The Role of Gut Microbiota in Insomnia: A Systematic Review of Case–Control Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Quality Assessment
2.5. Qualitative Synthesis
3. Results
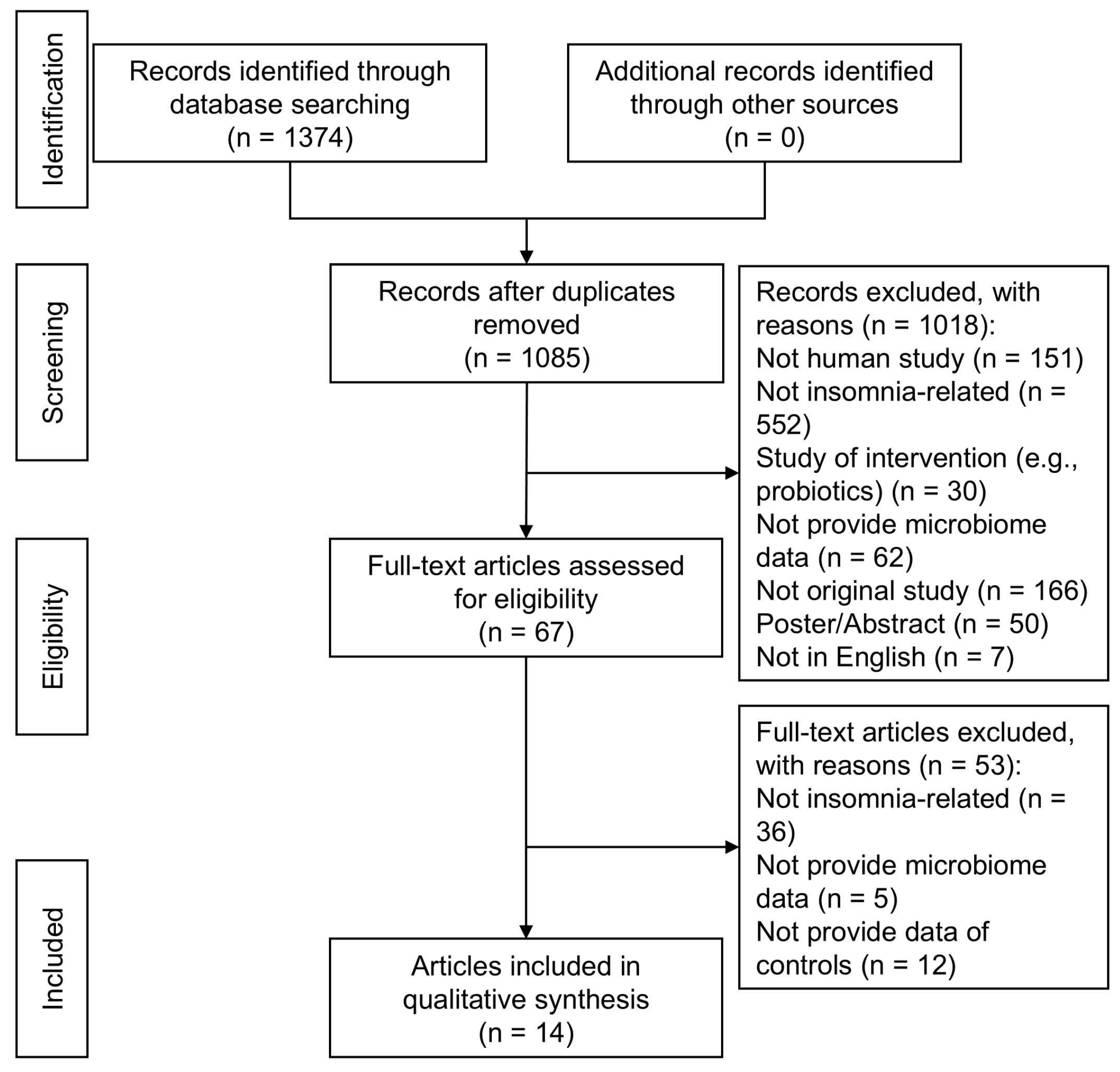
3.1. Study Selection and Literature Flow
3.2. Study Characteristics and Population Demographics
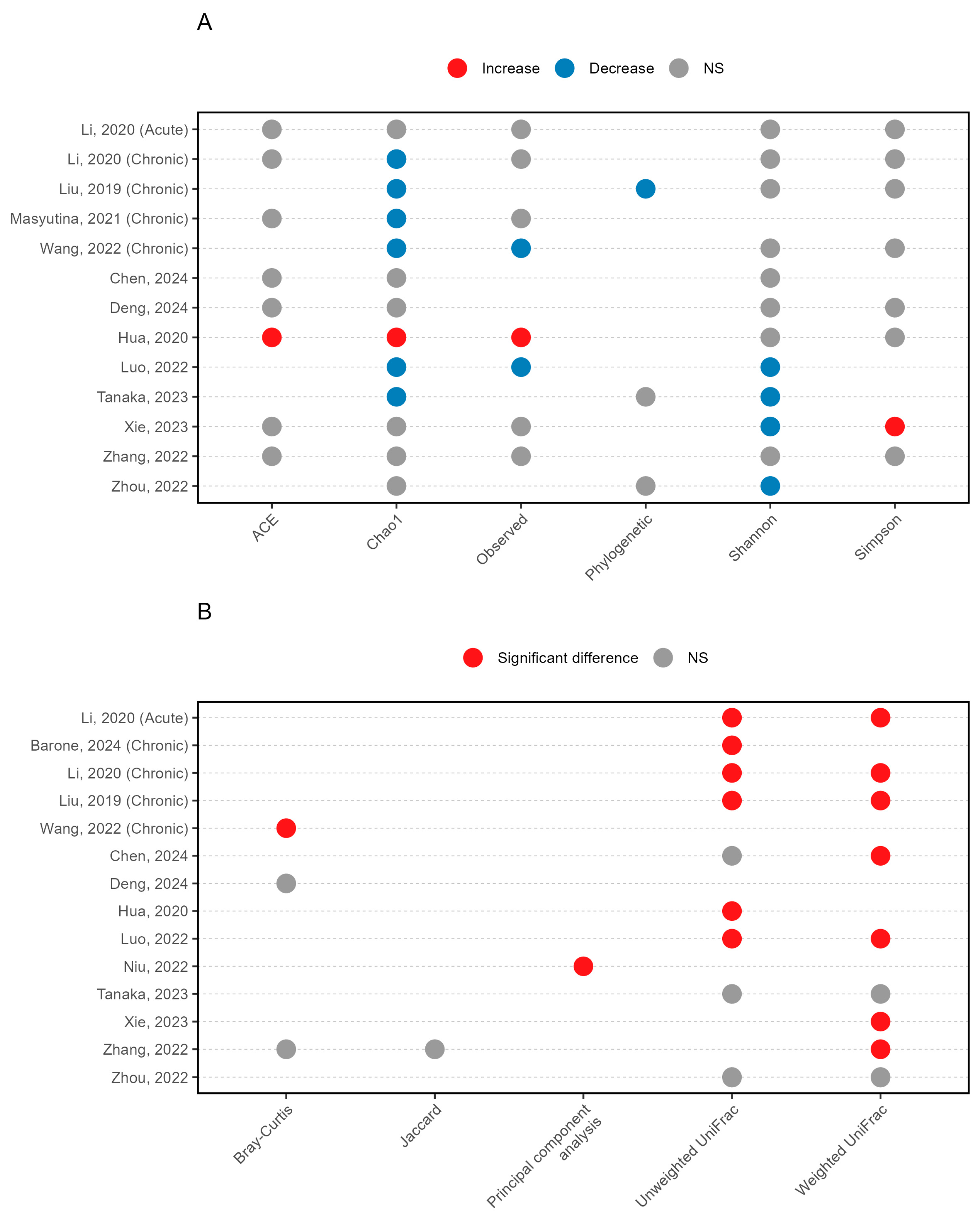
3.3. Microbial Diversity Patterns in Insomnia
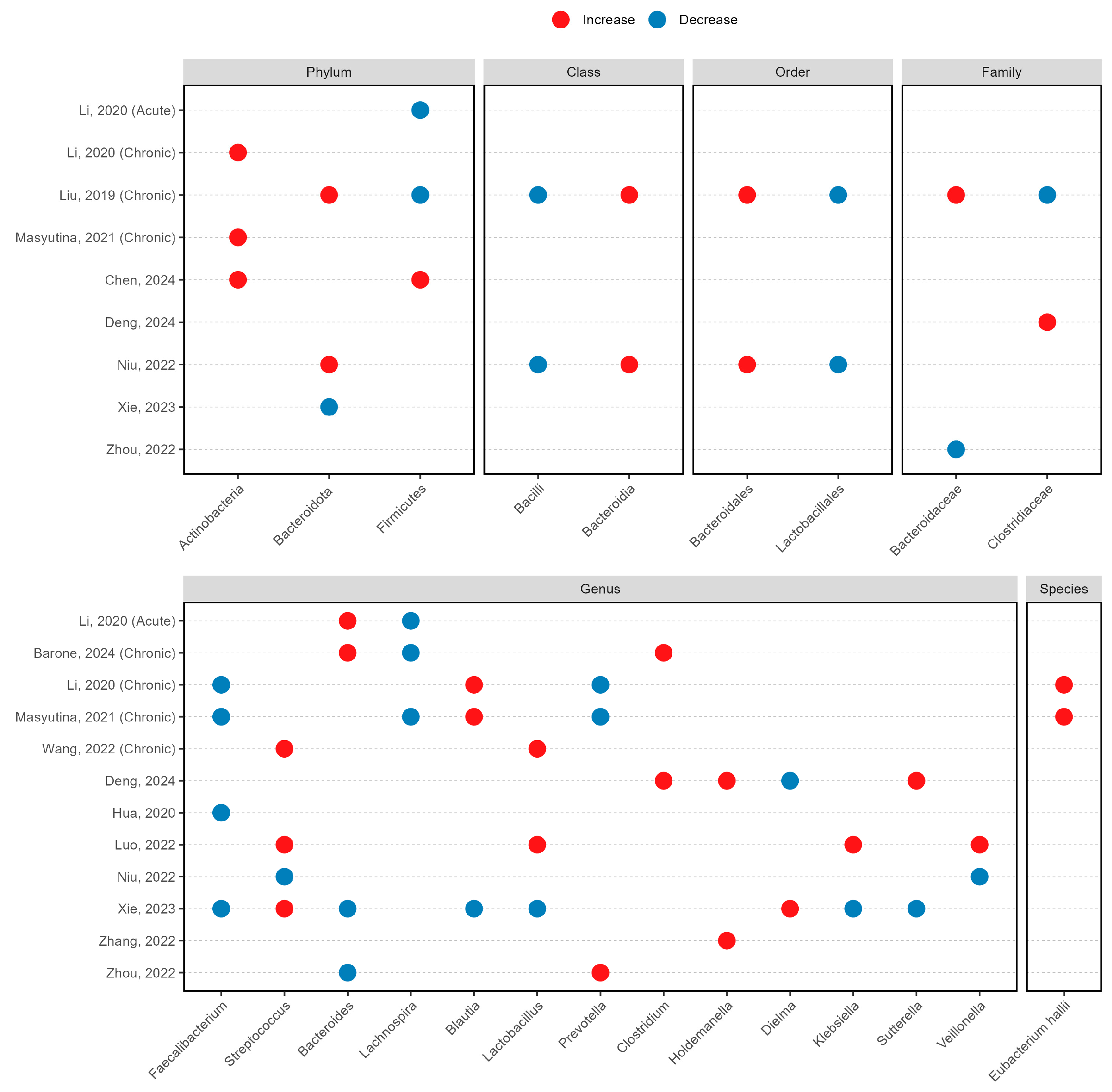
3.4. Taxonomic Alterations in Insomnia
3.5. Microbiota-Insomnia Severity Associations
3.6. Microbiome Signatures in Chronic Insomnia Disorders
3.7. Quality of Evidence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buysse, D.J. Insomnia. JAMA 2013, 309, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Jahrami, H.A.; Alhaj, O.A.; Humood, A.M.; Alenezi, A.F.; Fekih-Romdhane, F.; AlRasheed, M.M.; Saif, Z.Q.; Bragazzi, N.L.; Pandi-Perumal, S.R.; BaHammam, A.S.; et al. Sleep disturbances during the COVID-19 pandemic: A systematic review, meta-analysis, and meta-regression. Sleep Med. Rev. 2022, 62, 101591. [Google Scholar] [CrossRef]
- Riemann, D.; Nissen, C.; Palagini, L.; Otte, A.; Perlis, M.L.; Spiegelhalder, K. The neurobiology, investigation, and treatment of chronic insomnia. Lancet Neurol. 2015, 14, 547–558. [Google Scholar] [CrossRef]
- Chen, S.J.; Que, J.Y.; Chan, N.Y.; Shi, L.; Li, S.X.; Chan, J.W.Y.; Huang, W.; Chen, C.X.; Tsang, C.C.; Ho, Y.L.; et al. Effectiveness of app-based cognitive behavioral therapy for insomnia on preventing major depressive disorder in youth with insomnia and subclinical depression: A randomized clinical trial. PLoS Med. 2025, 22, e1004510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lam, S.P.; Li, S.X.; Yu, M.W.; Li, A.M.; Ma, R.C.; Kong, A.P.; Wing, Y.K. Long-term outcomes and predictors of chronic insomnia: A prospective study in Hong Kong Chinese adults. Sleep Med. 2012, 13, 455–462. [Google Scholar] [CrossRef]
- Agirman, G.; Yu, K.B.; Hsiao, E.Y. Signaling inflammation across the gut-brain axis. Science 2021, 374, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Góralczyk-Bińkowska, A.; Szmajda-Krygier, D.; Kozłowska, E. The Microbiota-Gut-Brain Axis in Psychiatric Disorders. Int. J. Mol. Sci. 2022, 23, 11245. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Lu, T.; Chen, W.; Yan, W.; Yuan, K.; Shi, L.; Liu, X.; Zhou, X.; Shi, J.; et al. The microbiota-gut-brain axis in sleep disorders. Sleep Med. Rev. 2022, 65, 101691. [Google Scholar] [CrossRef]
- Li, C.; Chen, S.; Wang, Y.; Su, Q. Microbiome-Based Therapeutics for Insomnia. Int. J. Mol. Sci. 2024, 25, 13208. [Google Scholar] [CrossRef]
- Han, M.; Yuan, S.; Zhang, J. The interplay between sleep and gut microbiota. Brain Res. Bull. 2022, 180, 131–146. [Google Scholar] [CrossRef]
- Zhu, R.; Fang, Y.; Li, H.; Liu, Y.; Wei, J.; Zhang, S.; Wang, L.; Fan, R.; Wang, L.; Li, S.; et al. Psychobiotic Lactobacillus plantarum JYLP-326 relieves anxiety, depression, and insomnia symptoms in test anxious college via modulating the gut microbiota and its metabolism. Front. Immunol. 2023, 14, 1158137. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Lin, W.; Chen, S.; Xiang, T.; Yang, Y.; Yin, Y.; Xu, G.; Liu, Z.; Liu, L.; Pan, J.; et al. Gut Microbiota as an Objective Measurement for Auxiliary Diagnosis of Insomnia Disorder. Front. Microbiol. 2019, 10, 1770. [Google Scholar] [CrossRef]
- De Simone, M.; De Feo, R.; Choucha, A.; Ciaglia, E.; Fezeu, F. Enhancing Sleep Quality: Assessing the Efficacy of a Fixed Combination of Linden, Hawthorn, Vitamin B1, and Melatonin. Med. Sci. 2023, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, B.; Zhou, Y.; Wang, D.; Liu, X.; Li, L.; Wang, T.; Zhang, Y.; Jiang, M.; Tang, H.; et al. Gut Microbiota Changes and Their Relationship with Inflammation in Patients with Acute and Chronic Insomnia. Nat. Sci. Sleep 2020, 12, 895–905. [Google Scholar] [CrossRef]
- Masyutina, A.A.; Gumenyuk, L.N.; Fatovenko Yu, V.; Sorokina, L.E.; Bayramova, S.S.; Alekseenko, A.I.; Shavrov Yu, V.; Romanova, A.A.; Seydametova, D.I. Changes in gut microbiota composition and their associations with cortisol, melatonin and interleukin 6 in patients with chronic insomnia. Bull. Russ. State Med. Univ. 2021, 18–24. [Google Scholar] [CrossRef]
- Hua, X.; Zhu, J.; Yang, T.; Guo, M.; Li, Q.; Chen, J.; Li, T. The Gut Microbiota and Associated Metabolites Are Altered in Sleep Disorder of Children With Autism Spectrum Disorders. Front. Psychiatry 2020, 11, 855. [Google Scholar] [CrossRef]
- Xie, H.; Chen, J.; Chen, Q.; Zhao, Y.; Liu, J.; Sun, J.; Hu, X. The Diagnostic Value of Gut Microbiota Analysis for Post-Stroke Sleep Disorders. Diagnostics 2023, 13, 2970. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ Clin. Res. Ed. 2021, 372, n71. [Google Scholar] [CrossRef]
- Barone, M.; Martucci, M.; Sciara, G.; Conte, M.; Medina, L.S.J.; Iattoni, L.; Miele, F.; Fonti, C.; Franceschi, C.; Brigidi, P.; et al. Towards a personalized prediction, prevention and therapy of insomnia: Gut microbiota profile can discriminate between paradoxical and objective insomnia in post-menopausal women. EPMA J. 2024, 15, 471–489. [Google Scholar] [CrossRef]
- Chen, Q.; Fan, R.; Song, L.; Wang, S.; You, M.; Cai, M.; Wu, Y.; Li, Y.; Xu, M. Association of methyl donor nutrients dietary intake and sleep disorders in the elderly revealed by the intestinal microbiome. Food Funct. 2024, 15, 6335–6346. [Google Scholar] [CrossRef]
- Deng, Z.; Liu, L.; Liu, W.; Liu, R.; Ma, T.; Xin, Y.; Xie, Y.; Zhang, Y.; Zhou, Y.; Tang, Y. Alterations in the fecal microbiota of methamphetamine users with bad sleep quality during abstinence. BMC Psychiatry 2024, 24, 324. [Google Scholar] [CrossRef]
- Luo, M.; Hu, F.R.; Xin, R.J.; Yao, L.; Hu, S.J.; Bai, F.H. Altered gut microbiota is associated with sleep disturbances in patients with minimal hepatic encephalopathy caused by hepatitis B-related liver cirrhosis. Expert Rev. Gastroenterol. Hepatol. 2022, 16, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Sanada, K.; Miyaho, K.; Tachibana, T.; Kurokawa, S.; Ishii, C.; Noda, Y.; Nakajima, S.; Fukuda, S.; Mimura, M.; et al. The relationship between sleep, gut microbiota, and metabolome in patients with depression and anxiety: A secondary analysis of the observational study. PLoS ONE 2023, 18, e0296047. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, B.; Sheng, D.; Yang, J.; Fu, S.; Wang, J.; Zhao, C.; Wang, Y.; Gai, X.; Wang, J.; et al. Multiomics Analysis Reveals Aberrant Metabolism and Immunity Linked Gut Microbiota with Insomnia. Microbiol. Spectr. 2022, 10, e0099822. [Google Scholar] [CrossRef] [PubMed]
- Zhanfeng, N.; Liang, W.; Jing, K.; Jinbo, B.; Yanjun, C.; Hechun, X. Regulation of sleep disorders in patients with traumatic brain injury by intestinal flora based on the background of brain-gut axis. Front. Neurosci. 2022, 16, 934822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, X.; Zhang, K.; Lu, X.; Yuan, G.; Yang, H.; Guo, H.; Zhu, Z.; Wang, T.; Hao, J.; et al. The Component and Functional Pathways of Gut Microbiota Are Altered in Populations with Poor Sleep Quality—A Preliminary Report. Pol. J. Microbiol. 2022, 71, 241–250. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, X.; Li, Z.; Zou, Z.; Dou, S.; Li, G.; Yan, F.; Chen, B.; Li, Y. Alterations in Gut Microbiota Are Correlated With Serum Metabolites in Patients With Insomnia Disorder. Front. Cell. Infect. Microbiol. 2022, 12, 722662. [Google Scholar] [CrossRef]
- Li, Z.; Lu, G.; Li, Z.; Wu, B.; Luo, E.; Qiu, X.; Guo, J.; Xia, Z.; Zheng, C.; Su, Q.; et al. Altered Actinobacteria and Firmicutes Phylum Associated Epitopes in Patients With Parkinson’s Disease. Front. Immunol. 2021, 12, 632482. [Google Scholar] [CrossRef]
- Fan, J.; Zhou, Y.; Meng, R.; Tang, J.; Zhu, J.; Aldrich, M.C.; Cox, N.J.; Zhu, Y.; Li, Y.; Zhou, D. Cross-talks between gut microbiota and tobacco smoking: A two-sample Mendelian randomization study. BMC Med. 2023, 21, 163. [Google Scholar] [CrossRef]
- Sutanto, C.N.; Loh, W.W.; Kim, J.E. The impact of tryptophan supplementation on sleep quality: A systematic review, meta-analysis, and meta-regression. Nutr. Rev. 2022, 80, 306–316. [Google Scholar] [CrossRef]
- Qiu, X.; Zhang, M.; Yang, X.; Hong, N.; Yu, C. Faecalibacterium prausnitzii upregulates regulatory T cells and anti-inflammatory cytokines in treating TNBS-induced colitis. J. Crohns Colitis 2013, 7, e558–e568. [Google Scholar] [CrossRef] [PubMed]
- Li, H.B.; Xu, M.L.; Xu, X.D.; Tang, Y.Y.; Jiang, H.L.; Li, L.; Xia, W.J.; Cui, N.; Bai, J.; Dai, Z.M.; et al. Faecalibacterium prausnitzii Attenuates CKD via Butyrate-Renal GPR43 Axis. Circ. Res. 2022, 131, e120–e134. [Google Scholar] [CrossRef]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K.; et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Miquel, S.; Martín, R.; Bridonneau, C.; Robert, V.; Sokol, H.; Bermúdez-Humarán, L.G.; Thomas, M.; Langella, P. Ecology and metabolism of the beneficial intestinal commensal bacterium Faecalibacterium prausnitzii. Gut Microbes 2014, 5, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252. [Google Scholar] [CrossRef]
- Nikolova, V.L.; Smith, M.R.B.; Hall, L.J.; Cleare, A.J.; Stone, J.M.; Young, A.H. Perturbations in Gut Microbiota Composition in Psychiatric Disorders: A Review and Meta-analysis. JAMA Psychiatry 2021, 78, 1343–1354. [Google Scholar] [CrossRef]
- Gao, T.; Wang, Z.; Dong, Y.; Cao, J.; Lin, R.; Wang, X.; Yu, Z.; Chen, Y. Role of melatonin in sleep deprivation-induced intestinal barrier dysfunction in mice. J. Pineal Res. 2019, 67, e12574. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Wang, X.; Wang, R.; Hao, Y.; Ren, F.; Wang, P.; Fang, B. Faecalibacterium prausnitzii Supplementation Prevents Intestinal Barrier Injury and Gut Microflora Dysbiosis Induced by Sleep Deprivation. Nutrients 2024, 16, 1100. [Google Scholar] [CrossRef]
- Forbes, J.D.; Chen, C.Y.; Knox, N.C.; Marrie, R.A.; El-Gabalawy, H.; de Kievit, T.; Alfa, M.; Bernstein, C.N.; Van Domselaar, G. A comparative study of the gut microbiota in immune-mediated inflammatory diseases-does a common dysbiosis exist? Microbiome 2018, 6, 221. [Google Scholar] [CrossRef]
- Sokol, H.; Seksik, P.; Furet, J.P.; Firmesse, O.; Nion-Larmurier, I.; Beaugerie, L.; Cosnes, J.; Corthier, G.; Marteau, P.; Doré, J. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm. Bowel Dis. 2009, 15, 1183–1189. [Google Scholar] [CrossRef]
- Wagner-Skacel, J.; Dalkner, N.; Moerkl, S.; Kreuzer, K.; Farzi, A.; Lackner, S.; Painold, A.; Reininghaus, E.Z.; Butler, M.I.; Bengesser, S. Sleep and Microbiome in Psychiatric Diseases. Nutrients 2020, 12, 2198. [Google Scholar] [CrossRef]
- Gebara, M.A.; Siripong, N.; DiNapoli, E.A.; Maree, R.D.; Germain, A.; Reynolds, C.F.; Kasckow, J.W.; Weiss, P.M.; Karp, J.F. Effect of insomnia treatments on depression: A systematic review and meta-analysis. Depress. Anxiety 2018, 35, 717–731. [Google Scholar] [CrossRef]
- Mirchandaney, R.; Barete, R.; Asarnow, L.D. Moderators of Cognitive Behavioral Treatment for Insomnia on Depression and Anxiety Outcomes. Curr. Psychiatry Rep. 2022, 24, 121–128. [Google Scholar] [CrossRef]
- Yuan, X.; Chen, B.; Duan, Z.; Xia, Z.; Ding, Y.; Chen, T.; Liu, H.; Wang, B.; Yang, B.; Wang, X.; et al. Depression and anxiety in patients with active ulcerative colitis: Crosstalk of gut microbiota, metabolomics and proteomics. Gut Microbes 2021, 13, 1987779. [Google Scholar] [CrossRef]
- Li, J.; Ma, Y.; Bao, Z.; Gui, X.; Li, A.N.; Yang, Z.; Li, M.D. Clostridiales are predominant microbes that mediate psychiatric disorders. J. Psychiatr. Res. 2020, 130, 48–56. [Google Scholar] [CrossRef]
- Shetty, S.A.; Zuffa, S.; Bui, T.P.N.; Aalvink, S.; Smidt, H.; De Vos, W.M. Reclassification of Eubacterium hallii as Anaerobutyricum hallii gen. nov., comb. nov., and description of Anaerobutyricum soehngenii sp. nov., a butyrate and propionate-producing bacterium from infant faeces. Int. J. Syst. Evol. Microbiol. 2018, 68, 3741–3746. [Google Scholar] [CrossRef]
- Udayappan, S.; Manneras-Holm, L.; Chaplin-Scott, A.; Belzer, C.; Herrema, H.; Dallinga-Thie, G.M.; Duncan, S.H.; Stroes, E.S.G.; Groen, A.K.; Flint, H.J.; et al. Oral treatment with Eubacterium hallii improves insulin sensitivity in db/db mice. NPJ Biofilms Microbiomes 2016, 2, 16009. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, Q.; Hou, Y.; Zhang, X.; Yin, Z.; Cai, X.; Wei, W.; Wang, J.; He, D.; Wang, G.; et al. Bacteroides species differentially modulate depression-like behavior via gut-brain metabolic signaling. Brain Behav. Immun. 2022, 102, 11–22. [Google Scholar] [CrossRef]
- Ho, Y.T.; Tsai, Y.C.; Kuo, T.B.J.; Yang, C.C.H. Effects of Lactobacillus plantarum PS128 on Depressive Symptoms and Sleep Quality in Self-Reported Insomniacs: A Randomized, Double-Blind, Placebo-Controlled Pilot Trial. Nutrients 2021, 13, 2820. [Google Scholar] [CrossRef]
- Tett, A.; Pasolli, E.; Masetti, G.; Ercolini, D.; Segata, N. Prevotella diversity, niches and interactions with the human host. Nat. Rev. Microbiol. 2021, 19, 585–599. [Google Scholar] [CrossRef]
- Hosomi, K.; Saito, M.; Park, J.; Murakami, H.; Shibata, N.; Ando, M.; Nagatake, T.; Konishi, K.; Ohno, H.; Tanisawa, K.; et al. Oral administration of Blautia wexlerae ameliorates obesity and type 2 diabetes via metabolic remodeling of the gut microbiota. Nat. Commun. 2022, 13, 4477. [Google Scholar] [CrossRef]

| Study | Country | Comorbid Diseases | Diagnosis Criteria for Insomnia | Age Group | Subtype | No. of Insomnia Cases | No. of Control Cases | Type of Specimen | DNA Extraction Method | Microbiome Assessment Method |
|---|---|---|---|---|---|---|---|---|---|---|
| Barone, 2024 [19] | Italy | NR | ICSD-3 | Adult | Chronic | 54 (F) | 42 (F) | Stool | QIAamp DNA Stool Mini Kit (QIAGEN, Hilden, Germany) | 16S rRNA V3-V4 |
| Chen, 2024 [20] | China | NR | PSQI ≥ 5 | Adult | NR | 26 (M) 65 (F) | 42 (M) 105 (F) | Stool | MGIEasy fecal genomic DNA (meta) extraction kit (BGI, Shenzhen, China) | 16S rRNA V3-V4 |
| Deng, 2024 [21] | China | Methamphetamine users during abstinence | DSM-5 PSQI ≥ 7 | Adult | NR | 14 (M) 7 (F) | 35 (M) 14 (F) | Stool | DNA extraction kit (MN® NucleoSpin 96 Soi kit, Düren, Germany) | 16S rRNA V3-V4 |
| Hua, 2020 [16] | China | Autism | CSHQ ≥ 41 | Child | NR | 48 (M) 12 (F) | 52 (M) 8 (F) | Stool | OMEGA DNA Kit (Omega Bio-Tek, Norcross, GA, USA) | 16S rRNA V3-V4 |
| Li, 2020 [14] | China | NR | DSM-5 | Adult | Acute | 5 (M) 15 (F) | 20 (M) 18 (F) | Stool | HiPure Stool DNA Kits B (D3141-03B, Guangzhou meiji biotechnology Co., Ltd., Guangzhou, China) | 16S rRNA V3-V4 |
| Li, 2020 [14] | China | NR | DSM-5 | Adult | Chronic | 13 (M) 25 (F) | 20 (M) 18 (F) | Stool | HiPure Stool DNA Kits B (D3141-03B, Guangzhou meiji biotechnology Co., Ltd., Guangzhou, China) | 16S rRNA V3-V4 |
| Liu, 2019 [12] | China | NR | ICSD-3 | Adult | Chronic | 10 | 10 | Stool | ZR Fecal DNA Kit (Zymo Research, Irvine, CA, United States) | 16S rRNA V3-V4 |
| Luo, 2022 [22] | China | Minimal hepatic encephalopathy | PSQI > 5 | Adult | NR | 37 (M) 28 (F) | 45 (M) 33 (F) | Stool | QIAamp Fast DNA Stool Mini Kit (Qiagen, Germantown, MD, USA) | 16S rRNA V3-V4 |
| Masyutina, 2021 [15] | Russia | NR | ICSD-3 | Adult | Chronic | 23 (M) 32 (F) | 16 (M) 34 (F) | Stool | Means of phenol extraction | 16S rRNA |
| Tanaka, 2023 [23] | Japan | Depression and anxiety | PSQI ≥ 9 | Adult | NR | 7 (M) 13 (F) | 10 (M) 10 (F) | Stool | In-house method | 16S rRNA V1-V2 |
| Wang, 2022 [24] | China | NR | DSM-5 | Adult | Chronic | 13 (M) 27 (F) | 10 (M) 30 (F) | Stool | Modified cetyl trimethyl-ammonium bromide (CTAB) methods | 16S rRNA V1-V2 |
| Xie, 2023 [17] | China | Ischemic stroke | PSQI > 5 | Adult | NR | 46 (M) 28 (F) | 92 (M) 39 (F) | Stool | E.Z.N.A.® soil DNA Kit (Omega Bio-Tek, Norcross, GA, USA) | 16S rRNA |
| Niu, 2022 [25] | China | Traumatic brain injury | PSQI | Adult | NR | 11 (M) 3 (F) | 11 (M) 3 (F) | Stool | NR | 16S rRNA |
| Zhang, 2022 [26] | China | NR | PSQI > 7 | Adult | NR | 10 (M) 7 (F) | 7 (M) 10 (F) | Stool | QIAamp DNA stool Mini Kit (Qiagen, Hilden, Germany) | 16S rRNA V3-V4 |
| Zhou, 2022 [27] | China | NR | DSM-5 PSQI ≥ 11 | Adult | NR | 24 | 22 | Stool | QIAamp® Fast DNA stool mini kit (Qiagen, Hilden, Germany) | 16S rRNA V3-V4 |
| Selection | Comparability | Outcome | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Is the Case Definition Adequate? | Representativeness of the Cases | Selection of Controls | Definition of Controls | Comparability of Baseline Characteristic 1 (Gender) | Comparability of Baseline Characteristic 2 (Age) | Ascertainment of Exposure | Same Method of Ascertainment for Cases and Controls | Non-Response Rate |
| Barone, 2024 [19] | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 |
| Chen, 2024 [20] | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 |
| Deng, 2024 [21] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 |
| Hua, 2020 [16] | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 |
| Li, 2020 [14] | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 |
| Li, 2020 [14] | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 |
| Liu, 2019 [12] | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Luo, 2022 [22] | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 |
| Masyutina, 2021 [15] | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 |
| Tanaka, 2023 [23] | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 |
| Wang, 2022 [24] | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Xie, 2023 [17] | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 |
| Niu, 2022 [25] | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 |
| Zhang, 2022 [26] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 |
| Zhou, 2022 [27] | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xie, S.; Chen, S.; Li, C.; Chan, Y.L.; Chan, N.Y.; Wing, Y.K.; Chan, F.K.L.; Su, Q.; Ng, S.C. The Role of Gut Microbiota in Insomnia: A Systematic Review of Case–Control Studies. Life 2025, 15, 1086. https://doi.org/10.3390/life15071086
Wang Y, Xie S, Chen S, Li C, Chan YL, Chan NY, Wing YK, Chan FKL, Su Q, Ng SC. The Role of Gut Microbiota in Insomnia: A Systematic Review of Case–Control Studies. Life. 2025; 15(7):1086. https://doi.org/10.3390/life15071086
Chicago/Turabian StyleWang, Yun, Suyi Xie, Sizhe Chen, Chenyu Li, Yeuk Lam Chan, Ngan Yin Chan, Yun Kwok Wing, Francis K. L. Chan, Qi Su, and Siew C. Ng. 2025. "The Role of Gut Microbiota in Insomnia: A Systematic Review of Case–Control Studies" Life 15, no. 7: 1086. https://doi.org/10.3390/life15071086
APA StyleWang, Y., Xie, S., Chen, S., Li, C., Chan, Y. L., Chan, N. Y., Wing, Y. K., Chan, F. K. L., Su, Q., & Ng, S. C. (2025). The Role of Gut Microbiota in Insomnia: A Systematic Review of Case–Control Studies. Life, 15(7), 1086. https://doi.org/10.3390/life15071086








