Ablation of the Evolutionarily Acquired Functions of the Atp1b4 Gene Increases Metabolic Capacity and Reduces Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Generation and Maintenance of Atp1b4 Knockout Mice
2.2. Metabolic Parameters
2.3. Intraperitoneal Glucose Tolerance Test
2.4. Intraperitoneal Insulin Tolerance Test
2.5. Body Composition
2.6. Energy Balance Measurements
2.7. Ex Vivo Palmitate Oxidation
2.8. Protein Isolation and Western Blotting
2.9. Semi-Quantitative and Quantitative Real Time-PCR (qRT-PCR)
2.10. Statistical Analysis
3. Results
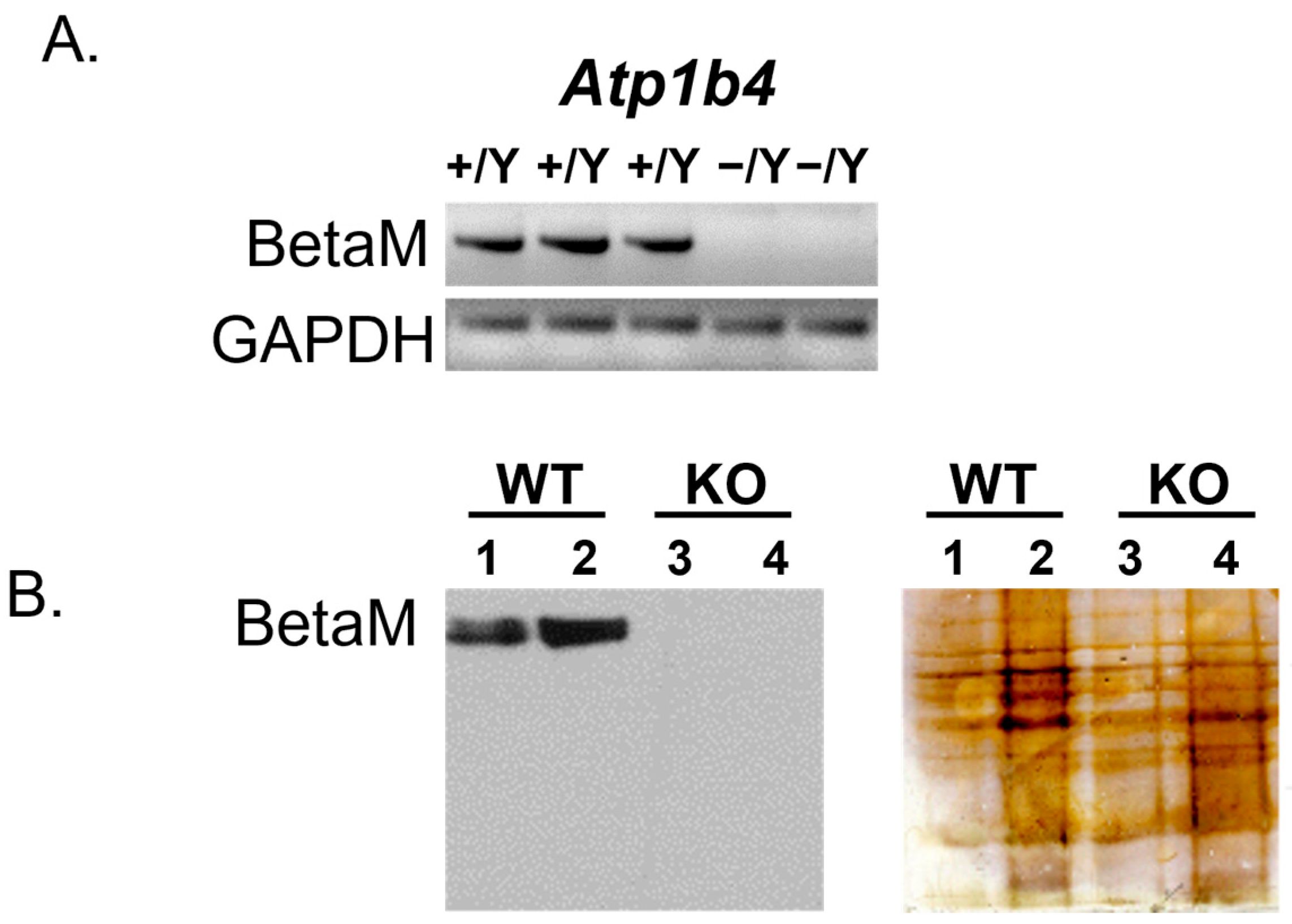
3.1. Construction of the BetaM Knockout Mouse
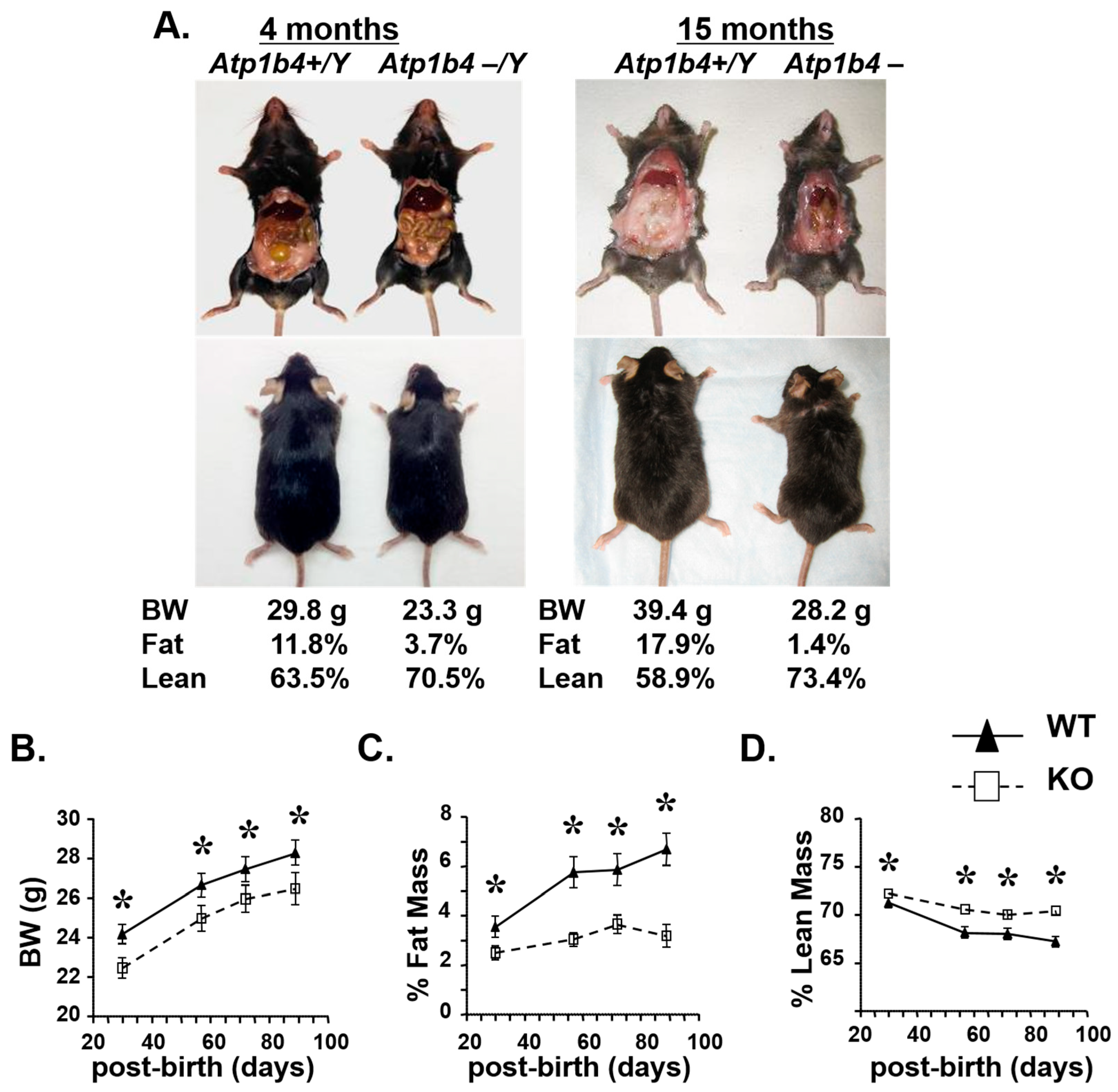
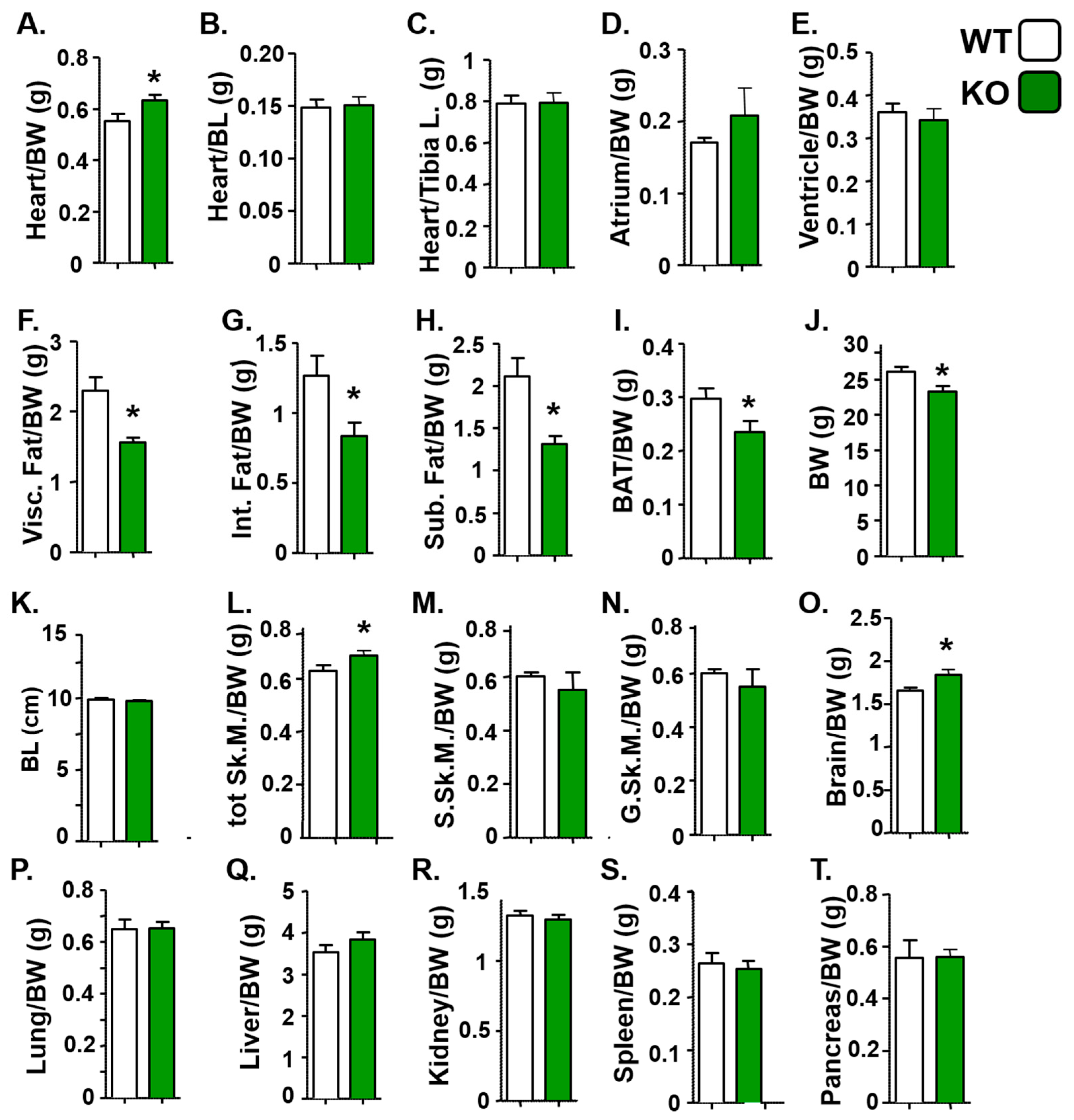
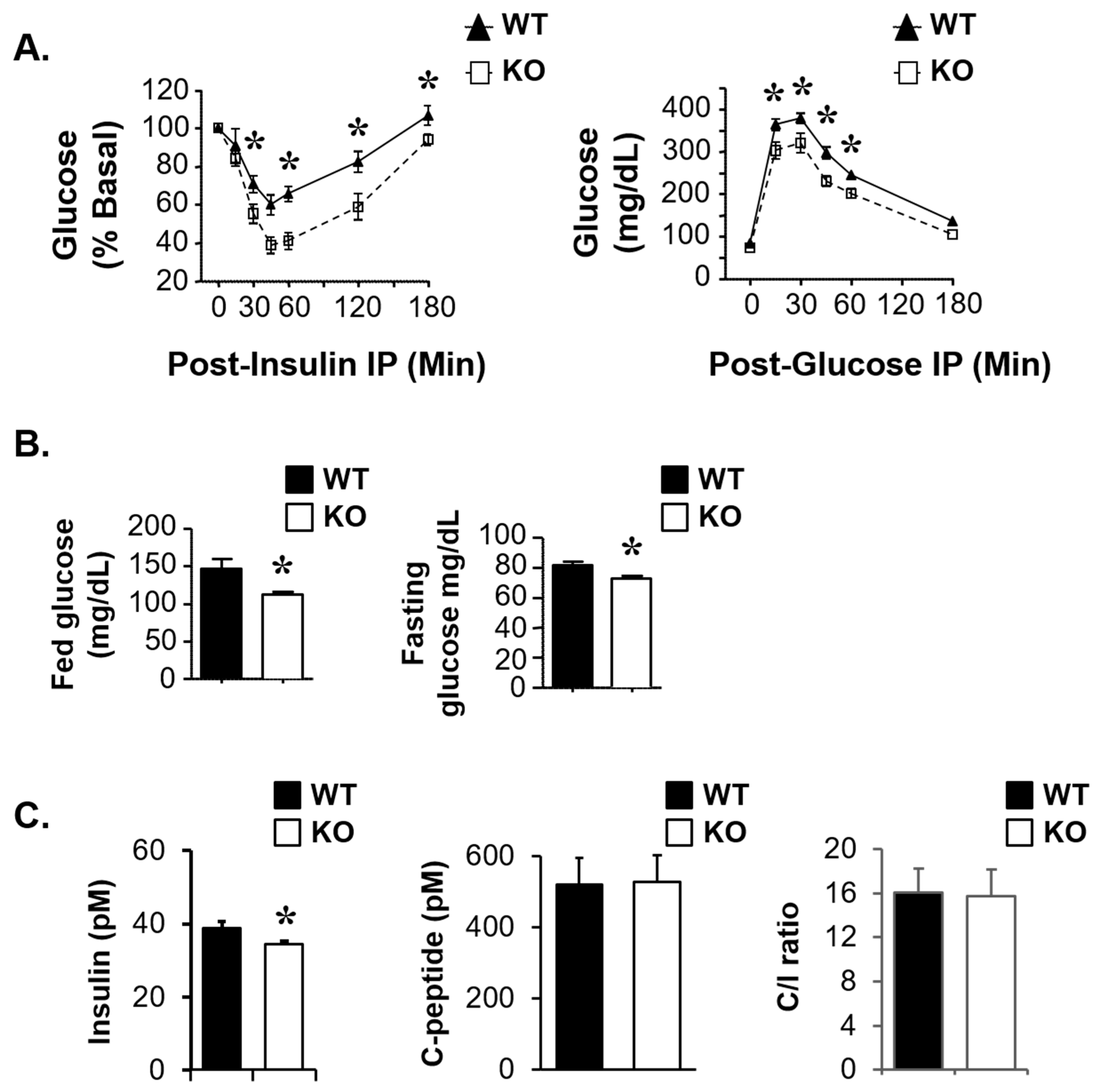
3.2. Mice Deficient in Atp1b4 Exhibit Reduced Body Weight and Adiposity
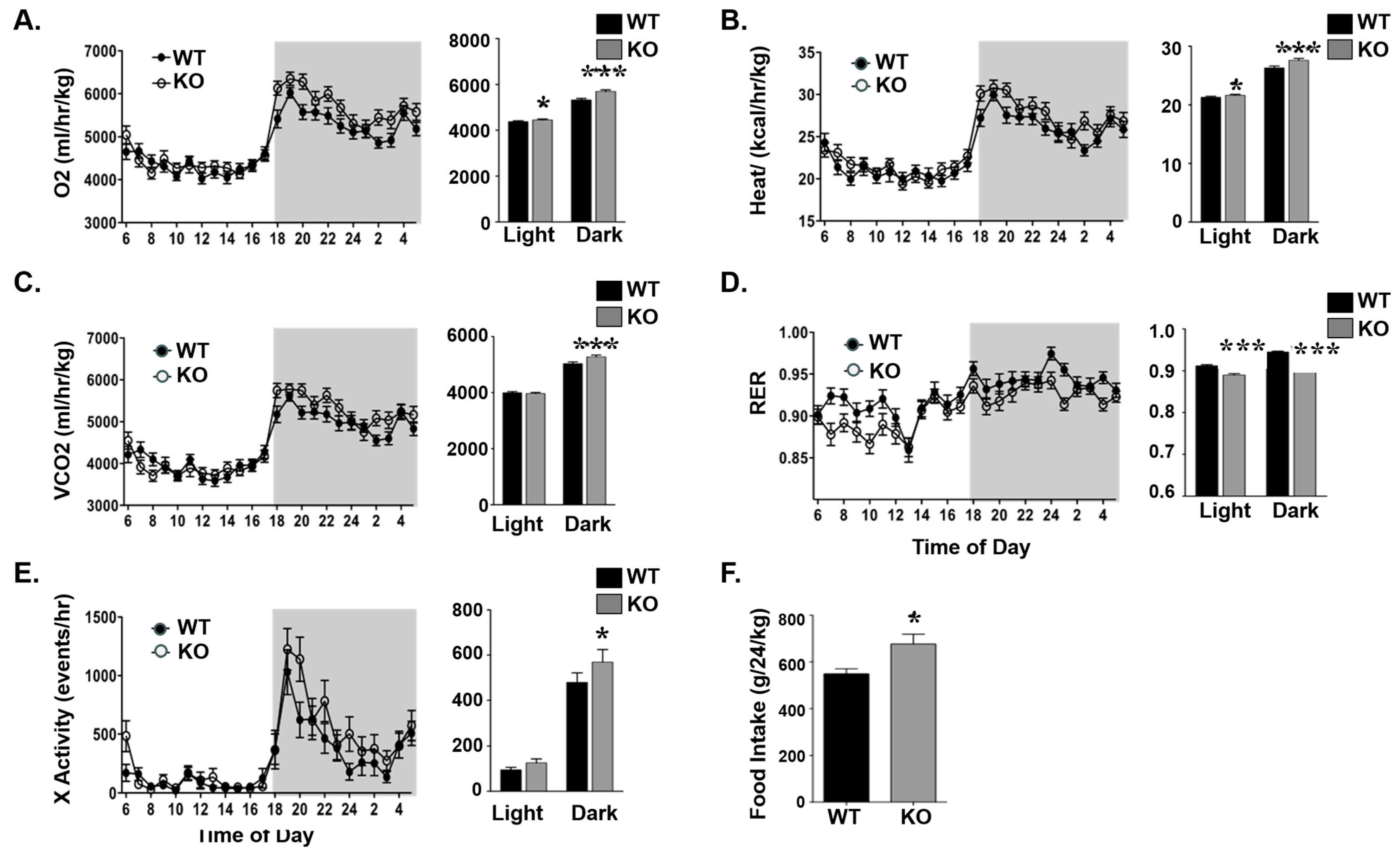
3.3. Elevated Energy Expenditure in Atp1b4-Deficient Male Mice
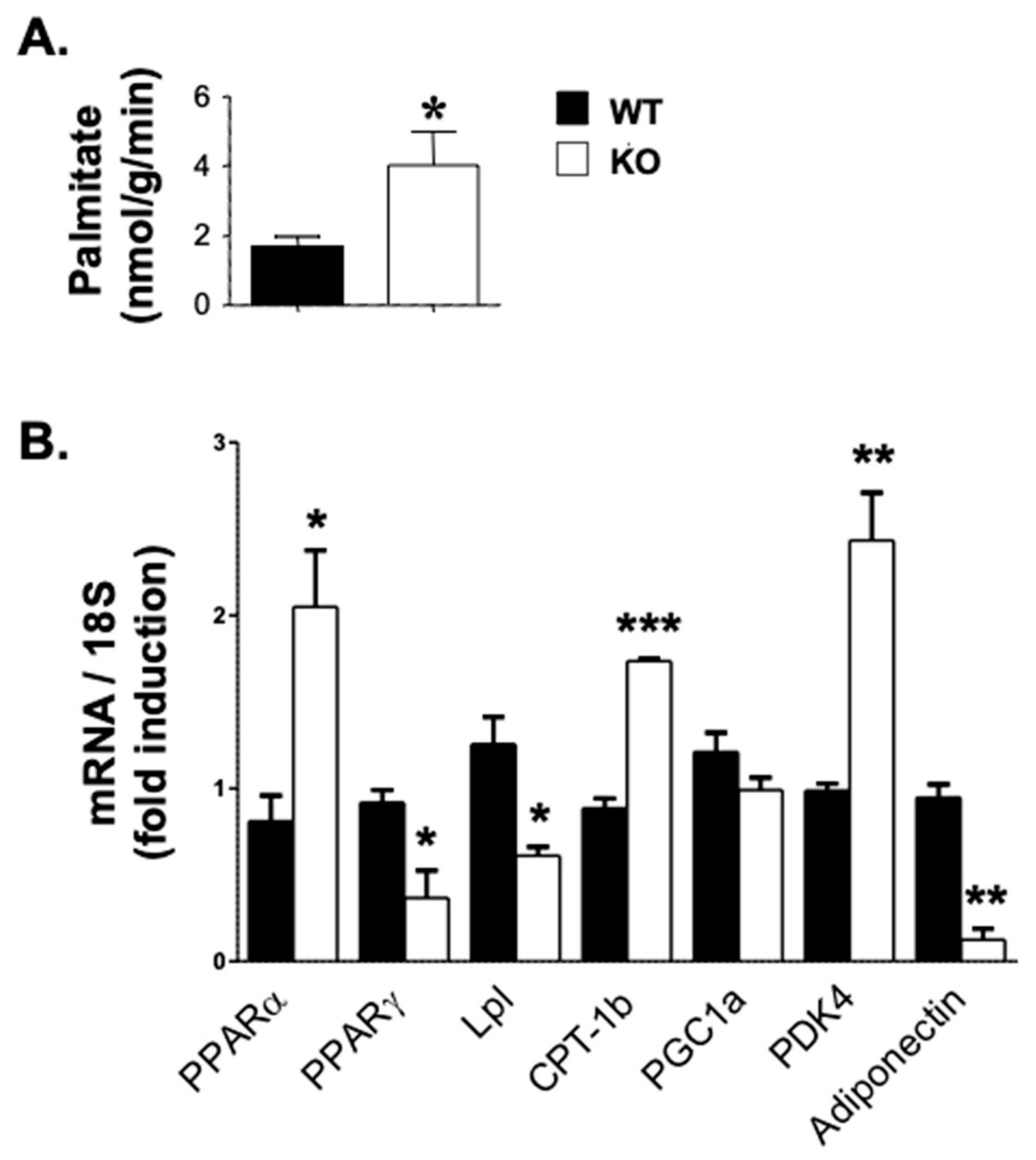
3.4. Elevated Fatty Acid β-Oxidation and Reduced Lipid Accumulation in Atp1b4 KO Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pestov, N.B.; Ahmad, N.; Korneenko, T.V.; Zhao, H.; Radkov, R.; Schaer, D.; Roy, S.; Bibert, S.; Geering, K.; Modyanov, N.N. Evolution of Na,K-ATPase beta m-subunit into a coregulator of transcription in placental mammals. Proc. Natl. Acad. Sci. USA 2007, 104, 11215–11220. [Google Scholar] [CrossRef] [PubMed]
- Lusk, C.P.; Blobel, G.; King, M.C. Highway to the inner nuclear membrane: Rules for the road. Nat. Rev. Mol. Cell Biol. 2007, 8, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Geering, K. The functional role of beta subunits in oligomeric P-type ATPases. J. Bioenerg. Biomembr. 2001, 33, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Pestov, N.B.; Adams, G.; Shakhparonov, M.I.; Modyanov, N.N. Identification of a novel gene of the X,K-ATPase beta-subunit family that is predominantly expressed in skeletal and heart muscles. FEBS Lett. 1999, 456, 243–248. [Google Scholar] [CrossRef]
- Pestov, N.B.; Korneenko, T.V.; Zhao, H.; Adams, G.; Kostina, M.B.; Shakhparonov, M.I.; Modyanov, N.N. The betam protein, a member of the X,K-ATPase beta-subunits family, is located intracellularly in pig skeletal muscle. Arch. Biochem. Biophys. 2001, 396, 80–88. [Google Scholar] [CrossRef]
- Zhao, H.; Pestov, N.B.; Korneenko, T.V.; Shakhparonov, M.I.; Modyanov, N.N. Accumulation of beta (m), a structural member of X,K-ATPase beta-subunit family, in nuclear envelopes of perinatal myocytes. Am. J. Physiol. Cell Physiol. 2004, 286, C757–C767. [Google Scholar] [CrossRef][Green Version]
- Faux, N.G.; Bottomley, S.P.; Lesk, A.M.; Irving, J.A.; Morrison, J.R.; de la Banda, M.G.; Whisstock, J.C. Functional insights from the distribution and role of homopeptide repeat-containing proteins. Genome Res. 2005, 15, 537–551. [Google Scholar] [CrossRef]
- Garza, A.S.; Ahmad, N.; Kumar, R. Role of intrinsically disordered protein regions/domains in transcriptional regulation. Life Sci. 2009, 84, 189–193. [Google Scholar] [CrossRef]
- Pestov, N.B.; Korneenko, T.V.; Zhao, H.; Adams, G.; Shakhparonov, M.I.; Modyanov, N.N. Immunochemical demonstration of a novel beta-subunit isoform of X, K-ATPase in human skeletal muscle. Biochem. Biophys. Res. Commun. 2000, 277, 430–435. [Google Scholar] [CrossRef]
- Pestov, N.B.; Crambert, G.; Zhao, H.; Korneenko, T.V.; Shakhparonov, M.I.; Geering, K.; Modyanov, N.N. The muscle-specific beta m protein is functionally different from other members of the X,K-ATPase beta-subunit family. Ann. N. Y. Acad. Sci. 2003, 986, 304–305. [Google Scholar] [CrossRef]
- Pestov, N.B.; Zhao, H.; Basrur, V.; Modyanov, N.N. Isolation and characterization of BetaM protein encoded by ATP1B4--a unique member of the Na,K-ATPase beta-subunit gene family. Biochem. Biophys. Res. Commun. 2011, 412, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Korneenko, T.V.; Pestov, N.B.; Ahmad, N.; Okkelman, I.A.; Dmitriev, R.I.; Shakhparonov, M.I.; Modyanov, N.N. Evolutionary diversification of the BetaM interactome acquired through co-option of the ATP1B4 gene in placental mammals. Sci. Rep. 2016, 6, 22395. [Google Scholar] [CrossRef]
- Ahmad, N.; de la Serna, I.L.; Marathe, H.G.; Fan, X.; Dube, P.; Zhang, S.; Haller, S.T.; Kennedy, D.J.; Pestov, N.B.; Modyanov, N.N. Eutherian-Specific Functions of BetaM Acquired through Atp1b4 Gene Co-Option in the Regulation of MyoD Expression. Life 2023, 13, 414. [Google Scholar] [CrossRef]
- Testa, G.; Schaft, J.; van der Hoeven, F.; Glaser, S.; Anastassiadis, K.; Zhang, Y.; Hermann, T.; Stremmel, W.; Stewart, A.F. A reliable lacZ expression reporter cassette for multipurpose, knockout-first alleles. Genesis 2004, 38, 151–158. [Google Scholar] [CrossRef]
- Al-Share, Q.Y.; DeAngelis, A.M.; Lester, S.G.; Bowman, T.A.; Ramakrishnan, S.K.; Abdallah, S.L.; Russo, L.; Patel, P.R.; Kaw, M.K.; Raphael, C.K.; et al. Forced Hepatic Overexpression of CEACAM1 Curtails Diet-Induced Insulin Resistance. Diabetes 2015, 64, 2780–2790. [Google Scholar] [CrossRef]
- Patel, P.R.; Ramakrishnan, S.K.; Kaw, M.K.; Raphael, C.K.; Ghosh, S.; Marino, J.S.; Heinrich, G.; Lee, S.J.; Bourey, R.E.; Hill, J.W.; et al. Increased metabolic rate and insulin sensitivity in male mice lacking the carcino-embryonic antigen-related cell adhesion molecule 2. Diabetologia 2012, 55, 763–772. [Google Scholar] [CrossRef]
- Sorensen, B.K.; Hojrup, P.; Ostergard, E.; Jorgensen, C.S.; Enghild, J.; Ryder, L.R.; Houen, G. Silver staining of proteins on electroblotting membranes and intensification of silver staining of proteins separated by polyacrylamide gel electrophoresis. Anal. Biochem. 2002, 304, 33–41. [Google Scholar] [CrossRef]
- Barker, D.J. The developmental origins of chronic adult disease. Acta Paediatr. Suppl. 2004, 93, 26–33. [Google Scholar] [CrossRef]
- Horikoshi, M.; Beaumont, R.N.; Day, F.R.; Warrington, N.M.; Kooijman, M.N.; Fernandez-Tajes, J.; Feenstra, B.; van Zuydam, N.R.; Gaulton, K.J.; Grarup, N.; et al. Genome-wide associations for birth weight and correlations with adult disease. Nature 2016, 538, 248–252. [Google Scholar] [CrossRef]
- Kersten, S. Integrated physiology and systems biology of PPARalpha. Mol. Metab. 2014, 3, 354–371. [Google Scholar] [CrossRef]
- Wynn, R.M.; Kato, M.; Chuang, J.L.; Tso, S.C.; Li, J.; Chuang, D.T. Pyruvate dehydrogenase kinase-4 structures reveal a metastable open conformation fostering robust core-free basal activity. J. Biol. Chem. 2008, 283, 25305–25315. [Google Scholar] [CrossRef]
- Rakhshandehroo, M.; Knoch, B.; Muller, M.; Kersten, S. Peroxisome proliferator-activated receptor alpha target genes. PPAR Res. 2010, 2010, 612089. [Google Scholar] [CrossRef]
- Ahmadian, M.; Suh, J.M.; Hah, N.; Liddle, C.; Atkins, A.R.; Downes, M.; Evans, R.M. PPARgamma signaling and metabolism: The good, the bad and the future. Nat. Med. 2013, 19, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Mead, J.R.; Irvine, S.A.; Ramji, D.P. Lipoprotein lipase: Structure, function, regulation, and role in disease. J. Mol. Med. 2002, 80, 753–769. [Google Scholar] [CrossRef] [PubMed]
- Diez, J.J.; Iglesias, P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur. J. Endocrinol. 2003, 148, 293–300. [Google Scholar] [CrossRef]
- True, J.R.; Carroll, S.B. Gene co-option in physiological and morphological evolution. Annu. Rev. Cell Dev. Biol. 2002, 18, 53–80. [Google Scholar] [CrossRef] [PubMed]
- Charge, S.B.; Rudnicki, M.A. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 2004, 84, 209–238. [Google Scholar] [CrossRef]
- Zhao, P.; Hoffman, E.P. Embryonic myogenesis pathways in muscle regeneration. Dev. Dyn. 2004, 229, 380–392. [Google Scholar] [CrossRef]
- Shi, X.; Garry, D.J. Muscle stem cells in development, regeneration, and disease. Genes. Dev. 2006, 20, 1692–1708. [Google Scholar] [CrossRef]
- Marin, P.; Andersson, B.; Krotkiewski, M.; Bjorntorp, P. Muscle fiber composition and capillary density in women and men with NIDDM. Diabetes Care 1994, 17, 382–386. [Google Scholar] [CrossRef]
- Tanner, C.J.; Barakat, H.A.; Dohm, G.L.; Pories, W.J.; MacDonald, K.G.; Cunningham, P.R.; Swanson, M.S.; Houmard, J.A. Muscle fiber type is associated with obesity and weight loss. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E1191–E1196. [Google Scholar] [CrossRef]
- Rinaudo, P.; Wang, E. Fetal programming and metabolic syndrome. Annu. Rev. Physiol. 2012, 74, 107–130. [Google Scholar] [CrossRef]
- Lee, J.S.; Pinnamaneni, S.K.; Eo, S.J.; Cho, I.H.; Pyo, J.H.; Kim, C.K.; Sinclair, A.J.; Febbraio, M.A.; Watt, M.J. Saturated, but not n-6 polyunsaturated, fatty acids induce insulin resistance: Role of intramuscular accumulation of lipid metabolites. J. Appl. Physiol. 2006, 100, 1467–1474. [Google Scholar] [CrossRef]
- Merz, K.E.; Thurmond, D.C. Role of Skeletal Muscle in Insulin Resistance and Glucose Uptake. Compr. Physiol. 2020, 10, 785–809. [Google Scholar] [CrossRef] [PubMed]
- Najjar, S.M.; Caprio, S.; Gastaldelli, A. Insulin Clearance in Health and Disease. Annu. Rev. Physiol. 2023, 85, 363–381. [Google Scholar] [CrossRef] [PubMed]
- Patarrao, R.S.; Meneses, M.J.; Ghadieh, H.E.; Herrera, L.; Duarte, S.; Ribeiro, R.T.; Raposo, J.F.; Schmitt, V.; Singer, B.B.; Gastaldelli, A.; et al. Insights into circulating CEACAM1 in insulin clearance and disease progression: Evidence from the Portuguese PREVADIAB2 study. Eur. J. Clin. Investig. 2024, 54 (Suppl. 2), e14344. [Google Scholar] [CrossRef]
- Lin, Y.; Shi, H.; Yang, R.; Li, S.; Tang, J.; Li, S. A transcriptomic analysis of incisional hernia based on high-throughput sequencing technology. Hernia 2024, 28, 1899–1907. [Google Scholar] [CrossRef]
- Bai, X.; Huang, Z.; Tan, H.; Gu, Y.; Wang, X.; Jin, L.; Shang, P.; Long, K.; Li, D.; Li, M. Insights into high-altitude adaptation and meat quality regulation by gastrointestinal metabolites in Tibetan and black pigs. Front. Vet. Sci. 2025, 12, 1569196. [Google Scholar] [CrossRef]
- Oraha, J.; Enriquez, R.F.; Herzog, H.; Lee, N.J. Sex-specific changes in metabolism during the transition from chow to high-fat diet feeding are abolished in response to dieting in C57BL/6J mice. Int. J. Obes. 2022, 46, 1749–1758. [Google Scholar] [CrossRef]
- Casimiro, I.; Stull, N.D.; Tersey, S.A.; Mirmira, R.G. Phenotypic sexual dimorphism in response to dietary fat manipulation in C57BL/6J mice. J. Diabetes Complicat. 2021, 35, 107795. [Google Scholar] [CrossRef]
- Neel, J.V. Diabetes mellitus: A “thrifty” genotype rendered detrimental by “progress”? Am. J. Hum. Genet. 1962, 14, 353–362. [Google Scholar] [PubMed]
- MacMillen, R.E.; Nelson, J.E. Bioenergetics and body size in dasyruid marsupials. Am. J. Physiol. 1969, 217, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- Keipert, S.; Gaudry, M.J.; Kutschke, M.; Keuper, M.; Dela Rosa, M.A.S.; Cheng, Y.; Monroy Kuhn, J.M.; Laterveer, R.; Cotrim, C.A.; Giere, P.; et al. Two-stage evolution of mammalian adipose tissue thermogenesis. Science 2024, 384, 1111–1117. [Google Scholar] [CrossRef]
- Modyanov, N.; de la Serna, I.; Ahmad, N.; Russo, L.; Pestov, N.; Najjar, S. Impact of ATP1B4 Gene Co-option on Perinatal Development of Placental Mammals. FASEB J. 2015, 29, 684.1. [Google Scholar] [CrossRef]
| Primer | Forward Sequence | Reverse Sequence |
|---|---|---|
| 18S | TTCGAACGTCTGCCCTATCAA | ATGGTAGGCACGGCGACT |
| Ppar-a | TGCTGGTATCGGCTCAATAA | TCCTGCCACTTGCTCACTAC |
| Ppar-g | AGATCATCTACACGATGCTGGCCT | ATAAAGTCACCAAAGGGCTTCCGC |
| Lpl | AAGGTCAGAGCCAAGAGAAGCA | CCAGAAAAGTGAATCTTGACTTGGT |
| Cpt-1b | CAGCGCTTTGGGAACCACAT | CACTGCCTCAAGAGCTGTTCTC |
| Pgck-1a | AACAAGCACTTCGGTCATCCCTG | TTACTGAAGTCGCCATCCCTTAG |
| Pdk4 | TTTCTCGTCTCTACGCCAAG | GATACACCAGTCATCAGCTTCG |
| Adiponectin | GGCCGTTCTCTTCACCTACG | TGGAGGAGCACAGAGCCAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modyanov, N.N.; Russo, L.; Lester, S.G.; Castañeda, T.R.; Marathe, H.G.; Fedorova, L.V.; Bourey, R.E.; Najjar, S.M.; de la Serna, I.L. Ablation of the Evolutionarily Acquired Functions of the Atp1b4 Gene Increases Metabolic Capacity and Reduces Obesity. Life 2025, 15, 1103. https://doi.org/10.3390/life15071103
Modyanov NN, Russo L, Lester SG, Castañeda TR, Marathe HG, Fedorova LV, Bourey RE, Najjar SM, de la Serna IL. Ablation of the Evolutionarily Acquired Functions of the Atp1b4 Gene Increases Metabolic Capacity and Reduces Obesity. Life. 2025; 15(7):1103. https://doi.org/10.3390/life15071103
Chicago/Turabian StyleModyanov, Nikolai N., Lucia Russo, Sumona Ghosh Lester, Tamara R. Castañeda, Himangi G. Marathe, Larisa V. Fedorova, Raymond E. Bourey, Sonia M. Najjar, and Ivana L. de la Serna. 2025. "Ablation of the Evolutionarily Acquired Functions of the Atp1b4 Gene Increases Metabolic Capacity and Reduces Obesity" Life 15, no. 7: 1103. https://doi.org/10.3390/life15071103
APA StyleModyanov, N. N., Russo, L., Lester, S. G., Castañeda, T. R., Marathe, H. G., Fedorova, L. V., Bourey, R. E., Najjar, S. M., & de la Serna, I. L. (2025). Ablation of the Evolutionarily Acquired Functions of the Atp1b4 Gene Increases Metabolic Capacity and Reduces Obesity. Life, 15(7), 1103. https://doi.org/10.3390/life15071103










