific responses are absent. The occurrence of responses in ASSR may be attributed to sensory artifacts, non-neural responses, and/or short-latency vestibular responses [19]. ASSR evaluation is based on the detection of a peak in a frequency spectrum and receives contributions from multiple generators, which can, unfortunately, result in false-positive results at high levels of sound stimuli in individuals with profound hearing loss due to cochlear nerve malformations, which could be caused by the presence of electrical artifacts [71].
There is still controversy about whether ASSR is a more effective method for determining hearing thresholds than Frequency-Specific ABR. Recommendations for the use of ASSR as being more accurate have already been mentioned [10,20]. Rodrigues et al. [29] underline that ASSR can overcome limitations of Frequency-Specific ABR [72], especially by allowing the evaluation of four frequencies at the same time, making recordings faster. However, there are other researchers who suggest the opposite, favoring the use of Frequency-Specific ABR over ASSR [22,28,29]. Nevertheless, more than half the articles included in this review have concluded that, when applied to the pediatric population, ASSR has better diagnostic efficiency [20,23,24,26,30,31,32,33,35,36]. To reduce the response collection time, a novel assessment technique known as Parallel Auditory Brainstem Response (pABR) has recently been suggested; however, it still needs to be evaluated in the pediatric population due to its low-frequency restrictions [73].
6. Conclusions
This research has confirmed the effectiveness of ASSR as a diagnostic tool for babies, infants, and children; however, ABR is still considered the gold standard within the audiological diagnostic process. Technological innovations such as second-generation CE-Chirp stimuli have led to better detection sensitivity, facilitating the differential diagnosis of mild hearing loss or normal auditory threshold. Although there are still large uncertainties associated with the use of bone conduction, ASSR appears to be a very effective tool in the pediatric population we studied. Furthermore, it is relevant to mention that, in cases where children do not sleep, ASSR is not a viable assessment option; therefore, evaluation through cortical auditory evoked potentials could be employed.
Author Contributions
Conceptualization, M.F.P.M. and M.D.S.; data curation, M.F.P.M., C.D., A.J.T.d.S. and M.D.S.; formal analysis, M.F.P.M., C.D. and M.D.S.; investigation, M.F.P.M., C.D., D.G. and M.D.S.; methodology, M.F.P.M., C.D. and M.D.S.; project administration, D.G., P.H.S. and M.D.S.; supervision, M.D.S.; funding acquisition, M.D.S. and P.H.S.; validation, P.H.S., A.N.d.A. and M.D.S.; visualization, M.D.S. and P.H.S.; writing—original draft, M.F.P.M., C.D., A.J.T.d.S., A.N.d.A., D.G. and M.D.S.; writing—review and editing, M.F.P.M., C.D. and M.D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ABR | auditory brainstem response |
| AC | air conduction |
| ASSR | Auditory Steady-State Response |
| BA | behavioral audiometry |
| BC | bone conduction |
| CE | Chirp-Evoked |
| CE-Chirp | Chirp-Evoked Stimulus |
| CG | control group |
| CPR | cochleopalpebral reflex |
| d | days |
| dB | decibel |
| dB eHL | decibel estimated hearing level |
| dB HL | decibel hearing level |
| DPOAE | distortion product otoacoustic emissions |
| EG | experimental group |
| Equip | equipment |
| F | female |
| Freq | frequency |
| FS | frequency-specific |
| FS-ABR (ND) | Frequency-Specific Auditory Brainstem Response (with type of stimulus not detailed) |
| HL | hearing loss |
| Hz | hertz |
| ICU | intensive care unit |
| Intens | intensity |
| LE | left Ear |
| M | male |
| MeSH | Medical Subject Headings |
| mo | months |
| Mod Rate | modulation rate |
| NB | narrowband |
| NH | normal hearing |
| OAE | otoacoustic emissions |
| OAE-ND | otoacoustic emissions—not detailed |
| PDOAE | distortion product otoacoustic emission |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RE | right ear |
| SF-ABR | Specific Frequency Auditory Brainstem Response |
| Stim | stimulation |
| TOAE | transient otoacoustic emissions |
| Tymp | tympanometry |
| Tymp ND | tympanometry–type of probe not detailed |
| VRA | visual reinforcement audiometry |
| y | years |
References
- Sharma, A.; Nash, A.A.; Dorman, M. Cortical development, plasticity and re-organization in children with cochlear implants. J. Commun. Disord. 2009, 42, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Kral, A.; Sharma, A. Developmental neuroplasticity after cochlear implantation. Trends Neurosci. 2012, 35, 111–122. [Google Scholar] [CrossRef]
- Gilley, P.M.; Sharma, A.; Dorman, M.F. Cortical reorganization in children with cochlear implants. Brain Res. 2008, 6, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Monshizadeh, L.; Vameghi, R.; Rahimi, M.; Sajedi, F.; Hashemi, S.B.; Yadegari, F.; Kasbi, F. Is There Any Association Between Language Acquisition and Cognitive Development in Cochlear-Implanted Children? J. Int. Adv. Otol. 2021, 17, 195–199. [Google Scholar] [CrossRef]
- Northern, J.L.; Downs, M.P. Hearing in Children; Plural Publishing: San Diego, CA, USA, 2014; Volume 6, pp. 10–702. ISBN 978-1-59756-392-5. [Google Scholar]
- Brennan, S.; Lightfoot, G.; Ferm, I.; Fritzgerald, J. Practice Guidance Guidelines for the Early Audiological Assessment and Management of Babies Referred from the Newborn Hearing Screening Programme. Br. Soc. Audiol. 2021, 15, 860. [Google Scholar]
- The Joint Committee on Infant Hearing. Year 2019 Position Statement: Principles and Guidelines for Early Hearing Detection and Intervention Programs. JEHDI 2019, 4, 1–44. [Google Scholar]
- Andrade, A.N.; Soares, A.; Skarzyinski, P.H.; Sanfins, M.D. Childhood audiological assessment (part II): Recommended procedures in the first two years of life. Mendincus Bull. 2024, 14, 1–12. [Google Scholar]
- Bower, C.; Reilly, B.K.; Richerson, J.; Hecht, J.L.; Committee on Practice & Ambulatory Medicine; Section on Otolaryngology–Head and Neck Surgery. Hearing assessment in infants, children, and adolescents: Recommendations beyond neonatal screening. Pediatrics 2023, 152, e2023063288. [Google Scholar] [CrossRef] [PubMed]
- Sanfins, M.D.; Andrade, A.N.; Skarzynski, P.H.; Matas, C.G.; Colella-Santos, M.F. Use of auditory brainstem potentials to measure auditory thresholds: Type of stimulus and use of sedation. Medincus 2023, 9. [Google Scholar] [CrossRef]
- Stapells, D.R. Threshold Estimation by the Tone-Evoked Auditory Brainstem Response: A Literature Meta-Analysis. J. Speech-Lang. Pathol. Audiol. 2000, 24, 74–83. [Google Scholar] [CrossRef]
- Elberling, C.; Don, M. Auditory brainstem responses to a chirp stimulus designed from derived-band latencies in normal-hearing subjects. J. Acoust. Soc. Am. 2008, 124, 3022–3037. [Google Scholar] [CrossRef]
- Findlen, U.M.; Hounam, G.M.; Alexy, E.; Adunka, O.F. Early Hearing Detection and Intervention: Timely Diagnosis, Timely Management. Ear Hear. 2019, 40, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Biagio-de Jager, L.; van Dyk, Z.; Vinck, B.H. Diagnostic accuracy of CE Chirp. Int. J. Pediatr. Otorhinolaryngol. 2020, 135, 110071. [Google Scholar] [CrossRef] [PubMed]
- Ehrmann-Müller, D.; Shehata-Dieler, W.; Alzoubi, A.; Hagen, R.; Cebulla, M. Using ASSR with narrow-band chirps to evaluate hearing in children and adults. Eur. Arch. Otorhinolaryngol. 2021, 278, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Rance, G.; Briggs, R.J. Assessment of hearing in infants with moderate to profound impairment: The Melbourne experience with auditory steady-state evoked potential testing. Ann. Otol. Rhinol. Laryngol. Suppl. 2002, 189, 22–28. [Google Scholar] [CrossRef]
- Beck, R.M.; Ramos, B.F.; Grasel, S.S.; Ramos, H.F.; Moraes, M.F.; Almeida, E.R.; Bento, R.F. Comparative study between pure tone audiometry and auditory steady-state responses in normal hearing subjects. Braz. J. Otorhinolaryngol. 2014, 80, 35–40. [Google Scholar] [CrossRef]
- Lopes, M.B.; Bueno, C.D.; Dinodé, D.D.; Sleifer, P. Comparison between click and CE-CHIRP® stimuli in neonatal hearing screening. J. Hum. Growth Dev. 2020, 30, 260–265. [Google Scholar] [CrossRef]
- British Society of Audiology. Auditory Steady State Response (ASSR) Testing. 2023. Available online: https://www.thebsa.org.uk/resources/ (accessed on 12 June 2025).
- Sininger, Y.S.; Hunter, L.L.; Hayes, D.; Roush, P.A.; Uhler, K.M. Evaluation of Speed and Accuracy of Next-Generation Auditory Steady State Response and Auditory Brainstem Response Audiometry in Children with Normal Hearing and Hearing Loss. Ear Hear. 2018, 39, 1207–1223. [Google Scholar] [CrossRef]
- Sideri, K.P.; Chiriboga, L.F.; Skarzynski, P.H.; Skarzynska, M.B.; Sanfins, M.D.; Colella-Santos, M.F. Correlations Between ASSR Based on Narrow-Band CE® Chirp, Click ABR, and Tone-Burst ABR in Audiological Evaluation of Children Under Anesthesia. Life 2025, 15, 860. [Google Scholar] [CrossRef]
- Valeriote, H.; Small, S.A. Comparisons of Auditory Steady State and Auditory Brainstem Response Thresholds in Infants with Normal Hearing and Conductive Hearing Loss. MedRxiv 2023. [Google Scholar] [CrossRef]
- Lu, P.; Huang, Y.; Chen, W.X.; Jiang, W.; Hua, N.Y.; Wang, Y.; Wang, B.; Xu, Z.M. Measurement of Thresholds Using Auditory Steady-State Response and Cochlear Microphonics in Children with Auditory Neuropathy. J. Am. Acad. Audiol. 2019, 30, 672–676. [Google Scholar] [CrossRef]
- Sousa, A.C.; Didoné, D.D.; Sleifer, P. Longitudinal Comparison of Auditory Steady-State Evoked Potentials in Preterm and Term Infants: The Maturation Process. Int. Arch. Otorhinolaryngol. 2017, 21, 200–205. [Google Scholar] [CrossRef]
- Torres-Fortuny, A.; Hernández-Pérez, H.; Ramírez, B.; Alonso, I.; Eimil, E.; Guerrero-Aranda, A.; Mijares, E. Comparing auditory steady-state responses amplitude evoked by simultaneous air- and bone-conducted stimulation in newborns. Int. J. Audiol. 2016, 55, 375–379. [Google Scholar] [CrossRef]
- François, M.; Dehan, E.; Carlevan, M.; Dumont, H. Use of auditory steady-state responses in children and comparison with other electrophysiological and behavioral tests. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2016, 133, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Venail, F.; Artaud, J.P.; Blanchet, C.; Uziel, A.; Mondain, M. Refining the audiological assessment in children using narrow-band CE-Chirp-evoked auditory steady state responses. Int. J. Audiol. 2015, 54, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Casey, K.A.; Small, S.A. Comparisons of auditory steady state response and behavioral air conduction and bone conduction thresholds for infants and adults with normal hearing. Ear Hear. 2014, 35, 423–439. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.R.; Lewis, D.R. Establishing auditory steady-state response thresholds to narrow band CE-chirps in full-term neonates. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 238–243. [Google Scholar] [CrossRef]
- Panahi, R.; Jafari, Z.; Hasani, S. Relationship between behavioral hearing thresholds and estimated auditory steady-state response thresholds in children with a history of neonatal hyperbilirubinemia. Eur. Arch. Otorhinolaryngol. 2014, 271, 2385–2392. [Google Scholar] [CrossRef]
- Ribeiro, F.M.; Carvallo, R.M.; Marcoux, A.M. Auditory steady-state evoked responses for preterm and term neonates. Audiol. Neurootol. 2010, 15, 97–110. [Google Scholar] [CrossRef]
- Choi, J.M.; Purcell, D.W.; John, M.S. Phase stability of auditory steady-state responses in newborn infants. Ear Hear. 2011, 32, 593–604. [Google Scholar] [CrossRef]
- Nodarse, M.M.E.; Alonso, H.D.; Vázquez, G.J.; Febles, S.E.; Abalo, P.M.C.; Alarcón, M.L.; Terry, R.R. Cribado auditivo neonatal con potenciales evocados auditivos de estado estable a múltiples frecuencias. Acta Otorrinolaringol. Esp. 2011, 62, 87–94. [Google Scholar] [CrossRef]
- Porto, M.A.A.; Azevedo, M.F.; Gil, D. Auditory evoked potentials in premature and full-term infants. Braz. J. Otorhinolaryngol. 2011, 77, 622–627. [Google Scholar] [CrossRef]
- Alaerts, J.; Luts, H.; Van Dun, B.; Desloovere, C.; Wouters, J. Latencies of auditory steady-state responses recorded in early infancy. Audiol. Neurootol. 2010, 15, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Van Maanen, A.; Stapells, D.R. Normal multiple auditory steady-state response thresholds to air-conducted stimuli in infants. J. Am. Acad. Audiol. 2009, 20, 196–207. [Google Scholar] [CrossRef]
- Van Maanen, A.; Stapells, D.R. Comparison of multiple auditory steady-state responses (80 versus 40 Hz) and slow cortical potentials for threshold estimation in hearing-impaired adults. Int. J. Audiol. 2005, 44, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Zheng, Z.; Wang, M.; Zhang, Y.; Tang, M.; Yang, Y.; Liu, Y. Comparison of ASSR and frequency specificity ABR induced by NB CE-Chirp for prediction of behavioral hearing thresholds in children with conductive hearing loss. Int. J. Pediatr. Otorhinolaryngol. 2024, 176, 111826. [Google Scholar] [CrossRef]
- Hatzopoulos, S.; Petruccelli, J.; Śliwa, L.; Jędrzejczak, W.W.; Kochanek, K.; Skarżyński, H. Hearing threshold prediction with Auditory Steady State Responses and estimation of correction functions to compensate for differences with behavioral data, in adult subjects. Part 1: Audera and CHARTR EP devices. Med. Sci. Monit. 2012, 18, MT47–MT53. [Google Scholar] [CrossRef] [PubMed]
- Cebulla, M.; Lurz, H.; Shehata-Dieler, W. Evaluation of waveform, latency and amplitude values of chirp ABR in newborns. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 631–636. [Google Scholar] [CrossRef]
- Tomlin, D.; Rance, G. Maturation of the Central Auditory Nervous System in Children with Auditory Processing Disorder. Semin. Hear. 2016, 37, 74–83. [Google Scholar] [CrossRef]
- Cho, S.W.; Han, K.H.; Jang, H.K.; Chang, S.O.; Jung, H.; Lee, J.H. Auditory brainstem responses to CE-Chirp® stimuli for normal ears and those with sensorineural hearing loss. Int. J. Audiol. 2015, 54, 700–704. [Google Scholar] [CrossRef]
- Swanepoel, D.; Ebrahim, S. Auditory steady-state response and auditory brainstem response thresholds in children. Eur. Arch. Otorhinolaryngol. 2009, 266, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Stürzebecher, E.; Cebulla, M.; Elberling, C. Automated auditory response detection: Statistical problems with repeated testing. Int. J. Audiol. 2005, 44, 110–117. [Google Scholar] [CrossRef]
- Cone, B.; Garinis, A. Auditory steady state responses and speech feature discrimination in infants. J. Am. Acad. Audiol. 2009, 20, 629–643. [Google Scholar] [CrossRef]
- Kurtzberg, D.; Hilpert, P.L.; Kreuzer, J.A.; Vaughan, H.G., Jr. Differential maturation of cortical auditory evoked potentials to speech sounds in normal full-term and very low-birth weight infants. Dev. Med. Child Neurol. 1984, 26, 466–475. [Google Scholar] [CrossRef]
- Novak, G.P.; Kurtzberg, D.; Kreuzer, J.A.; Vaughan, H.F., Jr. Cortical responses to speech sounds and their formants in normal infants: Maturational sequence and spatiotemporal analysis. Electroencephalogr. Clin. Neurophysiol. 1989, 73, 295–305. [Google Scholar] [CrossRef]
- Choudhury, N.; Benasich, A.A. Maturation of auditory evoked potentials from 6 to 48 months: Prediction to 3- and 4-year language and cognitive abilities. Clin. Neurophysiol. 2011, 122, 320–338. [Google Scholar] [CrossRef] [PubMed]
- Rance, G.; Rickards, F.W.; Cohen, L.T.; Vidi, S.; Clark, G.M. The automated prediction of hearing thresholds in sleeping subjects using auditory steady-state evoked potentials. Ear Hear. 1995, 16, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Jalaei, B.; Shaabani, M.; Zakaria, M.N. Mode of recording and modulation frequency effects of auditory steady state response thresholds. Braz. J. Otorhinolaryngol. 2017, 83, 10–15. [Google Scholar] [CrossRef]
- Picton, T.W.; John, M.S.; Dimitrijevic, A.; Purcell, D. Human auditory steady-state responses. Int. J. Audiol. 2003, 42, 177–219. [Google Scholar] [CrossRef]
- Picton, T.W.; Skinner, C.R.; Champagne, S.C.; Kellett, A.J.; Maiste, A.C. Potentials evoked by the sinusoidal modulation of the amplitude or frequency of a tone. J. Acoust. Soc. Am. 1987, 82, 165–178. [Google Scholar] [CrossRef]
- Mathew, R.; Bajo, F.R.; Hatton, N.; Buttfield, L.; Gowrishankar, S.; Vickers, D.; Donnelly, N.; Tysome, J.; Bance, M.; Axon, P. Assessment of the cochlear implant pathway for newborn hearing screening referrals. Cochlear Implant. Int. 2021, 22, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Lightfoot, G.; Cairns, A.; Stevens, J. Noise Levels Required to Mask Stimuli Used in Auditory Brainstem Response Testing. Int. J. Audiol. 2010, 49, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gao, N.; Yin, Y.; Yang, L.; Xie, Y.; Chen, Y.; Dai, P.; Zhang, T. Bone conduction hearing in congenital aural atresia. Eur. Arch. Otorhinolaryngol. 2016, 273, 1697–1703. [Google Scholar] [CrossRef]
- Small, S.A.; Stapells, D.R. Multiple auditory steady-state responses to bone-conduction stimuli in adults with normal hearing. J. Am. Acad. Audiol. 2005, 16, 172–183. [Google Scholar] [CrossRef]
- Hatton, J.L.; Janssen, R.M.; Stapells, D.R. Auditory brainstem response to bone-conducted brief tones in young children with conductive or sensorineural hearing loss. Int. J. Otolaryngol. 2012, 2012, 284864. [Google Scholar] [CrossRef]
- Hulecki, L.R.; Small, S.A. Behavioral bone-conduction thresholds for infants with normal hearing. J. Am. Acad. Audiol. 2011, 22, 81–92. [Google Scholar] [CrossRef]
- Hunter, L.L.; Sanford, C.A. Tympanometry and Wideband Acoustic Immittance. In Handbook of Clinical Audiology, 7th ed.; Katz, J., Ed.; Wolters Kluwer: Philadelphia, PA, USA, 2015. [Google Scholar]
- British Society of Audiology (BSA). Recommended Procedure: Tympanometry and Acoustic Reflex Thresholds; Minor Amendment February 2025; The British Society of Audiology: Seafield, UK, 2025. [Google Scholar]
- Sanford, C.A.; Keefe, D.H.; Liu, Y.W.; Fitzpatrick, D.; McCreery, R.W.; Lewis, D.E.; Gorga, M.P. Sound-conduction effects on distortion-product otoacoustic emission screening outcomes in newborn infants: Test performance of wideband acoustic trans-fer functions and 1-kHz tympanometry. Ear Hear. 2009, 30, 635–652. [Google Scholar] [CrossRef]
- Aithal, S.; Aithal, V.; Kei, J. Effect of ear canal pressure and age on wideband absorbance in young infants. Int. J. Audiol. 2017, 56, 346–355. [Google Scholar] [CrossRef]
- Hunter, L.L.; Keefe, D.H.; Feeney, M.P.; Fitzpatrick, D.F.; Lin, L. Longitudinal development of wideband reflectance tympanometry in normal and at-risk infants. Hear. Res. 2016, 340, 3–14. [Google Scholar] [CrossRef]
- Zimatore, G.; Skarzynski, P.H.; Di Berardino, F.; Filipponi, E.; Hatzopoulos, S. Differences between pressurized and non-pres-surized transient-evoked otoacoustic emissions in neonatal subjects. Audiol. Neuro-Otol. 2021, 26, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Wadhera, R.; Hernot, S.; Gulati, S.P.; Kalra, V. A controlled comparison of auditory steady-state responses and pure-tone audiometry in patients with hearing loss. Ear Nose Throat J. 2017, 96, E47–E52. [Google Scholar] [CrossRef]
- Erdem, M.Z.; Garça, M.F. Comparison of the Efficacy of Auditory Steady-State Response (ASSR) and Otoacoustic Emission (OAE) in Neonatal Hearing Screening. East. J. Med. 2024, 29, 467–477. [Google Scholar] [CrossRef]
- Mahmoudian, S.; Farhadi, M.; Kadivar, M.; Ghalehbaghi, B.; Rahimi, F.; Hemami, M.R.; Kamrava, S.K.; Asghari, A.; Amintehran, E.; Mohagheghi, P. Prognostic validity of dichotic multiple frequencies auditory steadystate responses versus distortion product otoacoustic emissions in hearing screening of high risk neonates. Int. J. Pediatr. Otorhinolaryngol. 2011, 75, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.; Sanfins, M.D.; Pinto, J.D.; Skarzynski, P.H.; Skarzyńska, M.B.; Vieira Biaggio, E.P. Congenital toxoplasmosis and auditory disorders: A literature review. Front. Psychol. 2024, 14, 1286211. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.; Valadão, M.; Skarzynski, P.; Sanfins, M.; Biaggio, E. Effect of congenital toxoplasmosis on the encoding of speech in infants. Int. J. Pediatr. Otorhinolaryngol. 2020, 129, 109767. [Google Scholar] [CrossRef]
- Lightfoot, G.; Norman, G. ASSR Results in Auditory Neuropathy: A Case of Unexpectedly Good ASSR Thresholds. In Proceedings of the XXVI IERASG Biennial Symposium, Sydney, Australia, 30 June–4 July 2019. [Google Scholar]
- Eder, K.; Polterauer, D.; Semmelbauer, S.; Schuster, M.; Rader, T.; Hoster, E.; Flatz, W. Comparison of ABR and ASSR using narrow-band-chirp-stimuli in children with cochlear malformation and/or cochlear nerve hypoplasia suffering from severe/profound hearing loss. Eur. Arch. Otorhinolaryngol. 2022, 279, 2845–2855. [Google Scholar] [CrossRef]
- Rodrigues, G.R.I.; Lewis, D.R.; Fichino, S.N. Steady-state auditory evoked responses in audiological diagnosis in children: A comparison with brainstem evoked auditory responses. Braz. J. Otorhinolaryngol. 2010, 76, 96–101. [Google Scholar] [CrossRef]
- Polonenko, M.J.; Maddox, R.K. Optimizing Parameters for Using the Parallel Auditory Brainstem Response to Quickly Estimate Hearing Thresholds. Ear Hear. 2022, 43, 646–658. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).