Guidance on the Surgical Management of Rectal Cancer: An Umbrella Review
Abstract
1. Introduction
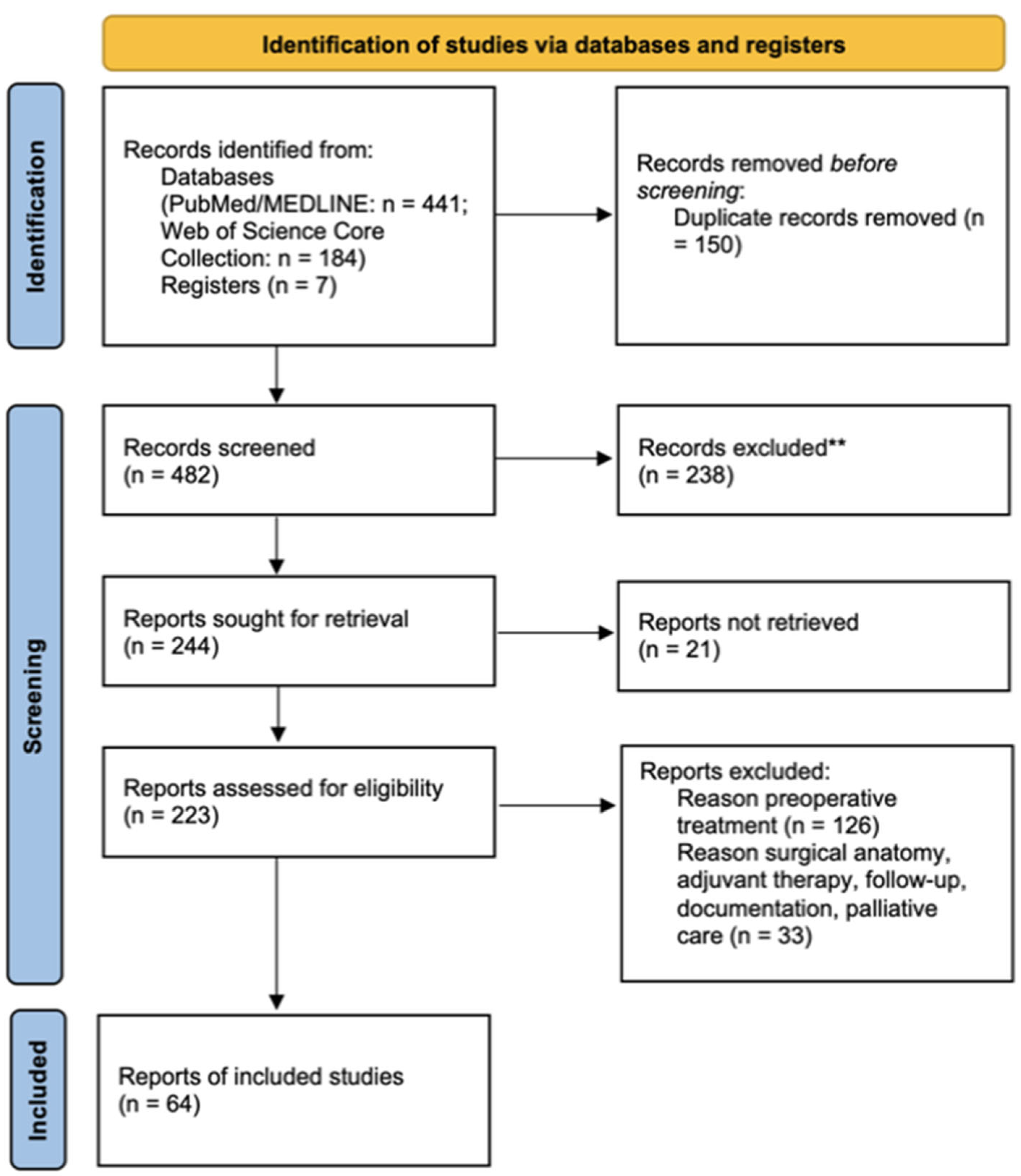
2. Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Strategy
2.3. Quality Appraisal
2.4. Data Synthesis
3. Results
3.1. Preoperative Optimization and Surgical Planning
3.1.1. Mechanical Bowel Preparation and Oral Antibiotics to Reduce Surgical Complications
3.1.2. Enhanced Recovery After Surgery (ERAS) Protocols
3.1.3. Venous Thromboembolism (VTE) Prophylaxis Strategies and Their Duration
3.2. Surgical Techniques and Approaches
3.2.1. Open and Minimally Invasive Approaches
3.2.2. Vascular Division
3.2.3. Mesorectal Excision
3.3. Surgical Expertise, Training, and Institutional Volume
3.4. Technical Aspects and Intraoperative Decision-Making in Low Rectal Cancers
3.5. Management of Lateral Pelvic Lymph Nodes
4. Discussions
4.1. Short-Term Outcomes of Surgical Approaches
4.2. Pathological Outcomes of Surgical Approaches
4.3. Long-Term Oncological Outcomes of Surgical Approaches
4.4. Limitations of the Current Study
5. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2019 Colorectal Cancer Collaborators. Global, Regional, and National Burden of Colorectal Cancer and Its Risk Factors, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol. Hepatol. 2022, 7, 627–647. [Google Scholar] [CrossRef]
- Mangone, L.; Zizzo, M.; Nardecchia, M.; Marinelli, F.; Bisceglia, I.; Braghiroli, M.B.; Banzi, M.C.; Damato, A.; Cerullo, L.; Pellegri, C.; et al. Impact of Multidisciplinary Team Management on Survival and Recurrence in Stage I–III Colorectal Cancer: A Population-Based Study in Northern Italy. Biology 2024, 13, 928. [Google Scholar] [CrossRef]
- Harji, D.; Vallance, A.; Ibitoye, T.; Wilkin, R.; Boyle, J.; Clifford, R.; Convie, L.; Duff, M.; Elavia, K.; Evans, M.; et al. IMPACT Organizational Survey Highlighting Provision of Services for Patients with Locally Advanced and Recurrent Colorectal Cancer across Great Britain and Ireland. Color. Dis. 2024, 26, 2033–2038. [Google Scholar] [CrossRef]
- Ma, H.; Li, H.; Xu, T.; Gao, Y.; Liu, S.; Wang, W.; Wei, L.; Wang, X.; Jiang, L.; Chi, Y.; et al. Multidisciplinary Team Quality Improves the Survival Outcomes of Locally Advanced Rectal Cancer Patients: A Post Hoc Analysis of the STELLAR Trial. Radiother. Oncol. 2024, 200, 110524. [Google Scholar] [CrossRef]
- Krzeszowiak, J.; Pach, R.; Richter, P.; Lorenc, Z.; Rutkowski, A.; Ochwat, K.; Zegarski, W.; Frączek, M.; Szczepanik, A. The Impact of Oncological Package Implementation on the Treatment of Rectal Cancer in Years 2013–2019 in Poland—Multicenter Study. Pol. Przegl. Chir. 2024, 96, 18–25. [Google Scholar] [CrossRef]
- Negoi, I. Guidance for the Management of Rectal Cancer: An Umbrella Review of Existing Guidelines Regarding Surgical Anatomy, Adjuvant Therapy, Follow-up and Surveillance, Specific Considerations, Documentation, and Palliative Care. Cureus 2025, 17, e83345. [Google Scholar] [CrossRef]
- REACCT Collaborative; Zaborowski, A.M.; Abdile, A.; Adamina, M.; Aigner, F.; d’Allens, L.; Allmer, C.; Álvarez, A.; Anula, R.; Andric, M.; et al. Characteristics of Early-Onset vs. Late-Onset Colorectal Cancer: A Review. JAMA Surg. 2021, 156, 865–874. [Google Scholar] [CrossRef]
- Peters, G.W.; Thomas, G.; Applegarth, J.A.; Wasvary, J.; Bohler, F.; Callahan, R.E.; Bergeron, S.; Wasvary, H.J. The Effect of the Adoption of the National Accreditation Program for Rectal Cancer Process on Compliance Standards at a Single Institution. Am. Surg. 2024, 91, 345–350. [Google Scholar] [CrossRef]
- Scott, A.J.; Kennedy, E.B.; Berlin, J.; Brown, G.; Chalabi, M.; Cho, M.T.; Cusnir, M.; Dorth, J.; George, M.; Kachnic, L.A.; et al. Management of Locally Advanced Rectal Cancer: ASCO Guideline. J. Clin. Oncol. 2024, 42, 3355–3375. [Google Scholar] [CrossRef]
- Belbasis, L.; Bellou, V.; Ioannidis, J.P.A. Conducting Umbrella Reviews. BMJ Med. 2022, 1, e000071. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Linder, C.; Nepogodiev, D.; GAIT 2024 Collaborative Group. Generative Artificial Intelligence Transparency in Scientific Writing: The GAIT 2024 Guidance. Impact Surg. 2025, 2, 6–11. [Google Scholar] [CrossRef]
- Moran, B.; Cunningham, C.; Singh, T.; Sagar, P.; Bradbury, J.; Geh, I.; Karandikar, S. Association of Coloproctology of Great Britain & Ireland (ACPGBI): Guidelines for the Management of Cancer of the Colon, Rectum and Anus (2017)—Surgical Management. Color. Dis. 2017, 19 (Suppl. 1), 18–36. [Google Scholar] [CrossRef]
- Rybakov, E.; Nagudov, M.; Sukhina, M.; Shelygin, Y. Impact of Oral Antibiotic Prophylaxis on Surgical Site Infection after Rectal Surgery: Results of Randomized Trial. Int. J. Color. Dis. 2021, 36, 323–330. [Google Scholar] [CrossRef]
- Kiran, R.P.; Murray, A.C.A.; Chiuzan, C.; Estrada, D.; Forde, K. Combined Preoperative Mechanical Bowel Preparation with Oral Antibiotics Significantly Reduces Surgical Site Infection, Anastomotic Leak, and Ileus after Colorectal Surgery. Ann. Surg. 2015, 262, 416–425; discussion 423–425. [Google Scholar] [CrossRef]
- Anjum, N.; Ren, J.; Wang, G.; Li, G.; Wu, X.; Dong, H.; Wu, Q.; Li, J. A Randomized Control Trial of Preoperative Oral Antibiotics as Adjunct Therapy to Systemic Antibiotics for Preventing Surgical Site Infection in Clean Contaminated, Contaminated, and Dirty Type of Colorectal Surgeries. Dis. Colon Rectum 2017, 60, 1291–1298. [Google Scholar] [CrossRef]
- Johnson, G.; Ziegler, J.; Helewa, R.; Askin, N.; Rabbani, R.; Abou-Setta, A.M. Preoperative Oral Fluoroquinolone Antibiotics in Elective Colorectal Surgery to Prevent Surgical Site Infections: A Systematic Review and Meta-Analysis. Can. J. Surg. 2023, 66, E21–E31. [Google Scholar] [CrossRef]
- Nygren, J.; Thacker, J.; Carli, F.; Fearon, K.C.H.; Norderval, S.; Lobo, D.N.; Ljungqvist, O.; Soop, M.; Ramirez, J.; Enhanced Recovery After Surgery Society. Guidelines for Perioperative Care in Elective Rectal/Pelvic Surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations. Clin. Nutr. 2012, 31, 801–816. [Google Scholar] [CrossRef]
- Hanna, H.H.; Abdelhalim, S.; Khairy, A.; Al-Abbasi, R.M.A. Enhanced Recovery After Colo-Rectal Surgeries (ERAS) V.S Conventional Care A Systematic Review and Meta-Analysis. QJM 2021, 114, i143. [Google Scholar] [CrossRef]
- Bakker, N.; Doodeman, H.J.; Dunker, M.S.; Schreurs, W.H.; Houdijk, A.P.J. Improving Postoperative Outcome in Rectal Cancer Surgery: Enhanced Recovery After Surgery in an Era of Increasing Laparoscopic Resection. Langenbeck’s Arch. Surg. 2021, 406, 2769–2779. [Google Scholar] [CrossRef]
- Teeuwen, P.H.E.; Bleichrodt, R.P.; Strik, C.; Groenewoud, J.J.M.; Brinkert, W.; van Laarhoven, C.J.H.M.; van Goor, H.; Bremers, A.J.A. Enhanced Recovery after Surgery (ERAS) versus Conventional Postoperative Care in Colorectal Surgery. J. Gastrointest. Surg. 2010, 14, 88–95. [Google Scholar] [CrossRef]
- Ban, K.A.; Berian, J.R.; Ko, C.Y. Does Implementation of Enhanced Recovery after Surgery (ERAS) Protocols in Colorectal Surgery Improve Patient Outcomes? Clin. Colon Rectal Surg. 2019, 32, 109–113. [Google Scholar] [CrossRef]
- Elsenosy, A.M.; Hassan, E.; Abdelgader, M.; Elgamily, O.S.; Hegazy, A. Enhanced Recovery after Surgery (ERAS) Approach: A Medical Complex Experience. Cureus 2023, 15, e51208. [Google Scholar] [CrossRef]
- Bel Diaz, J.; Barbero Mielgo, M.; Pérez Garnelo, A.; Guzmán Carranza, R.; García Fernández, J. Analysis of Protocol Adherence and Outcomes of an Enhanced Recovery Program in Colorectal Surgery after 5 Years of Implementation. J. Health Qual. Res. 2025, 40, 101111. [Google Scholar] [CrossRef]
- Olive, M.; Portilho, A.; Tustumi, F.; Seid, V.; Araujo, S. The Impact of Enhanced Recovery After Surgery (ERAS) Compliance in Colorectal Surgery for Cancer. Eur. J. Surg. Oncol. 2024, 50, 109057. [Google Scholar] [CrossRef]
- Hayes, J.W.; Ryan, É.J.; Boland, P.A.; Creavin, B.; Kelly, M.E.; Beddy, D. The Prevalence of Venous Thromboembolism in Rectal Surgery: A Systematic Review and Meta-Analysis. Int. J. Color. Dis. 2019, 34, 849–860. [Google Scholar] [CrossRef]
- Polk, H.C., Jr.; O’Brien, S.; Qadan, M. Finally, a More Balanced View of Venous Thromboembolism Prophylaxis. Dis. Colon Rectum 2019, 62, 1269–1270. [Google Scholar] [CrossRef]
- Rausa, E.; Kelly, M.E.; Asti, E.; Aiolfi, A.; Bonitta, G.; Winter, D.C.; Bonavina, L. Extended versus Conventional Thromboprophylaxis after Major Abdominal and Pelvic Surgery: Systematic Review and Meta-Analysis of Randomized Clinical Trials. Surgery 2018, 164, 1234–1240. [Google Scholar] [CrossRef]
- Benson, A.; Venook, A.; Adam, M.; Chang, G.; Chen, Y.; Ciombor, K.; Cohen, S.; Cooper, H.; Deming, D.; Garrido-Laguna, I.; et al. NCCN Guidelines Version 4.2024 Rectal Cancer. Available online: https://www.nccn.org/guidelines/category_1 (accessed on 30 November 2024).
- You, Y.N.; Hardiman, K.M.; Bafford, A.; Poylin, V.; Francone, T.D.; Davis, K.; Paquette, I.M.; Steele, S.R.; Feingold, D.L.; on behalf of the Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Rectal Cancer. Dis. Colon Rectum 2020, 63, 1191–1222. [Google Scholar] [CrossRef]
- Hashiguchi, Y.; Muro, K.; Saito, Y.; Ito, Y.; Ajioka, Y.; Hamaguchi, T.; Hasegawa, K.; Hotta, K.; Ishida, H.; Ishiguro, M.; et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2019 for the Treatment of Colorectal Cancer. Int. J. Clin. Oncol. 2020, 25, 1–42. [Google Scholar] [CrossRef]
- 1 Recommendations | Transanal Total Mesorectal Excision for Rectal Cancer | Guidance | NICE. Available online: https://www.nice.org.uk/guidance/ipg713 (accessed on 30 November 2024).
- Langenfeld, S.J.; Davis, B.R.; Vogel, J.D.; Davids, J.S.; Temple, L.K.F.; Cologne, K.G.; Hendren, S.; Hunt, S.; Garcia Aguilar, J.; Feingold, D.L.; et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Rectal Cancer 2023 Supplement. Dis. Colon Rectum 2024, 67, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Brillantino, A.; Skokowski, J.; Ciarleglio, F.A.; Vashist, Y.; Grillo, M.; Antropoli, C.; Herrera Kok, J.H.; Mosca, V.; De Luca, R.; Polom, K.; et al. Inferior Mesenteric Artery Ligation Level in Rectal Cancer Surgery beyond Conventions: A Review. Cancers 2023, 16, 72. [Google Scholar] [CrossRef] [PubMed]
- Charan, I.; Kapoor, A.; Singhal, M.K.; Jagawat, N.; Bhavsar, D.; Jain, V.; Kumar, V.; Kumar, H.S. High Ligation of Inferior Mesenteric Artery in Left Colonic and Rectal Cancers: Lymph Node Yield and Survival Benefit. Indian J. Surg. 2015, 77, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yu, M.-H.; Huang, Y.-Z.; Jing, R.; Qin, J.; Qin, S.-L.; Shah, J.N.; Zhong, M. Lymphadenectomy around Inferior Mesenteric Artery in Low-Tie vs. High-Tie Laparoscopic Anterior Resection: Short- and Long-Term Outcome of a Cohort of 614 Rectal Cancers. Cancer Manag. Res. 2021, 13, 3963–3971. [Google Scholar] [CrossRef]
- Kruszewski, W.J.; Szajewski, M.; Ciesielski, M.; Buczek, T.; Kawecki, K.; Walczak, J. Level of Inferior Mesenteric Artery Ligation Does Not Affect Rectal Cancer Treatment Outcomes despite Better Cancer-Specific Survival after Low Ligation-Randomized Trial Results. Color. Dis. 2021, 23, 2575–2583. [Google Scholar] [CrossRef]
- Nayeri, M.; Iskander, O.; Tabchouri, N.; Artus, A.; Michot, N.; Muller, O.; Giger-Pabst, U.; Bourlier, P.; Kraemer-Bucur, A.; Lecomte, T.; et al. Low Tie Compared to High Tie Vascular Ligation of the Inferior Mesenteric Artery in Rectal Cancer Surgery Decreases Postoperative Complications without Affecting Overall Survival. Anticancer Res. 2019, 39, 4363–4370. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Hsu, Y.-J.; Chern, Y.-J.; Jong, B.-K.; Liao, C.-K.; Hsieh, P.-S.; Tsai, W.-S.; You, J.-F. Potential Short-Term Outcome Advantage of Low vs. High Ligation of Inferior Mesenteric Artery for Sigmoid and Rectal Cancer: Propensity Score Matching Analysis. BMC Surg. 2023, 23, 33. [Google Scholar] [CrossRef]
- Fiori, E.; Crocetti, D.; Lamazza, A.; DE Felice, F.; Sterpetti, A.V.; Irace, L.; Mingoli, A.; Sapienza, P.; De Toma, G. Is Low Inferior Mesenteric Artery Ligation Worthwhile to Prevent Urinary and Sexual Dysfunction after Total Mesorectal Excision for Rectal Cancer? Anticancer Res. 2020, 40, 4223–4228. [Google Scholar] [CrossRef]
- Luo, W.; Li, F.; Qian, C.; Lu, T.; Xiao, Y.; Xu, Z.; Jia, Y. A Novel Technique for NO. 253 Lymph Node Dissection and Left Colic Artery Preservation to Avoid Potential Postoperative Internal Hernia in Laparoscopic Radical Resection for Rectal Cancer. BMC Surg. 2024, 24, 202. [Google Scholar] [CrossRef]
- You, X.; Liu, Q.; Wu, J.; Wang, Y.; Huang, C.; Cao, G.; Dai, J.; Chen, D.; Zhou, Y. High versus Low Ligation of Inferior Mesenteric Artery during Laparoscopic Radical Resection of Rectal Cancer: A Retrospective Cohort Study. Medicine 2020, 99, e19437. [Google Scholar] [CrossRef]
- Shaibu, Z.; Chen, Z.-H.; Theophilus, A.; Mzee, S.A.S. Preservation of the Arterial Arc Formed by Left Colic Artery, Proximal Inferior Mesenteric Artery, and the First Branch of Sigmoid Arteries in Anus Saving Treatment of Low Rectal Cancer. Am. Surg. 2021, 87, 1956–1964. [Google Scholar] [CrossRef] [PubMed]
- Yeo, H.L.; Abelson, J.S.; Mao, J.; O’Mahoney, P.R.A.; Milsom, J.W.; Sedrakyan, A. Surgeon Annual and Cumulative Volumes Predict Early Postoperative Outcomes after Rectal Cancer Resection. Ann. Surg. 2016, 265, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Chioreso, C.; Del Vecchio, N.; Schweizer, M.L.; Schlichting, J.; Gribovskaja-Rupp, I.; Charlton, M.E. Association between Hospital and Surgeon Volume and Rectal Cancer Surgery Outcomes in Patients with Rectal Cancer Treated since 2000: Systematic Literature Review and Meta-Analysis. Dis. Colon Rectum 2018, 61, 1320–1332. [Google Scholar] [CrossRef] [PubMed]
- Andric, M.; Stockheim, J.; Rahimli, M.; Al-Madhi, S.; Acciuffi, S.; Dölling, M.; Croner, R.S.; Perrakis, A. Influence of Certification Program on Treatment Quality and Survival for Rectal Cancer Patients in Germany: Results of 13 Certified Centers in Collaboration with AN Institute. Cancers 2024, 16, 1496. [Google Scholar] [CrossRef]
- Archampong, D.; Borowski, D.; Wille-Jørgensen, P.; Iversen, L.H. Workload and Surgeon’s Specialty for Outcome after Colorectal Cancer Surgery. Cochrane Database Syst. Rev. 2012, 2012, CD005391. [Google Scholar] [CrossRef]
- Boyle, J.M.; van der Meulen, J.; Kuryba, A.; Cowling, T.E.; Braun, M.S.; Aggarwal, A.; Walker, K.; Fearnhead, N.S. What Is the Impact of Hospital and Surgeon Volumes on Outcomes in Rectal Cancer Surgery? Color. Dis. 2023, 25, 1981–1993. [Google Scholar] [CrossRef]
- Gollins, S.; Moran, B.; Adams, R.; Cunningham, C.; Bach, S.; Myint, A.S.; Renehan, A.; Karandikar, S.; Goh, V.; Prezzi, D.; et al. Association of Coloproctology of Great Britain & Ireland (ACPGBI): Guidelines for the Management of Cancer of the Colon, Rectum and Anus (2017)—Multidisciplinary Management. Color. Dis. 2017, 19 (Suppl. 1), 37–66. [Google Scholar] [CrossRef]
- Negoi, I.; Hostiuc, S.; Paun, S.; Negoi, R.I.; Beuran, M. Extralevator vs. Conventional Abdominoperineal Resection for Rectal Cancer-A Systematic Review and Meta-Analysis. Am. J. Surg. 2016, 212, 511–526. [Google Scholar] [CrossRef]
- Kusters, M.; Marijnen, C.A.M.; van de Velde, C.J.H.; Rutten, H.J.T.; Lahaye, M.J.; Kim, J.H.; Beets-Tan, R.G.H.; Beets, G.L. Patterns of Local Recurrence in Rectal Cancer; a Study of the Dutch TME Trial. Eur. J. Surg. Oncol. 2010, 36, 470–476. [Google Scholar] [CrossRef]
- Williamson, J.S.; Quyn, A.J.; Sagar, P.M. Rectal Cancer Lateral Pelvic Sidewall Lymph Nodes: A Review of Controversies and Management. Br. J. Surg. 2020, 107, 1562–1569. [Google Scholar] [CrossRef]
- Perez, R.O.; São Julião, G.P.; Vailati, B.B.; Fernandez, L.M.; Mattacheo, A.E.; Konishi, T. Lateral Node Dissection in Rectal Cancer in the Era of Minimally Invasive Surgery: A Step-by-Step Description for the Surgeon Unacquainted with This Complex Procedure with the Use of the Laparoscopic Approach. Dis. Colon Rectum 2018, 61, 1237–1240. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S.; Takifuji, K.; Hotta, T.; Matsuda, K.; Watanabe, T.; Mitani, Y.; Ieda, J.; Yamaue, H. Survival Benefit of Lateral Lymph Node Dissection According to the Region of Involvement and the Number of Lateral Lymph Nodes Involved. Surg. Today 2014, 44, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Schaap, D.P.; Boogerd, L.S.F.; Konishi, T.; Cunningham, C.; Ogura, A.; Garcia-Aguilar, J.; Beets, G.L.; Suzuki, C.; Toda, S.; Lee, I.K.; et al. Rectal Cancer Lateral Lymph Nodes: Multicentre Study of the Impact of Obturator and Internal Iliac Nodes on Oncological Outcomes. Br. J. Surg. 2021, 108, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Ogura, A.; Konishi, T.; Cunningham, C.; Garcia-Aguilar, J.; Iversen, H.; Toda, S.; Lee, I.K.; Lee, H.X.; Uehara, K.; Lee, P.; et al. Neoadjuvant (Chemo)Radiotherapy with Total Mesorectal Excision Only Is Not Sufficient to Prevent Lateral Local Recurrence in Enlarged Nodes: Results of the Multicenter Lateral Node Study of Patients with Low CT3/4 Rectal Cancer. J. Clin. Oncol. 2019, 37, 33–43. [Google Scholar] [CrossRef]
- Ogura, A.; Konishi, T.; Beets, G.L.; Cunningham, C.; Garcia-Aguilar, J.; Iversen, H.; Toda, S.; Lee, I.K.; Lee, H.X.; Uehara, K.; et al. Lateral Nodal Features on Restaging Magnetic Resonance Imaging Associated with Lateral Local Recurrence in Low Rectal Cancer after Neoadjuvant Chemoradiotherapy or Radiotherapy. JAMA Surg. 2019, 154, e192172. [Google Scholar] [CrossRef]
- Kawai, K.; Shiratori, H.; Hata, K.; Nozawa, H.; Tanaka, T.; Nishikawa, T.; Murono, K.; Ishihara, S. Optimal Size Criteria for Lateral Lymph Node Dissection after Neoadjuvant Chemoradiotherapy for Rectal Cancer. Dis. Colon Rectum 2021, 64, 274–283. [Google Scholar] [CrossRef]
- Fujita, S.; Mizusawa, J.; Kanemitsu, Y.; Ito, M.; Kinugasa, Y.; Komori, K.; Ohue, M.; Ota, M.; Akazai, Y.; Shiozawa, M.; et al. Mesorectal Excision with or without Lateral Lymph Node Dissection for Clinical Stage II/III Lower Rectal Cancer (JCOG0212). Ann. Surg. 2017, 266, 201–207. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Fujita, S.; Ota, M.; Mizusawa, J.; Shida, D.; Kanemitsu, Y.; Ito, M.; Shiomi, A.; Komori, K.; Ohue, M.; et al. Long-Term Follow-up of the Randomized Trial of Mesorectal Excision with or without Lateral Lymph Node Dissection in Rectal Cancer (JCOG0212). Br. J. Surg. 2020, 107, 586–594. [Google Scholar] [CrossRef]
- Hajibandeh, S.; Hajibandeh, S.; Matthews, J.; Palmer, L.; Maw, A. Meta-Analysis of Survival and Functional Outcomes after Total Mesorectal Excision with or without Lateral Pelvic Lymph Node Dissection in Rectal Cancer Surgery. Surgery 2020, 168, 486–496. [Google Scholar] [CrossRef]
- Conticchio, M.; Papagni, V.; Notarnicola, M.; Delvecchio, A.; Riccelli, U.; Ammendola, M.; Currò, G.; Pessaux, P.; Silvestris, N.; Memeo, R. Laparoscopic vs. Open Mesorectal Excision for Rectal Cancer: Are These Approaches Still Comparable? A Systematic Review and Meta-Analysis. PLoS ONE 2020, 15, e0235887. [Google Scholar] [CrossRef]
- Chi, P.; Su, X.; Xu, J.; Qiu, H.; Wang, Z.; Kang, L.; Deng, H.; Chen, W.; Zhang, Q.; Du, X.; et al. Short-Term Outcomes of Laparoscopy-Assisted versus Open Surgery for Low Rectal Cancer (LASRE): A Multicenter, Randomized, Controlled Trial. J. Clin. Oncol. 2022, 40, 3516. [Google Scholar] [CrossRef]
- Martínez-Pérez, A.; Carra, M.C.; Brunetti, F.; de’Angelis, N. Pathologic Outcomes of Laparoscopic vs. Open Mesorectal Excision for Rectal Cancer: A Systematic Review and Meta-Analysis. JAMA Surg. 2017, 152, e165665. [Google Scholar] [CrossRef]
- Acuna, S.A.; Chesney, T.R.; Ramjist, J.K.; Shah, P.S.; Kennedy, E.D.; Baxter, N.N. Laparoscopic versus Open Resection for Rectal Cancer: A Noninferiority Meta-Analysis of Quality of Surgical Resection Outcomes. Ann. Surg. 2019, 269, 849–855. [Google Scholar] [CrossRef]
- de’Angelis, N.; Schena, C.A.; Azzolina, D.; Carra, M.C.; Khan, J.; Gronnier, C.; Gaujoux, S.; Bianchi, P.P.; Spinelli, A.; Rouanet, P.; et al. Histopathological Outcomes of Transanal, Robotic, Open, and Laparoscopic Surgery for Rectal Cancer Resection. A Bayesian Network Meta-Analysis of Randomized Controlled Trials. Eur. J. Surg. Oncol. 2025, 51, 109481. [Google Scholar] [CrossRef]
- Seow, W.; Dudi-Venkata, N.N.; Bedrikovetski, S.; Kroon, H.M.; Sammour, T. Outcomes of Open vs. Laparoscopic vs. Robotic vs. Transanal Total Mesorectal Excision (TME) for Rectal Cancer: A Network Meta-Analysis. Tech. Coloproctol. 2023, 27, 345–360. [Google Scholar] [CrossRef]
- Deijen, C.L.; Velthuis, S.; Tsai, A.; Mavroveli, S.; de Lange-de Klerk, E.S.M.; Sietses, C.; Tuynman, J.B.; Lacy, A.M.; Hanna, G.B.; Bonjer, H.J. COLOR III: A Multicentre Randomised Clinical Trial Comparing Transanal TME versus Laparoscopic TME for Mid and Low Rectal Cancer. Surg. Endosc. 2016, 30, 3210–3215. [Google Scholar] [CrossRef]
- Tsai, A.Y.-C.; Mavroveli, S.; Miskovic, D.; van Oostendorp, S.; Adamina, M.; Hompes, R.; Aigner, F.; Spinelli, A.; Warusavitarne, J.; Knol, J.; et al. Surgical Quality Assurance in COLOR III: Standardization and Competency Assessment in a Randomized Controlled Trial. Ann. Surg. 2019, 270, 768–774. [Google Scholar] [CrossRef]
- Kang, L.; Zeng, Z.; Liu, H.; Chinese Transanal Endoscopic Surgery Collaborative (CTESC) Group. Three-Year Disease-Free Survival after Transanal vs. Laparoscopic Total Mesorectal Excision for Rectal Cancer (TaLaR): A Randomized Clinical Trial. J. Clin. Oncol. 2024, 42, 3516. [Google Scholar] [CrossRef]
- Kong, M.; Chen, H.; Shan, K.; Sheng, H.; Li, L. Comparison of Survival among Adults with Rectal Cancer Who Have Undergone Laparoscopic vs. Open Surgery: A Meta-Analysis. JAMA Netw. Open 2022, 5, e2210861. [Google Scholar] [CrossRef]
- Lim, W.H.; Tan, D.J.H.; Ng, C.H.; Syn, N.; Tai, B.C.; Gu, T.; Xiao, J.; Chin, Y.H.; Wing Ow, Z.G.; Wong, N.W.; et al. Laparoscopic versus Open Resection for Rectal Cancer: An Individual Patient Data Meta Analysis of Randomized Controlled Trials. Eur. J. Surg. Oncol. 2022, 48, 1133–1143. [Google Scholar] [CrossRef]
- Cui, M.; Liu, S. Meta-Analysis of the Effect of Laparoscopic Surgery and Open Surgery on Long-Term Quality of Life in Patients with Colorectal Cancer. Medicine 2023, 102, e34922. [Google Scholar] [CrossRef]
- Park, J.W.; Kang, S.-B.; Hao, J.; Lim, S.-B.; Choi, H.S.; Kim, D.-W.; Chang, H.J.; Kim, D.Y.; Jung, K.H.; Kim, T.-Y.; et al. Open versus Laparoscopic Surgery for Mid or Low Rectal Cancer after Neoadjuvant Chemoradiotherapy (COREAN Trial): 10-Year Follow-up of an Open-Label, Non-Inferiority, Randomised Controlled Trial. Lancet Gastroenterol. Hepatol. 2021, 6, 569–577. [Google Scholar] [CrossRef]
- Kang, S.-B.; Park, J.W.; Jeong, S.-Y.; Nam, B.H.; Choi, H.S.; Kim, D.-W.; Lim, S.-B.; Lee, T.-G.; Kim, D.Y.; Kim, J.-S.; et al. Open versus Laparoscopic Surgery for Mid or Low Rectal Cancer after Neoadjuvant Chemoradiotherapy (COREAN Trial): Short-Term Outcomes of an Open-Label Randomised Controlled Trial. Lancet Oncol. 2010, 11, 637–645. [Google Scholar] [CrossRef]
- Jeong, S.-Y.; Park, J.W.; Nam, B.H.; Kim, S.; Kang, S.-B.; Lim, S.-B.; Choi, H.S.; Kim, D.-W.; Chang, H.J.; Kim, D.Y.; et al. Open versus Laparoscopic Surgery for Mid-Rectal or Low-Rectal Cancer after Neoadjuvant Chemoradiotherapy (COREAN Trial): Survival Outcomes of an Open-Label, Non-Inferiority, Randomised Controlled Trial. Lancet Oncol. 2014, 15, 767–774. [Google Scholar] [CrossRef]
- Domínguez-Garijo, P.; de Lacy, F.B.; Lacy, A.M. COREAN Trial: Long-Term Clinical Impact of Laparoscopic Surgery in Patients Undergoing Total Mesorectal Excision. Dig. Med. Res. 2022, 5, 1. [Google Scholar] [CrossRef]
- van der Pas, M.H.; Haglind, E.; Cuesta, M.A.; Fürst, A.; Lacy, A.M.; Hop, W.C.; Bonjer, H.J.; COlorectal cancer Laparoscopic or Open Resection II (COLOR II) Study Group. Laparoscopic versus Open Surgery for Rectal Cancer (COLOR II): Short-Term Outcomes of a Randomised, Phase 3 Trial. Lancet Oncol. 2013, 14, 210–218. [Google Scholar] [CrossRef]
- Bonjer, H.J.; Deijen, C.L.; Haglind, E.; COLOR II Study Group. A Randomized Trial of Laparoscopic Versus Open Surgery for Rectal Cancer. N. Engl. J. Med. 2015, 373, 194. [Google Scholar] [CrossRef]
- Fleshman, J.; Branda, M.E.; Sargent, D.J.; Boller, A.M.; George, V.V.; Abbas, M.A.; Peters, W.R., Jr.; Maun, D.C.; Chang, G.J.; Herline, A.; et al. Disease-Free Survival and Local Recurrence for Laparoscopic Resection Compared with Open Resection of Stage II to III Rectal Cancer: Follow-up Results of the ACOSOG Z6051 Randomized Controlled Trial. Ann. Surg. 2019, 269, 589–595. [Google Scholar] [CrossRef]
- Fleshman, J.; Branda, M.; Sargent, D.J.; Boller, A.M.; George, V.; Abbas, M.; Peters, W.R., Jr.; Maun, D.; Chang, G.; Herline, A.; et al. Effect of Laparoscopic-Assisted Resection vs. Open Resection of Stage II or III Rectal Cancer on Pathologic Outcomes: The ACOSOG Z6051 Randomized Clinical Trial. JAMA 2015, 314, 1346–1355. [Google Scholar] [CrossRef]
- Martínez-Pérez, A.; de’Angelis, N. Oncologic Results of Conventional Laparoscopic TME: Is the Intramesorectal Plane Really Acceptable? Tech. Coloproctol. 2018, 22, 831–834. [Google Scholar] [CrossRef]
- Acuna, S.A.; Chesney, T.R.; Baxter, N.N. ASO Author Reflections: Clarifying the Controversy Generated by Non-Inferiority Trials of Laparoscopic Surgery for Rectal Cancer. Ann. Surg. Oncol. 2019, 26, 545–546. [Google Scholar] [CrossRef]
- Tou, S.; Bergamaschi, R. Laparoscopic Rectal Cancer Resection: Inferior to Open or Not? Color. Dis. 2016, 18, 233. [Google Scholar] [CrossRef]
- Ludwig, A.D.; Fichera, A. Laparoscopy for Rectal Cancer: Is the Story Settled? J. Laparoendosc. Adv. Surg. Tech. A 2016, 26, 302–304. [Google Scholar] [CrossRef]
- Stevenson, A.R.L.; Solomon, M.J.; Lumley, J.W.; Hewett, P.; Clouston, A.D.; Gebski, V.J.; Davies, L.; Wilson, K.; Hague, W.; Simes, J.; et al. Effect of Laparoscopic-Assisted Resection vs. Open Resection on Pathological Outcomes in Rectal Cancer: The ALaCaRT Randomized Clinical Trial. JAMA 2015, 314, 1356–1363. [Google Scholar] [CrossRef]
- Mercieca-Bebber, R.; Eggins, R.; Brown, K.; Gebski, V.J.; Brewer, K.; Lai, L.; Bailey, L.; Solomon, M.J.; Lumley, J.W.; Hewett, P.; et al. Patient-Reported Bowel, Urinary, and Sexual Outcomes after Laparoscopic-Assisted Resection or Open Resection for Rectal Cancer: The Australasian Laparoscopic Cancer of the Rectum Randomized Clinical Trial (ALaCart). Ann. Surg. 2023, 277, 449–455. [Google Scholar] [CrossRef]
- Spinelli, A.; D’Hoore, A.; Panis, Y.; Bemelman, W.A.; Jayne, D.G.; Fürst, A. Critical Appraisal of Two Randomized Clinical Trials on Pathologic Outcomes. Coloproctology 2017, 39, 277. [Google Scholar] [CrossRef]
- Acuna, S.A.; Dossa, F.; Baxter, N.N. Frequency of Misinterpretation of Inconclusive Noninferiority Trials: The Case of the Laparoscopic vs. Open Resection for Rectal Cancer Trials. JAMA Surg. 2019, 154, 90–92. [Google Scholar] [CrossRef]
- Chi, P.; Jiang, W.; Su, X.; Xu, J.; Qiu, H.; Kang, L.; Deng, H.; Chen, W.; Zhang, Q.; Yang, C.; et al. Laparoscopic-Assisted vs. Open Surgery for Low Rectal Cancer: 5-Year Outcomes of the LASRE Randomized Clinical Trial. J. Clin. Oncol. 2024, 42, e15630. [Google Scholar] [CrossRef]
- Jiang, W.; Xu, J.; Cui, M.; Qiu, H.; Wang, Z.; Kang, L.; Deng, H.; Chen, W.; Zhang, Q.; Du, X.; et al. Laparoscopy-Assisted versus Open Surgery for Low Rectal Cancer (LASRE): 3-Year Survival Outcomes of a Multicentre, Randomised, Controlled, Non-Inferiority Trial. Lancet Gastroenterol. Hepatol. 2025, 10, 34–43. [Google Scholar] [CrossRef]
- Zhu, X.-M.; Bai, X.; Wang, H.-Q.; Dai, D.-Q. Comparison of Efficacy and Safety between Robotic-Assisted versus Laparoscopic Surgery for Locally Advanced Mid-Low Rectal Cancer Following Neoadjuvant Chemoradiotherapy: A Systematic Review and Meta-Analysis. Int. J. Surg. 2024, 111, 1154–1166. [Google Scholar] [CrossRef]
- Park, J.S.; Lee, S.M.; Choi, G.-S.; Park, S.Y.; Kim, H.J.; Song, S.H.; Min, B.S.; Kim, N.K.; Kim, S.H.; Lee, K.Y. Comparison of Laparoscopic versus Robot-Assisted Surgery for Rectal Cancers: The COLRAR Randomized Controlled Trial. Ann. Surg. 2023, 278, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yuan, W.; Li, T.; Tang, B.; Jia, B.; Zhou, Y.; Zhang, W.; Zhao, R.; Zhang, C.; Cheng, L.; et al. Robotic versus Laparoscopic Surgery for Middle and Low Rectal Cancer (REAL): Primary Outcome of a Multicenter Randomized Controlled Trial. J. Clin. Oncol. 2024, 42, 3617. [Google Scholar] [CrossRef]
- Xu, J.; Yuan, W.; Li, T.; Tang, B.; Jia, B.; Zhou, Y.; Zhang, W.; Zhao, R.; Zhang, C.; Cheng, L.; et al. Robotic versus Laparoscopic Surgery for Middle and Low Rectal Cancer (REAL): Short-Term Outcomes of a Multicenter Randomized Controlled Trial. J. Clin. Oncol. 2022, 40, 14. [Google Scholar] [CrossRef]
| Patients | Malignant tumors located in the rectum. |
| Intervention | A standardized approach, with pre-established clinical pathways to be used as clinical decision-making support by members of the multidisciplinary team (surgeons, imagists, gastroenterologists, medical oncologists, radiation oncologists, pathologists, etc.). |
| Comparison | No structured approach. |
| Outcomes | Oncological, quality of life, and financial costs. |
| PubMed search strategy combined keywords and indexed terms (MeSH/headings and free text): “rectal cancer”, “surgery”, “surgical technique”, “guideline”, “consensus”, “RCT”, and “meta-analysis”, supplemented by filters for date range (2010–2025) and English language. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negoi, I. Guidance on the Surgical Management of Rectal Cancer: An Umbrella Review. Life 2025, 15, 955. https://doi.org/10.3390/life15060955
Negoi I. Guidance on the Surgical Management of Rectal Cancer: An Umbrella Review. Life. 2025; 15(6):955. https://doi.org/10.3390/life15060955
Chicago/Turabian StyleNegoi, Ionut. 2025. "Guidance on the Surgical Management of Rectal Cancer: An Umbrella Review" Life 15, no. 6: 955. https://doi.org/10.3390/life15060955
APA StyleNegoi, I. (2025). Guidance on the Surgical Management of Rectal Cancer: An Umbrella Review. Life, 15(6), 955. https://doi.org/10.3390/life15060955






