Effects of a Self-Management Telehealth Program on Improving Strength and Hand Function in Systemic Sclerosis Patients: A Randomized Controlled Trial
Abstract
1. Introduction
2. Methods
2.1. Intervention
2.2. Data Collection
2.3. Operational Definitions
2.4. Statistical Analyses
2.5. Sample Size Calculations
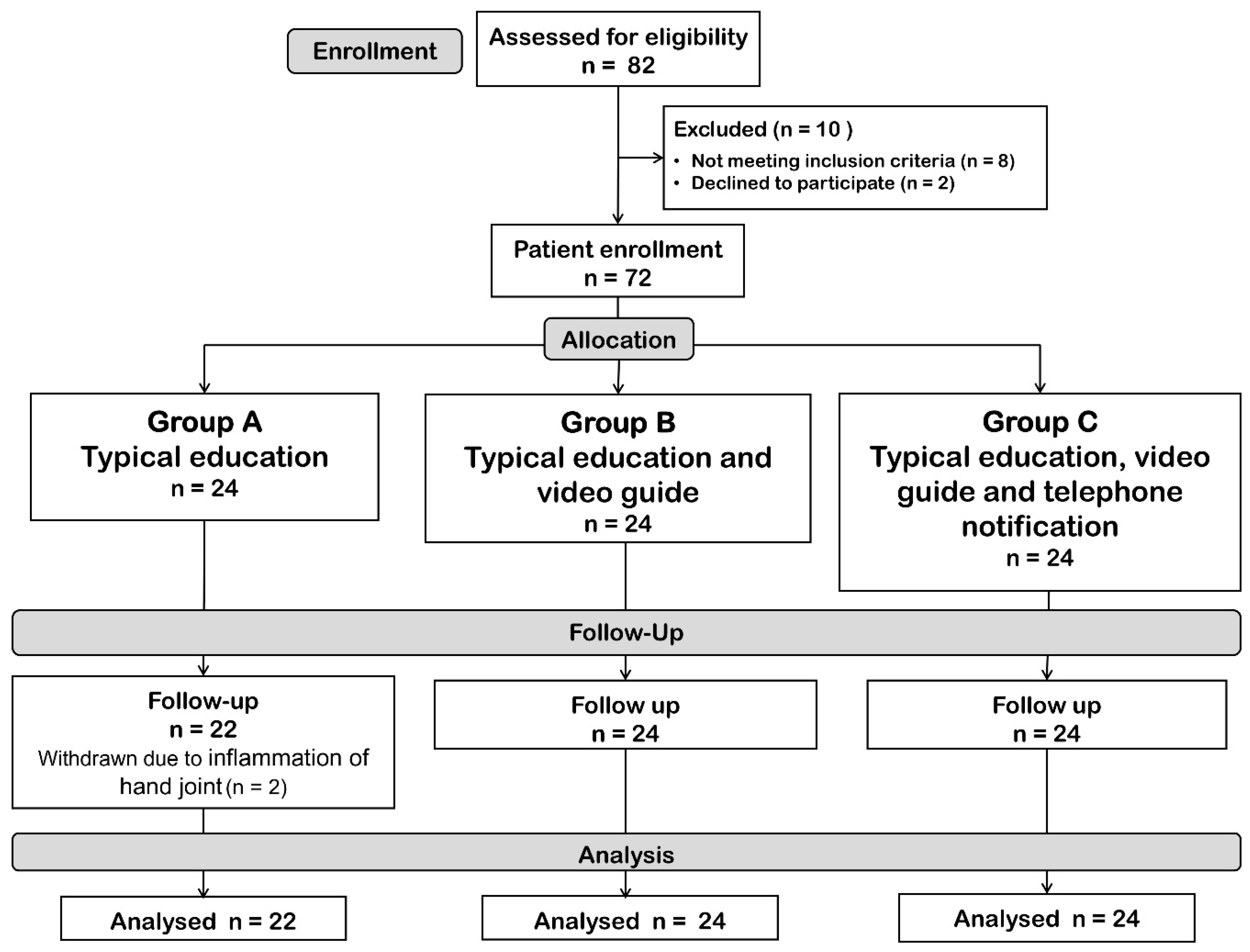
3. Results
3.1. Primary Endpoint
3.2. Secondary Endpoints
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, A.A.; Carl, H.M.; Lifchez, S.D. The Scleroderma Hand: Manifestations of Disease and Approach to Management. J. Hand Surg. 2018, 43, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Namas, R.; Dodge, C.; Khanna, D. Hand Impairment in Systemic Sclerosis: Various Manifestations and Currently Available Treatment. Curr. Treat. Opt. Rheumatol. 2016, 2, 252–269. [Google Scholar] [CrossRef] [PubMed]
- Mugii, N.; Matsushita, T.; Oohata, S.; Okita, H.; Yahata, T.; Someya, F.; Hasegawa, M.; Fujimoto, M.; Takehara, K.; Hamaguchi, Y. Long-Term Follow-up of Finger Passive Range of Motion in Japanese Systemic Sclerosis Patients Treated with Self-Administered Stretching. Mod. Rheumatol. 2019, 29, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Sandqvist, G.; Scheja, A.; Hesselstrand, R. Pain, Fatigue and Hand Function Closely Correlated to Work Ability and Employment Status in Systemic Sclerosis. Rheumatology 2010, 49, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Poole, J.L.; Santhanam, D.D.; Latham, A.L. Hand Impairment and Activity Limitations in Four Chronic Diseases. J. Hand Ther. 2013, 26, 232–237. [Google Scholar] [CrossRef]
- Sandqvist, G.; Eklund, M.; Åkesson, A.; Nordenskiöld, U. Daily Activities and Hand Function in Women with Scleroderma. Scand. J. Rheumatol. 2004, 33, 102–107. [Google Scholar] [CrossRef]
- Askew, L.J.; Beckett, V.L.; An, K.N.; Chao, E.Y. Objective Evaluation of Hand Function in Scleroderma Patients to Assess Effectiveness of Physical Therapy. Br. J. Rheumatol. 1983, 22, 224–232. [Google Scholar] [CrossRef]
- Sandqvist, G.; Åkesson, A.; Eklund, M. Evaluation of Paraffin Bath Treatment in Patients with Systemic Sclerosis. Disabil. Rehabil. 2004, 26, 981–987. [Google Scholar] [CrossRef]
- Mancuso, T.; Poole, J.L. The Effect of Paraffin and Exercise on Hand Function in Persons with Scleroderma: A Series of Single Case Studies. J. Hand Ther. 2009, 22, 71–78. [Google Scholar] [CrossRef]
- Vannajak, K. Physical Therapy in Systemic Sclerosis. Srinagarind Med. J. 2016, 31, 428–441. [Google Scholar]
- Occupational Therapy Integrated with a Self-Administered Stretching Program on Systemic Sclerosis Patients with Hand Involvement. Available online: https://www.clinexprheumatol.org/abstract.asp?a=9492 (accessed on 16 February 2025).
- Antonioli, C.M.; Bua, G.; Frigè, A.; Prandini, K.; Radici, S.; Scarsi, M.; Danieli, E.; Malvicini, A.; Airo, P. An Individualized Rehabilitation Program in Patients with Systemic Sclerosis May Improve Quality of Life and Hand Mobility. Clin. Rheumatol. 2009, 28, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Landim, S.; Bertolo, M.; Marcatto de Abreu, M.; Del Rio, A.P.; Mazon, C.; Marques-Neto, J.; Poole, J.; Magalhães, E. The Evaluation of a Home-Based Program for Hands in Patients with Systemic Sclerosis. J. Hand Ther. 2017, 32, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Mugii, N.; Hasegawa, M.; Matsushita, T.; Kondo, M.; Orito, H.; Yanaba, K.; Komura, K.; Hayakawa, I.; Hamaguchi, Y.; Ikuta, M.; et al. The Efficacy of Self-Administered Stretching for Finger Joint Motion in Japanese Patients with Systemic Sclerosis. J. Rheumatol. 2006, 33, 1586–1592. [Google Scholar] [PubMed]
- Mokhberdezfuli, M.; Ayatollahi, H.; Naser Moghadasi, A. A Smartphone-Based Application for Self-Management in Multiple Sclerosis. J. Healthc. Eng. 2021, 2021, 6749951. [Google Scholar] [CrossRef]
- Skrabal Ross, X.; Gunn, K.M.; Patterson, P.; Olver, I. A Smartphone Self-Management Program to Support Oral Chemotherapy Adherence in Young and Adult Cancer Patients: Design and Development Background [Internet]. ResearchGate. 2017. Available online: https://www.researchgate.net/publication/334318052_A_Smartphone_Self-Management_Program_to_Support_Oral_Chemotherapy_Adherence_in_Young_and_Adult_Cancer_Patients_Design_and_Development_Background (accessed on 21 June 2024).
- van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E.; et al. 2013 Classification Criteria for Systemic Sclerosis: An American College of Rheumatology/European League against Rheumatism Collaborative Initiative. Arthritis Rheum. 2013, 65, 2737–2747. [Google Scholar] [CrossRef]
- Chiang Mai University. การพัฒนารูปแบบการฟื้นฟูสมรรถภาพมือในผู้ป่วยโรคสเคลอโรเดอร์มาที่มีข้ออักเสบข้อนิ้วมือเฉียบพลัน [Internet]. Chiang Mai University Digital Collection. 2021. Available online: https://cmudc.library.cmu.ac.th/frontend/Info/item/dc:104795 (accessed on 21 June 2024).
- Scleroderma Foundation. Stretching Exercises for the Hands and Face [Internet]. 2021. Available online: https://scleroderma.org/wp-content/uploads/2021/12/Stretching-Exercises-for-the-Hands-and-Face-20211127.pdf (accessed on 21 June 2024).
- Sandqvist, G.; Eklund, M. Hand Mobility in Scleroderma (HAMIS) Test: The Reliability of a Novel Hand Function Test. Arthritis Care Res. 2000, 13, 369–374. [Google Scholar] [CrossRef]
- LeRoy, E.C.; Black, C.; Fleischmajer, R.; Jablonska, S.; Krieg, T.; Medsger, T.A.; Rowell, N.; Wollheim, F. Scleroderma (Systemic Sclerosis): Classification, Subsets and Pathogenesis. J. Rheumatol. 1988, 15, 202–205. [Google Scholar]
- Silver, R.M. Clinical Aspects of Systemic Sclerosis (Scleroderma). Ann. Rheum. Dis. 1991, 50, 854–861. [Google Scholar] [CrossRef]
- Foocharoen, C.; Peansukwech, U.; Mahakkanukrauh, A.; Suwannaroj, S.; Pongkulkiat, P.; Khamphiw, P.; Nanagara, R. Clinical Characteristics and Outcomes of 566 Thais with Systemic Sclerosis: A Cohort Study. Int. J. Rheum. Dis. 2020, 23, 945–957. [Google Scholar] [CrossRef]
- Parniyan, R.; Pasyar, N.; Rambod, M.; Momennasab, M.; Nazarinia, M. The Effect of a Self-Management Program on the Quality of Life of Patients with Scleroderma. J. Educ. Health Promot. 2023, 12, 440. [Google Scholar] [CrossRef]
- Godard, D. The Needs of Patients with Systemic Sclerosis—What Are the Difficulties Encountered? Autoimmun. Rev. 2011, 10, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Thombs, B.D.; Kwakkenbos, L.; Riehm, K.E.; Saadat, N.; Fedoruk, C. Comparison of Self-Efficacy for Managing Chronic Disease between Patients with Systemic Sclerosis and Other Chronic Conditions: A Systematic Review. Rheumatol. Int. 2017, 37, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Maddali-Bongi, S.; Landi, G.; Galluccio, F.; Del Rosso, A.; Miniati, I.; Conforti, M.L.; Casale, R.; Matucci-Cerinic, M. The Rehabilitation of Facial Involvement in Systemic Sclerosis: Efficacy of the Combination of Connective Tissue Massage, Kabat’s Technique and Kinesitherapy: A Randomized Controlled Trial. Rheumatol. Int. 2011, 31, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Bongi, S.M.; Del Rosso, A.; Galluccio, F.; Sigismondi, F.; Miniati, I.; Conforti, M.L.; Nacci, F.; Cerinic, M.M. Efficacy of Connective Tissue Massage and Mc Mennell Joint Manipulation in the Rehabilitative Treatment of the Hands in Systemic Sclerosis. Clin. Rheumatol. 2009, 28, 1167–1173. [Google Scholar] [CrossRef]
- Civi Karaaslan, T.; Tarakci, E.; Keles, O.; Aslan Keles, Y.; Ugurlu, S. Comparison of Telerehabilitation Methods for Patients with Systemic Sclerosis in the COVID-19 Era: A Randomized Controlled Study. J. Hand Ther. 2023, 36, 751–769. [Google Scholar] [CrossRef]
- Poole, J.L.; Macintyre, N.J.; Deboer, H.N. Evidence-Based Management of Hand and Mouth Disability in a Woman Living with Diffuse Systemic Sclerosis (Scleroderma). Physiother. Can. 2013, 65, 317–320. [Google Scholar] [CrossRef]
- Piga, M.; Tradori, I.; Pani, D.; Barabino, G.; Dessì, A.; Raffo, L.; Mathieu, A. Telemedicine Applied to Kinesiotherapy for Hand Dysfunction in Patients with Systemic Sclerosis and Rheumatoid Arthritis: Recovery of Movement and Telemonitoring Technology. J. Rheumatol. 2014, 41, 1324–1333. [Google Scholar] [CrossRef]
- Horváth, J.; Bálint, Z.; Szép, E.; Deiszinger, A.; Minier, T.; Farkas, N.; Török, E.; Horváthné Papp, É.; Komjáti, D.; Mándó, Z.; et al. Efficacy of Intensive Hand Physical Therapy in Patients with Systemic Sclerosis. Clin. Exp. Rheumatol. 2017, 35 (Suppl. 106), 159–166. [Google Scholar]
- Rannou, F.; Boutron, I.; Mouthon, L.; Sanchez, K.; Tiffreau, V.; Hachulla, E.; Thoumie, P.; Cabane, J.; Chatelus, E.; Sibilia, J.; et al. Personalized Physical Therapy Versus Usual Care for Patients With Systemic Sclerosis: A Randomized Controlled Trial. Arthritis Care Res. 2017, 69, 1050–1059. [Google Scholar] [CrossRef]
- Whitehead, L.; Seaton, P. The Effectiveness of Self-Management Mobile Phone and Tablet Apps in Long-Term Condition Management: A Systematic Review. J. Med. Internet Res. 2016, 18, e97. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Chen, X.; Zhang, Y.; Li, Y.; Hu, X. Effects of Web-Based Acceptance and Commitment Therapy on Health-Related Outcomes Among Patients With Lung Cancer: A Feasibility Randomized Controlled Trial. Psychooncology 2024, 33, e70045. [Google Scholar] [CrossRef]
| Clinical Characteristics | Total SSc n = 72 | Intervention | p-Value * | |||
|---|---|---|---|---|---|---|
| Group A Typical Education n = 24 | Group B Video Guide n = 24 | Group C Video and Telephone Notification n = 24 | ||||
| Age (years), mean (SD) | 55.9 (9.9) | 56.1 (10.3) | 53 (11.7) | 58.8 (6.9) | 0.14 | |
| Age at onset (years), mean (SD) | 50.3 (11.2) | 50.8 (11.6) | 46.8 (12.8) | 53.3 (8.3) | 0.13 | |
| Disease duration (years), median (IQR) | 5.9 (2–9) | 6.1 (2–8.5) | 6.2 (1.5–11) | 5.6 (2–8.5) | 0.92 | |
| Sex, n (%) | 0.62 | |||||
| Male | 52 (72.2) | 19 (79.2) | 17 (70.8) | 16 (66.7) | ||
| Female | 20 (27.8) | 5 (20.8) | 7 (29.2) | 8 (33.3) | ||
| Education level, n (%) | 0.95 | |||||
| Primary-high school | 36 (50) | 13 (54.2) | 11 (45.8) | 12 (50) | ||
| Diploma | 17 (23.6) | 5 (20.8) | 7 (29.2) | 5 (20.8) | ||
| Bachelor’s or higher | 19 (26.4) | 6 (25) | 6 (25) | 7 (29.2) | ||
| Occupation, n (%) | 0.27 | |||||
| Unemployed | 24 (33.3) | 11 (45.8) | 6 (25) | 7 (29.2) | ||
| Employee | 48 (66.7) | 13 (54.2) | 18 (75) | 17 (70.8) | ||
| SSc subset, n (%) | 0.78 | |||||
| lcSSc | 15 (20.8) | 5 (20.8) | 4 (16.7) | 6 (25) | ||
| dcSSc | 57 (79.2) | 19 (79.2) | 20 (83.3) | 18 (75) | ||
| Clinical presentation on study date, n (%) | ||||||
| Raynaud’s phenomenon | 53 (73.6) | 18 (75) | 20 (83.3) | 15 (62.5) | 0.26 | |
| Digital ulcer | 24 (33.3) | 9 (29.2) | 7 (29.2) | 10 (41.7) | 0.57 | |
| Salt and pepper skin | 35 (48.6) | 12 (50) | 13 (54.2) | 10 (41.7) | 0.68 | |
| Telangiectasia | 21 (29.2) | 9 (37.5) | 6 (25) | 6 (25) | 0.55 | |
| Smoking, n (%) | 4 (5.56) | 0 | 3 (12.5) | 1 (4.2) | 0.68 | |
| Organ involvement, n (%) | ||||||
| Interstitial lung disease | 44 (61.1) | 13 (54.2) | 16 (66.7) | 15 (62.5) | 0.67 | |
| Gastrointestinal | 23 (31.9) | 6 (25) | 9 (37.5) | 8 (33.3) | 0.64 | |
| Heart | 8 (11.1) | 4 (16.7) | 2 (8.3) | 2 (8.3) | 0.57 | |
| Renal | 2 (2.8) | 1 (4.2) | 0 | 1 (4.2) | 0.59 | |
| Arthralgia/Arthritis | 1 (1.4) | 1 (1.4) | 0 | 0 | 0.36 | |
| Muscle | 9 (12.5) | 5 (20.8) | 1 (4.2) | 3 (12.5) | 0.22 | |
| Serology, n (%) | ||||||
| Anti-topoisomerase positive | 60 of 67 (89.6) | 20 of 22 (90.9) | 20 of 22 (90.9) | 20 of 23 (87.0) | 0.88 | |
| Anti-centromere positive | 3 of 60 (5.0) | 1 of 18 (5.6) | 1 of 22 (4.6) | 1 of 20 (5.0) | 0.99 | |
| mRSS, mean (SD) | 10.1 (8.5) | 10.1 (8.6) | 10 (6.5) | 10.1 (10.5) | 0.99 | |
| Hand grip strength (kg), mean (SD) | ||||||
| Dominant hand | 18.3 (7.5) | 15.8 (1.16) | 19.2 (8.5) | 19.9 (8.2) | 0.16 | |
| Non-dominant hand | 17.2 (7.5) | 15.5 (5.7) | 17.5 (7.9) | 18.9 (8.2) | 0.19 | |
| HAMIS, mean (SD) | ||||||
| Dominant hand | 6.3 (4.5) | 6.6 (5.6) | 6.1 (3.4) | 6.3 (4.4) | 0.91 | |
| Non-dominant hand | 4.9 (4.2) | 5.2 (4.2) | 5.0 (3.7) | 4.6 (3.5) | 0.87 | |
| QoL by EQ-5D, n (%) | 0.34 | |||||
| Mobility | 20 (27.8) | 7 (29.2) | 9 (37.5) | 4 (16.7) | 0.55 | |
| Self-care | 27 (37.5) | 11 (45.8) | 10 (41.7) | 6 (25) | 0.29 | |
| Daily activities | 41 (56.9) | 15 (62.5) | 14 (58.3) | 12 (50) | 0.59 | |
| Pain or discomfort | 49 (68.1) | 18 (75) | 17 (70.8) | 14 (58.3) | 0.73 | |
| Anxiety or depression | 56 (77.8) | 17 (70.8) | 21 (87.5) | 18 (75) | 0.21 | |
| EQ-5D VAS, mean (SD) | 67.9 (13.3) | 71.6 (12.0) | 64.4 (13.3) | 67.7 (13.9) | 0.16 | |
| Self-management behavior, mean (SD) | 29.0 (5.4) | 28.1 (4.5) | 29.8 (6.3) | 29.2 (5.4) | 0.55 | |
| Clinical Characteristic | Intervention | p-Value * | ||||
|---|---|---|---|---|---|---|
| Group A Typical Education n = 22 | Group B Video Guide n = 24 | Group C Video and Telephone Notification n = 24 | ||||
| Hand grip strength (kg); mean (SD) | ||||||
| Dominant hand | 16.7 (5.4) | 21.4 (9.1) | 23.2 (9.1) | 0.172 a, 1.00 b, 0.028 c* | ||
| Non-dominant | 16.9 (5.4) | 20.3 (8.4) | 22.7 (9.0) | 0.471 a, 0.827 b, 0.044 c* | ||
| HAMIS, mean (SD) | ||||||
| Dominant hand | 5.5 (5.9) | 2.9 (3.0) | 3.7 (4.5) | 0.25 | ||
| Finger flexion | 0.50 (0.74) | 0.25 (0.53) | 0.25 (0.68) | 0.31 | ||
| Finger extension | 0.73 (0.93) | 0.63 (0.87) | 0.63 (0.71) | 0.67 | ||
| Thumb abduction | 0.74 (0.91) | 0.25 (0.44) | 0.38 (0.77) | 0.38 | ||
| Pincer grip | 0.86 (0.88) | 0.58 (0.77) | 0.54 (0.78) | 0.64 | ||
| Finger abduction | 0.41 (0.73) | 0.08 (0.28) | 0.29 (0.55) | 0.29 | ||
| Volar flexion | 0.50 (0.74) | 0.21 (0.51) | 0.33 (0.56) | 0.46 | ||
| Dorsal extension | 0.64 (0.84) | 0.33 (0.48) | 0.17 (0.38) | 0.15 | ||
| Pronation | 0.68 (1.04) | 0.17 (0.38) | 0.50 (0.93) | 0.21 | ||
| Supination | 0.77 (0.92) | 0.42 (0.65) | 0.75 (0.79) | 0.46 | ||
| Non-dominant | 4.0 (5.1) | 2.6 (3.3) | 1.6 (2.4) | 0.08 | ||
| Finger flexion | 0.41 (0.66) | 0.33 (0.64) | 0.13 (0.34) | 0.49 | ||
| Finger extension | 0.50 (0.86) | 0.42 (0.88) | 0.33 (0.56) | 0.46 | ||
| Thumb abduction | 0.40 (0.84) | 0.21 (0.41) | 0.13 (0.34) | 0.27 | ||
| Pincer grip | 0.82 (0.85) | 0.58 (0.78) | 0.21 (0.65) | 0.035 * | ||
| Finger abduction | 0.36 (0.73) | 0.08 (0.28) | 0.22 (0.51) | 0.38 | ||
| Volar flexion | 0.45 (0.67) | 0.25 (0.53) | 0.17 (0.38) | 0.45 | ||
| Dorsal extension | 0.55 (0.80) | 0.25 (0.44) | 0.13 (0.34) | 0.27 | ||
| Pronation | 0.27 (0.63) | 0.13 (0.34) | 0.04 (0.20) | 0.23 | ||
| Supination | 0.45 (0.59) | 0.37 (0.49) | 0.33 (0.56) | 0.74 | ||
| QoL by EQ5D, n (%) | ||||||
| Mobility | 6 (27.3) | 8 (33.3) | 4 (16.7) | 0.39 | ||
| Self-care | 8 (36.4) | 7 (29.2) | 4 (16.7) | 0.31 | ||
| Daily activities | 9 (40.9) | 6 (25) | 6 (25) | 0.40 | ||
| Pain or discomfort | 14 (63.6) | 13 (54.2) | 9 (37.5) | 0.43 | ||
| Anxiety/ depression | 6 (27.3) | 8 (33.3) | 4 (16.7) | 0.21 | ||
| EQ5D VAS, mean (SD) | 73.8 (11.5) | 75.6 (12) | 79.2 (12.7) | 0.32 | ||
| Hand care behaviors | ||||||
| Exercise habit, mean (SD) | 3.5 (0.6) | 3.8 (0.3) | 3.9 (0.3) | 0.15 | ||
| Keep warm, mean (SD) | 2.5 (1.1) | 3.1 (0.9) | 3.1 (0.8) | 0.19 | ||
| Skin moisture, mean (SD) | 3.6 (0.6) | 3.7 (0.8) | 3.7 (0.6) | 0.68 | ||
| Hand injury precautions, mean (SD) | 3.8 (0.4) | 4 (0) | 4 (0.2) | 0.014 * | ||
| Household modifications, mean (SD) | 3.5 (0.5) | 3.5 (0.9) | 3.8 (0.6) | 0.023 * | ||
| Emotional management, mean (SD) | 3.6 (0.6) | 3.9 (0.3) | 3.9 (0.3) | 0.11 | ||
| Clinical Characteristic | Group A Typical Education n = 22 | Group B Video Guide n = 24 | Group C Video and Telephone Notification n = 24 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Week 6 | Mean Difference (95%CI) | p-Value | Baseline | Week 6 | Mean Difference (95%CI) | p-Value | Baseline | Week 6 | Mean Difference (95%CI) | p-Value | ||
| Hand grip strength (kg); mean (SD) | |||||||||||||
| Dominant hand | 15.8 (5.4) | 16.7 (5.4) | −1.0 (−1.6, −0.3) | 0.004 * | 19.2 (8.5) | 21.4 (9.2) | −2.2 (−3.1, −1.3) | <0.001 * | 19.9 (8.2) | 23.2 (9.1) | −3.3 (−4.3, −2.3) | <0.001 * | |
| Non-dominant | 15.5 (5.7) | 16.9 (5.4) | −1.4 (−2.2, −0.6) | 0.001 * | 17.5 (7.9) | 20.3 (8.4) | −2.8 (−3.4, −2.1) | <0.001 * | 18.9 (8.2) | 22.7 (9.0) | −3.8 (−4.6, −2.9) | <0.001 * | |
| HAMIS, mean ± SD | |||||||||||||
| Dominant hand | 6.7 (5.8) | 5.6 (6.0) | 1.1 (0.3, 1.9) | 0.01 * | 6.1 (3.4) | 2.9 (3.0) | 3.2 (2.3, 4.1) | <0.001 * | 6.3 (4.4) | 3.8 (4.5) | 2.5 (1.7, 3.3) | <0.001 * | |
| Non-dominant | 5.3 (5.6) | 4.1 (5.1) | 1.2 (0.4, 2.0) | 0.01 * | 5.0 (3.7) | 2.6 (3.4) | 2.4 (1.4, 3.4) | <0.001 * | 4.6 (3.5) | 1.7 (2.4) | 3.0 (1.9, 4.0) | <0.001 * | |
| QoL by EQ-5D | 8 (1.8) | 7.3 (1.6) | 0.5 (−0.1, 1.2) | 0.076 | 8.2 (1.8) | 7.2 (1.5) | 1.1 (0.6, 1.6) | <0.001 * | 7.5 (1.4) | 6.5 (1.5) | 1.0 (0.5, 1.4) | <0.001 * | |
| EQ-5D VAS, mean ± SD | 72.7 (11.6) | 73.9 (11.5) | −1.1 (−2.1, −0.2) | 0.025 * | 64.4 (13.3) | 75.6 (12) | −11.3 (−13.5, −9.0) | <0.001 * | 67.7 (13.9) | 79.2 (12.7) | −11.5 (−13.0, −9.9) | <0.001 * | |
| Self-management behavior, mean ± SD | 28.1 (4.5) | 38.2 (4.2) | −10.2 (−12.2, −8.2) | 0.001 * | 29.8 (6.3) | 40.9 (3.9) | −11.1 (−13.7, −8.5) | 0.001 * | 29.2 (5.4) | 42.9 (3.1) | −13.8 (−16.3, −11.2) | 0.001 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wantha, O.; Mahakkanukrauh, A.; Suwannaroj, S.; Tuydaung, K.; Methakanjanasak, N.; Srichomphu, K.; Kraipoj, J.; Foocharoen, C. Effects of a Self-Management Telehealth Program on Improving Strength and Hand Function in Systemic Sclerosis Patients: A Randomized Controlled Trial. Life 2025, 15, 1087. https://doi.org/10.3390/life15071087
Wantha O, Mahakkanukrauh A, Suwannaroj S, Tuydaung K, Methakanjanasak N, Srichomphu K, Kraipoj J, Foocharoen C. Effects of a Self-Management Telehealth Program on Improving Strength and Hand Function in Systemic Sclerosis Patients: A Randomized Controlled Trial. Life. 2025; 15(7):1087. https://doi.org/10.3390/life15071087
Chicago/Turabian StyleWantha, Orathai, Ajanee Mahakkanukrauh, Siraphop Suwannaroj, Kwankaew Tuydaung, Nonglak Methakanjanasak, Kannika Srichomphu, Jinnaphat Kraipoj, and Chingching Foocharoen. 2025. "Effects of a Self-Management Telehealth Program on Improving Strength and Hand Function in Systemic Sclerosis Patients: A Randomized Controlled Trial" Life 15, no. 7: 1087. https://doi.org/10.3390/life15071087
APA StyleWantha, O., Mahakkanukrauh, A., Suwannaroj, S., Tuydaung, K., Methakanjanasak, N., Srichomphu, K., Kraipoj, J., & Foocharoen, C. (2025). Effects of a Self-Management Telehealth Program on Improving Strength and Hand Function in Systemic Sclerosis Patients: A Randomized Controlled Trial. Life, 15(7), 1087. https://doi.org/10.3390/life15071087









