Trophoblast Extracellular Vesicles as Modulators of Keratinocyte Stress Response and Senescence
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. TEV Enrichment
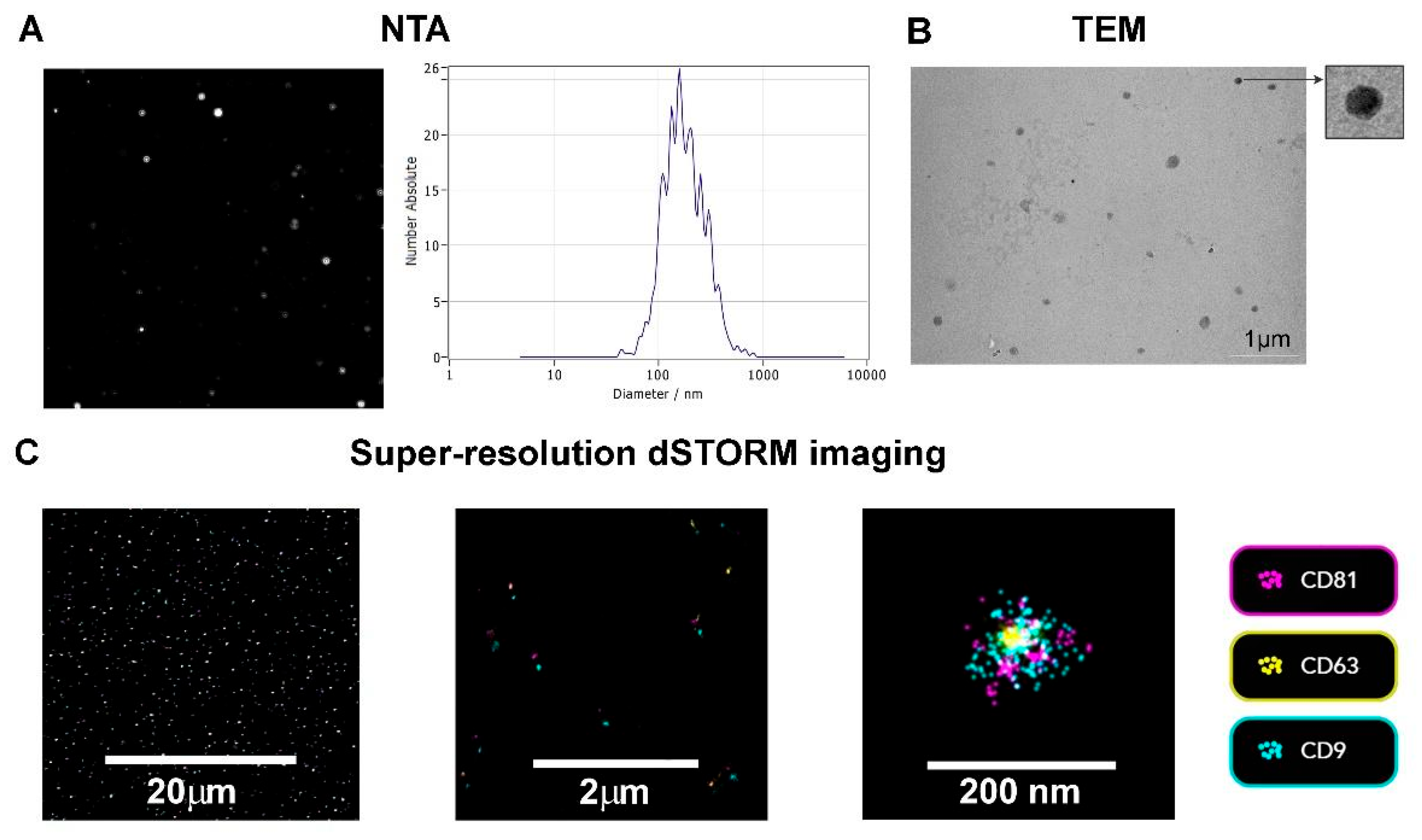
2.3. Nanoparticle Tracking Analysis (NTA)
2.4. Transmission Electron Microscopy (TEM)
2.5. TEV Super-Resolution Imaging
2.6. Experimental Design
2.7. Cell-Based ELISA Assay (cELISA)
2.8. 2′,7′–Dichlorofluorescin Diacetate (DCFH-DA) Assay
2.9. Senescence-Associated β-Galactosidase (SA-β-Gal) Staining
2.10. Alkaline Comet Assay
2.11. Flow Cytometry
2.12. qPCR Analysis
2.13. Wound Healing Assay
2.14. Statistical Analysis
3. Results
3.1. TEV Characterization
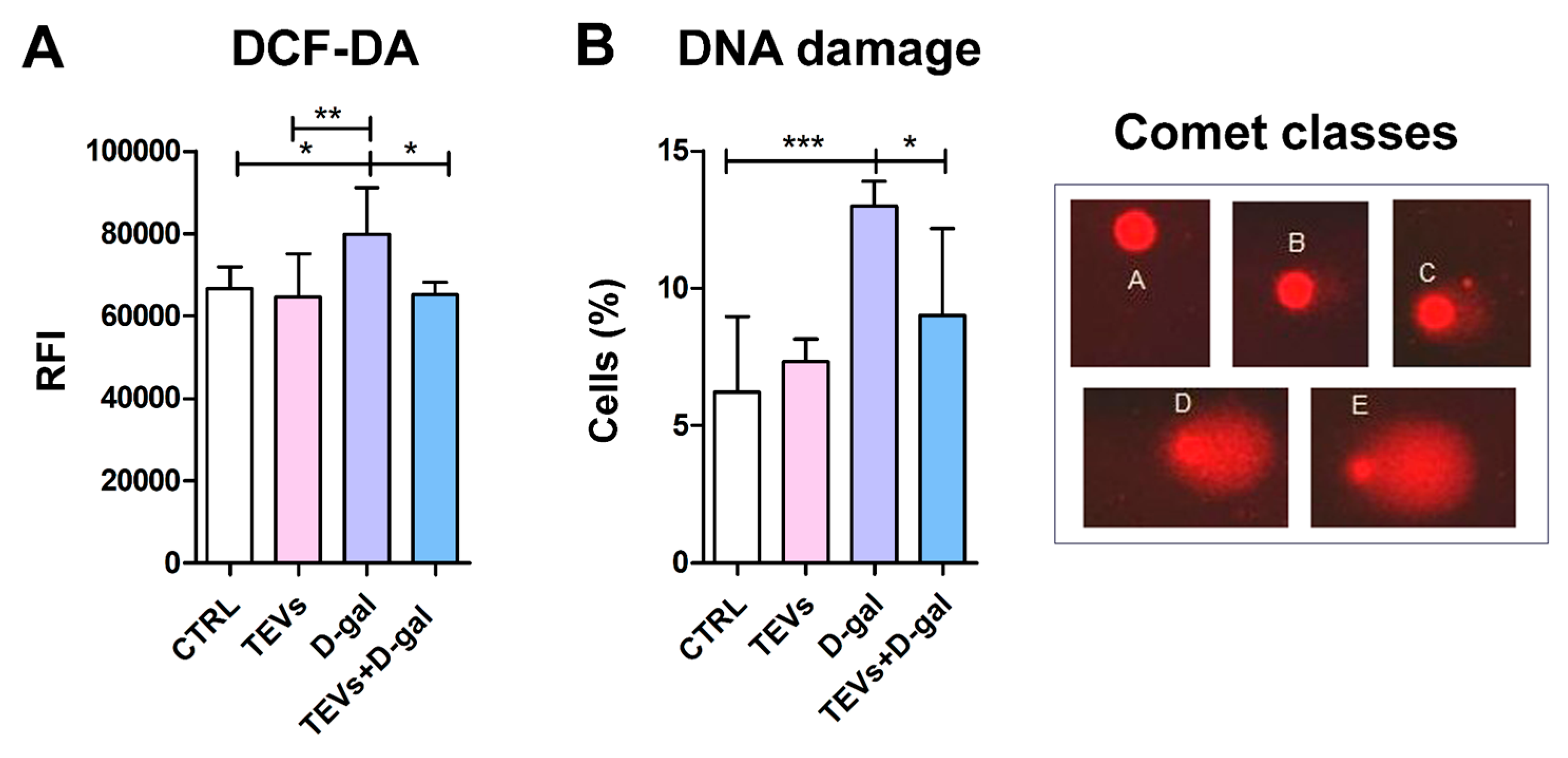
3.2. TEV Pretreatment Alleviates D-Gal-Induced OS and DNA Damage in Keratinocytes
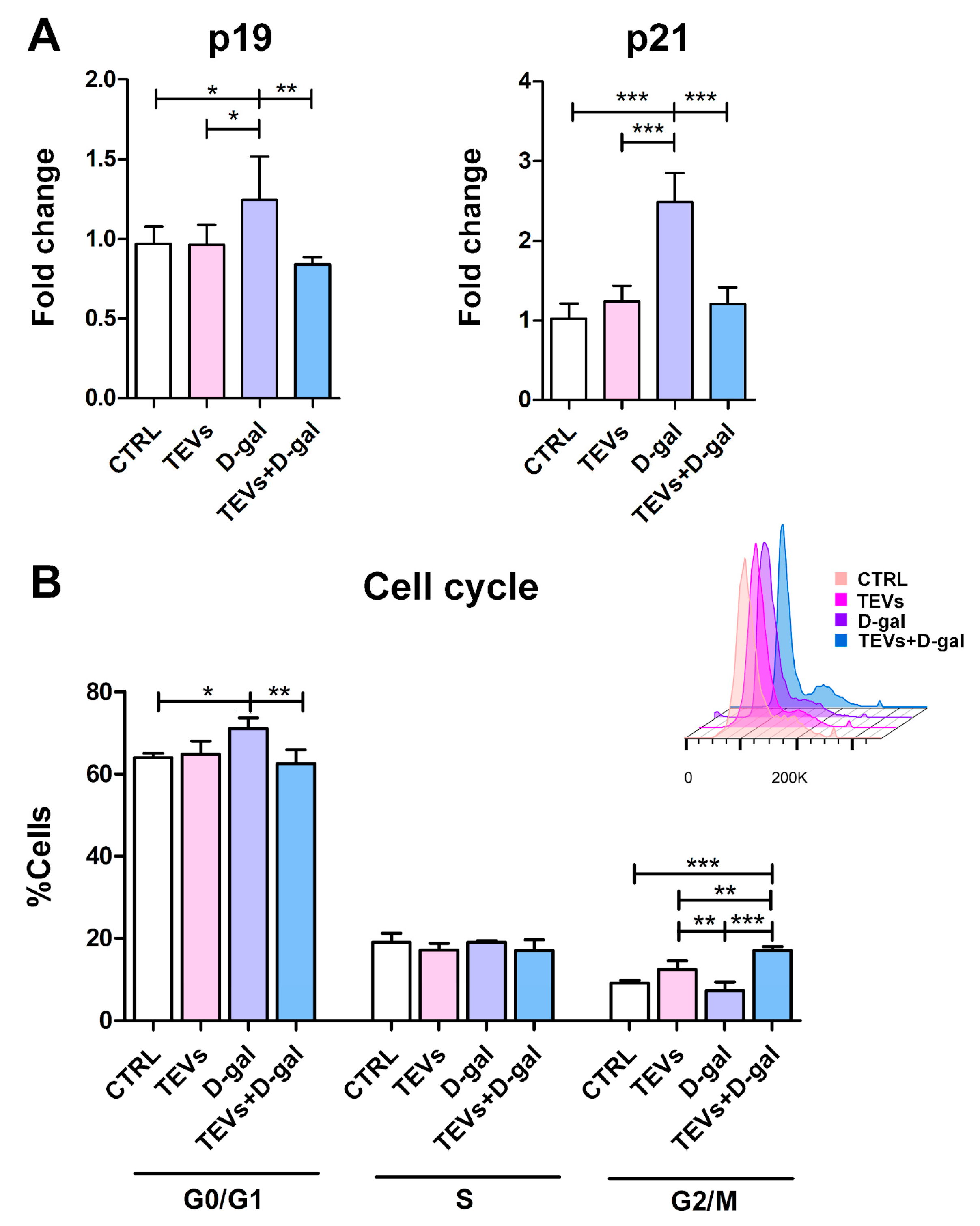
3.3. TEV Pretreatment Mitigates D-Gal-Induced Upregulation of Senescence Markers in Keratinocytes
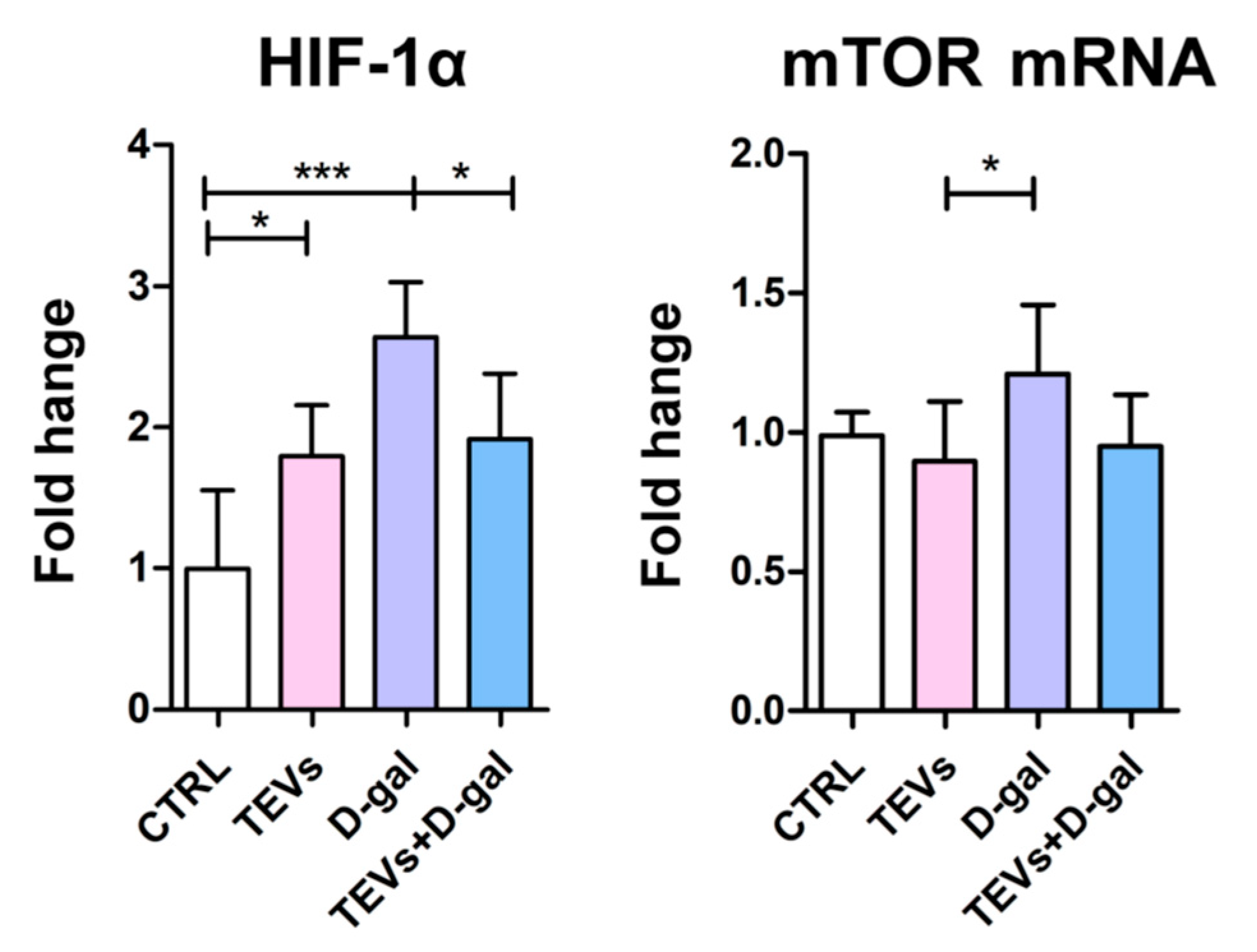
3.4. TEV Pretreatment Downregulates HIF-1α and mTOR Expression in D-Gal-Exposed Keratinocytes
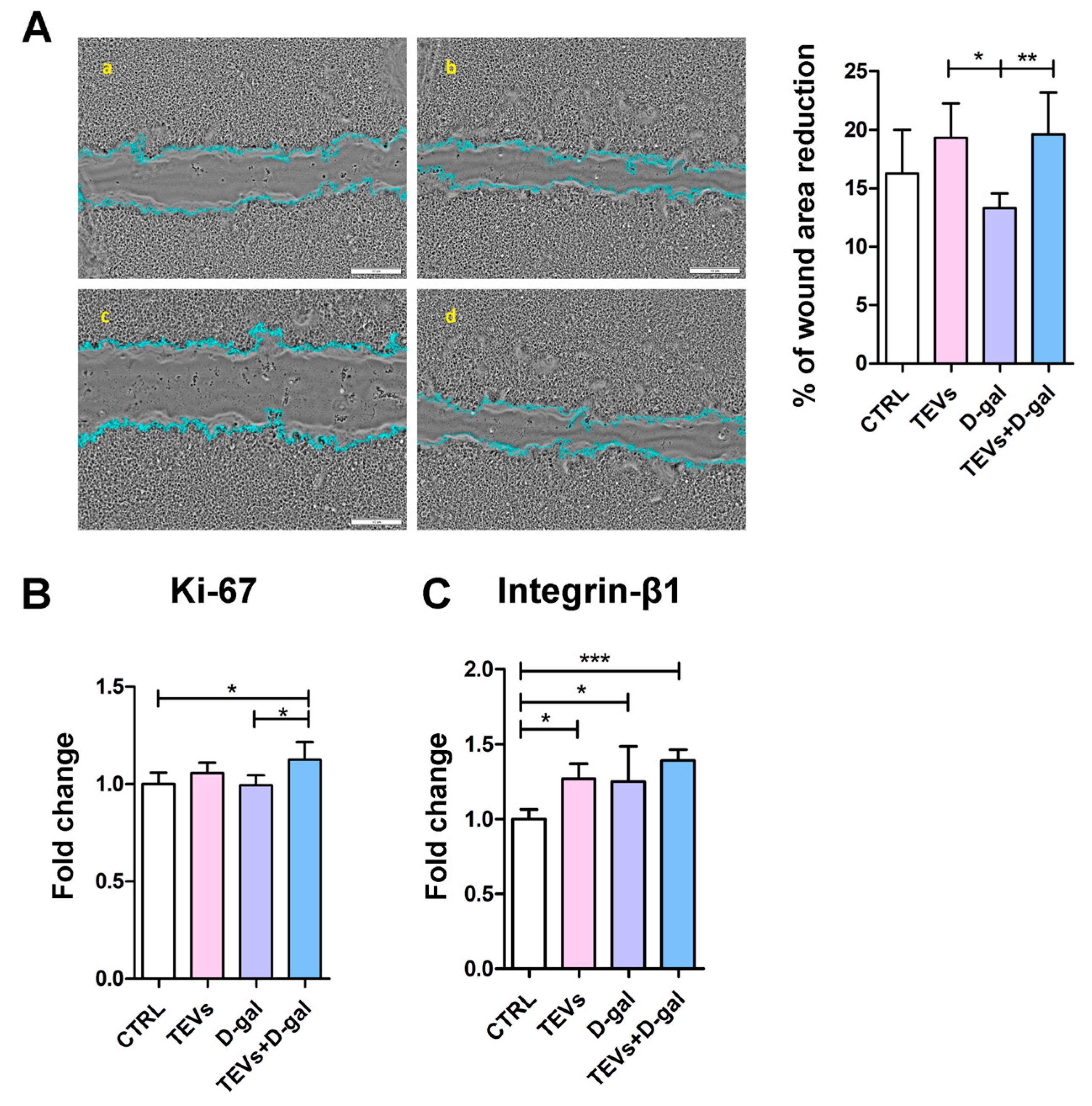
3.5. TEV Pretreatment Increases Keratinocyte Proliferation Rate, Migration, and Integrin-β1 Subunit Expression After D-Gal Exposure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kanitakis, J. Anatomy, Histology and Immunohistochemistry of Normal Human Skin. Eur. J. Dermatol. 2002, 12, 390–399; quiz 400–401. [Google Scholar] [PubMed]
- Wong, Q.Y.A.; Chew, F.T. Defining Skin Aging and Its Risk Factors: A Systematic Review and Meta-Analysis. Sci. Rep. 2021, 11, 22075. [Google Scholar] [CrossRef] [PubMed]
- Kuang, L.; Li, C. ΔNp63α-Mediated Epigenetic Regulation in Keratinocyte Senescence. Epigenetics 2023, 18, 2173931. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.P.; Katta, R. Sugar Sag: Glycation and the Role of Diet in Aging Skin. Ski. Ther. Lett. 2015, 20, 1–5. [Google Scholar]
- Danby, F.W. Nutrition and Aging Skin: Sugar and Glycation. Clin. Dermatol. 2010, 28, 409–411. [Google Scholar] [CrossRef]
- Umbayev, B.; Askarova, S.; Almabayeva, A.; Saliev, T.; Masoud, A.-R.; Bulanin, D. Galactose-Induced Skin Aging: The Role of Oxidative Stress. Oxid. Med. Cell. Longev. 2020, 2020, 7145656. [Google Scholar] [CrossRef]
- Dworkin, J.P.; Miller, S.L. A Kinetic Estimate of the Free Aldehyde Content of Aldoses. Carbohydr. Res. 2000, 329, 359–365. [Google Scholar] [CrossRef]
- Acosta, P.B.; Gross, K.C. Hidden Sources of Galactose in the Environment. Eur. J. Pediatr. 1995, 154, S87–S92. [Google Scholar] [CrossRef]
- Williams, C.A. Galactose. In Encyclopedia of Food Sciences and Nutrition; Caballero, B., Ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 2843–2846. [Google Scholar]
- Delwing-de Lima, D.; Hennrich, S.B.; Delwing-Dal Magro, D.; Aurélio, J.G.M.; Serpa, A.P.; Augusto, T.W.; Pereira, N.R. The Effect of D-Galactose Induced Oxidative Stress on in Vitro Redox Homeostasis in Rat Plasma and Erythrocytes. Biomed. Pharmacother. 2017, 86, 686–693. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.-X.; Liu, Y.-M.; Chen, K.-L.; Chen, G. A Comparative Study of Anti-Aging Properties and Mechanism: Resveratrol and Caloric Restriction. Oncotarget 2017, 8, 65717–65729. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Zhu, Q.; Li, T.; Lu, H.; Wei, N.; Huang, Y.; Shi, R.; Ma, X.; Wang, X.; et al. Anti-Skin-Aging Effect of Epigallocatechin Gallate by Regulating Epidermal Growth Factor Receptor Pathway on Aging Mouse Model Induced by d-Galactose. Mech. Ageing Dev. 2017, 164, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sukoyan, G.; Tsivtsivadze, E.; Golovach, V.; Kezeli, T.; Demina, N. Anti-Aging Effect of Cynara Cardunculus L. var. Cynara Scolymus L. Extract in D-Galactose-Induced Skin Aging Model in Rats. Pharmacol. Pharm. 2018, 9, 428–439. [Google Scholar] [CrossRef]
- Chin, T.; Lee, X.E.; Ng, P.Y.; Lee, Y.; Dreesen, O. The Role of Cellular Senescence in Skin Aging and Age-Related Skin Pathologies. Front. Physiol. 2023, 14, 1297637. [Google Scholar] [CrossRef]
- Pogozhykh, O.; Prokopyuk, V.; Figueiredo, C.; Pogozhykh, D. Placenta and Placental Derivatives in Regenerative Therapies: Experimental Studies, History, and Prospects. Stem Cells Int. 2018, 2018, 4837930. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.-B.; Kumar, R.; et al. Extracellular Vesicles as Tools and Targets in Therapy for Diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Godakumara, K.; Ord, J.; Lättekivi, F.; Dissanayake, K.; Viil, J.; Boggavarapu, N.R.; Faridani, O.R.; Jääger, K.; Velthut-Meikas, A.; Jaakma, Ü.; et al. Trophoblast Derived Extracellular Vesicles Specifically Alter the Transcriptome of Endometrial Cells and May Constitute a Critical Component of Embryo-Maternal Communication. Reprod. Biol. Endocrinol. 2021, 19, 115. [Google Scholar] [CrossRef]
- Manni, G.; Buratta, S.; Pallotta, M.T.; Chiasserini, D.; Di Michele, A.; Emiliani, C.; Giovagnoli, S.; Pascucci, L.; Romani, R.; Bellezza, I.; et al. Extracellular Vesicles in Aging: An Emerging Hallmark? Cells 2023, 12, 527. [Google Scholar] [CrossRef]
- Atay, S.; Gercel-Taylor, C.; Kesimer, M.; Taylor, D.D. Morphologic and Proteomic Characterization of Exosomes Released by Cultured Extravillous Trophoblast Cells. Exp. Cell Res. 2011, 317, 1192–1202. [Google Scholar] [CrossRef]
- Su, Y.; Li, Q.; Zhang, Q.; Li, Z.; Yao, X.; Guo, Y.; Xiao, L.; Wang, X.; Ni, H. Exosomes Derived from Placental Trophoblast Cells Regulate Endometrial Epithelial Receptivity in Dairy Cows during Pregnancy. J. Reprod. Dev. 2022, 68, 21–29. [Google Scholar] [CrossRef]
- Salomon, C.; Yee, S.; Scholz-Romero, K.; Kobayashi, M.; Vaswani, K.; Kvaskoff, D.; Illanes, S.E.; Mitchell, M.D.; Rice, G.E. Extravillous Trophoblast Cells-Derived Exosomes Promote Vascular Smooth Muscle Cell Migration. Front. Pharmacol. 2014, 5, 175. [Google Scholar] [CrossRef]
- Ćujić, D.; Kosanović, M.; Jovanović Krivokuća, M.; Vićovac, L.; Janković, M. Extracellular Presence/Release of Galectins from HTR-8/SVneo Extravillous Trophoblast Cells. Turk. J. Biol. 2017, 41, 843–848. [Google Scholar] [CrossRef]
- Panjwani, N. Role of Galectins in Re-Epithelialization of Wounds. Ann. Transl. Med. 2014, 2, 89. [Google Scholar] [PubMed]
- Hedlund, M.; Stenqvist, A.-C.; Nagaeva, O.; Kjellberg, L.; Wulff, M.; Baranov, V.; Mincheva-Nilsson, L. Human Placenta Expresses and Secretes NKG2D Ligands via Exosomes That Down-Modulate the Cognate Receptor Expression: Evidence for Immunosuppressive Function. J. Immunol. 2009, 183, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Salomon, C.; Ryan, J.; Sobrevia, L.; Kobayashi, M.; Ashman, K.; Mitchell, M.; Rice, G.E. Exosomal Signaling during Hypoxia Mediates Microvascular Endothelial Cell Migration and Vasculogenesis. PLoS ONE 2013, 8, e68451. [Google Scholar] [CrossRef]
- Ouyang, Y.; Bayer, A.; Chu, T.; Tyurin, V.A.; Kagan, V.E.; Morelli, A.E.; Coyne, C.B.; Sadovsky, Y. Isolation of Human Trophoblastic Extracellular Vesicles and Characterization of Their Cargo and Antiviral Activity. Placenta 2016, 47, 86–95. [Google Scholar] [CrossRef]
- Schürer, N.; Köhne, A.; Schliep, V.; Barlag, K.; Goerz, G. Lipid Composition and Synthesis of HaCaT Cells, an Immortalized Human Keratinocyte Line, in Comparison with Normal Human Adult Keratinocytes. Exp. Dermatol. 1993, 2, 179–185. [Google Scholar] [CrossRef]
- Micallef, L.; Belaubre, F.; Pinon, A.; Jayat-Vignoles, C.; Delage, C.; Charveron, M.; Simon, A. Effects of Extracellular Calcium on the Growth-Differentiation Switch in Immortalized Keratinocyte HaCaT Cells Compared with Normal Human Keratinocytes. Exp. Dermatol. 2009, 18, 143–151. [Google Scholar] [CrossRef]
- Neupane, Y.R.; Handral, H.K.; Alkaff, S.A.; Chng, W.H.; Venkatesan, G.; Huang, C.; Lee, C.K.; Wang, J.-W.; Sriram, G.; Dienzo, R.A.; et al. Cell-Derived Nanovesicles from Mesenchymal Stem Cells as Extracellular Vesicle-Mimetics in Wound Healing. Acta Pharm. Sin. B 2023, 13, 1887–1902. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, Y.; Han, S.; Zhang, W.; Zhou, Q.; Guan, H.; Liu, J.; Shi, J.; Su, L.; Hu, D. Exosomes Derived from Human Amniotic Epithelial Cells Accelerate Wound Healing and Inhibit Scar Formation. J. Mol. Histol. 2017, 48, 121–132. [Google Scholar] [CrossRef]
- Yang, L.; Shi, J.; Wang, X.; Zhang, R. Curcumin Alleviates D-Galactose-Induced Cardiomyocyte Senescence by Promoting Autophagy via the SIRT1/AMPK/MTOR Pathway. Evid. Based. Complement. Alternat. Med. 2022, 2022, 2990843. [Google Scholar] [CrossRef]
- Xu, W.; Xiang, X.; Lin, L.; Gong, Z.-H.; Xiao, W.-J. L-Theanine Delays d-Galactose-Induced Senescence by Regulating the Cell Cycle and Inhibiting Apoptosis in Rat Intestinal Cells. J. Sci. Food Agric. 2024, 104, 2073–2084. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Arnedo, A.; Torres Figueroa, F.; Clavijo, C.; Arbeláez, P.; Cruz, J.C.; Muñoz-Camargo, C. An Image J Plugin for the High Throughput Image Analysis of in Vitro Scratch Wound Healing Assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, E.; Théry, C.; Zimmermann, P. Tetraspanins Affect Membrane Structures and the Trafficking of Molecular Partners: What Impact on Extracellular Vesicles? Biochem. Soc. Trans. 2025, 53, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Davies, K.J.A.; Medeiros, M.H.; Di Mascio, P.; Wagner, J.R. Formation and Repair of Oxidatively Generated Damage in Cellular DNA. Free Radic. Biol. Med. 2017, 107, 13–34. [Google Scholar] [CrossRef]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A Simple Technique for Quantitation of Low Levels of DNA Damage in Individual Cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Lee, B.Y.; Han, J.A.; Im, J.S.; Morrone, A.; Johung, K.; Goodwin, E.C.; Kleijer, W.J.; DiMaio, D.; Hwang, E.S. Senescence-Associated Beta-Galactosidase Is Lysosomal Beta-Galactosidase. Aging Cell 2006, 5, 187–195. [Google Scholar] [CrossRef]
- Liu, L.; Xie, H.; Chen, X.; Shi, W.; Xiao, X.; Lei, D.; Li, J. Differential Response of Normal Human Epidermal Keratinocytes and HaCaT Cells to Hydrogen Peroxide-Induced Oxidative Stress. Clin. Exp. Dermatol. 2012, 37, 772–780. [Google Scholar] [CrossRef]
- Yang, N.-C.; Hu, M.-L. The Limitations and Validities of Senescence Associated-Beta-Galactosidase Activity as an Aging Marker for Human Foreskin Fibroblast Hs68 Cells. Exp. Gerontol. 2005, 40, 813–819. [Google Scholar] [CrossRef]
- Yegorov, Y.E.; Akimov, S.S.; Hass, R.; Zelenin, A.V.; Prudovsky, I.A. Endogenous Beta-Galactosidase Activity in Continuously Nonproliferating Cells. Exp. Cell Res. 1998, 243, 207–211. [Google Scholar] [CrossRef]
- Untergasser, G.; Gander, R.; Rumpold, H.; Heinrich, E.; Plas, E.; Berger, P. TGF-Beta Cytokines Increase Senescence-Associated Beta-Galactosidase Activity in Human Prostate Basal Cells by Supporting Differentiation Processes, but Not Cellular Senescence. Exp. Gerontol. 2003, 38, 1179–1188. [Google Scholar] [CrossRef]
- Chung, M.G.; Kim, Y.; Cha, Y.K.; Park, T.H.; Kim, Y. Bitter Taste Receptors Protect against Skin Aging by Inhibiting Cellular Senescence and Enhancing Wound Healing. Nutr. Res. Pract. 2022, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Nepovimova, E.; Heger, Z.; Valko, M.; Wu, Q.; Kuca, K.; Adam, V. Role of Hypoxia in Cellular Senescence. Pharmacol. Res. 2023, 194, 106841. [Google Scholar] [CrossRef]
- Hong, W.X.; Hu, M.S.; Esquivel, M.; Liang, G.Y.; Rennert, R.C.; McArdle, A.; Paik, K.J.; Duscher, D.; Gurtner, G.C.; Lorenz, H.P.; et al. The Role of Hypoxia-Inducible Factor in Wound Healing. Adv. Wound Care 2014, 3, 390–399. [Google Scholar] [CrossRef]
- Cho, Y.-S.; Bae, J.-M.; Chun, Y.-S.; Chung, J.-H.; Jeon, Y.-K.; Kim, I.-S.; Kim, M.-S.; Park, J.-W. HIF-1alpha Controls Keratinocyte Proliferation by up-Regulating P21(WAF1/Cip1). Biochim. Biophys. Acta 2008, 1783, 323–333. [Google Scholar] [CrossRef]
- Sakamoto, T.; Weng, J.S.; Hara, T.; Yoshino, S.; Kozuka-Hata, H.; Oyama, M.; Seiki, M. Hypoxia-Inducible Factor 1 Regulation through Cross Talk between MTOR and MT1-MMP. Mol. Cell. Biol. 2014, 34, 30–42. [Google Scholar] [CrossRef]
- Xu, C.; Huang, X.; Tong, Y.; Feng, X.; Wang, Y.; Wang, C.; Jiang, Y. Icariin Modulates the Sirtuin/NF-κB Pathway and Exerts Anti-aging Effects in Human Lung Fibroblasts. Mol. Med. Rep. 2020, 22, 3833–3839. [Google Scholar] [CrossRef]
- Thedieck, K.; Holzwarth, B.; Prentzell, M.T.; Boehlke, C.; Kläsener, K.; Ruf, S.; Sonntag, A.G.; Maerz, L.; Grellscheid, S.-N.; Kremmer, E.; et al. Inhibition of MTORC1 by Astrin and Stress Granules Prevents Apoptosis in Cancer Cells. Cell 2013, 154, 859–874. [Google Scholar] [CrossRef]
- Sun, X.; Kaufman, P.D. Ki-67: More than a Proliferation Marker. Chromosoma 2018, 127, 175–186. [Google Scholar] [CrossRef]
- Borghesan, M.; O’Loghlen, A. Integrins in Senescence and Aging. Cell Cycle 2017, 16, 909–910. [Google Scholar] [CrossRef]
- Levy, L.; Broad, S.; Diekmann, D.; Evans, R.D.; Watt, F.M. Beta1 Integrins Regulate Keratinocyte Adhesion and Differentiation by Distinct Mechanisms. Mol. Biol. Cell 2000, 11, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Lü, D.; Li, Z.; Gao, Y.; Luo, C.; Zhang, F.; Zheng, L.; Wang, J.; Sun, S.; Long, M. Β1 Integrin Signaling in Asymmetric Migration of Keratinocytes under Mechanical Stretch in a Co-Cultured Wound Repair Model. Biomed. Eng. Online 2016, 15, 130. [Google Scholar] [CrossRef] [PubMed]
- Yudintceva, N.; Bobkov, D.; Sulatsky, M.; Mikhailova, N.; Oganesyan, E.; Vinogradova, T.; Muraviov, A.; Remezova, A.; Bogdanova, E.; Garapach, I.; et al. Mesenchymal Stem Cells-Derived Extracellular Vesicles for Therapeutics of Renal Tuberculosis. Sci. Rep. 2024, 14, 4495. [Google Scholar] [CrossRef] [PubMed]
- Borosch, S.; Dahmen, E.; Beckers, C.; Stoppe, C.; Buhl, E.M.; Denecke, B.; Goetzenich, A.; Kraemer, S. Characterization of Extracellular Vesicles Derived from Cardiac Cells in an in Vitro Model of Preconditioning. J. Extracell. Vesicles 2017, 6, 1390391. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e18. [Google Scholar] [CrossRef]
- Mathieu, M.; Névo, N.; Jouve, M.; Valenzuela, J.I.; Maurin, M.; Verweij, F.J.; Palmulli, R.; Lankar, D.; Dingli, F.; Loew, D.; et al. Specificities of Exosome versus Small Ectosome Secretion Revealed by Live Intracellular Tracking of CD63 and CD9. Nat. Commun. 2021, 12, 4389. [Google Scholar] [CrossRef]
- Barranco, I.; Padilla, L.; Parrilla, I.; Álvarez-Barrientos, A.; Pérez-Patiño, C.; Peña, F.J.; Martínez, E.A.; Rodriguez-Martínez, H.; Roca, J. Extracellular Vesicles Isolated from Porcine Seminal Plasma Exhibit Different Tetraspanin Expression Profiles. Sci. Rep. 2019, 9, 11584. [Google Scholar] [CrossRef]
- Vornic, I.; Buciu, V.; Furau, C.G.; Gaje, P.N.; Ceausu, R.A.; Dumitru, C.-S.; Barb, A.C.; Novacescu, D.; Cumpanas, A.A.; Latcu, S.C.; et al. Oxidative Stress and Placental Pathogenesis: A Contemporary Overview of Potential Biomarkers and Emerging Therapeutics. Int. J. Mol. Sci. 2024, 25, 12195. [Google Scholar] [CrossRef]
- Duan, J.; Liu, X.; Shen, S.; Tan, X.; Wang, Y.; Wang, L.; Kang, L.; Wang, K.; Wei, Z.; Qi, Y.; et al. Trophoblast Stem-Cell-Derived Exosomes Alleviate Cardiotoxicity of Doxorubicin via Improving Mfn2-Mediated Mitochondrial Fusion. Cardiovasc. Toxicol. 2023, 23, 23–31. [Google Scholar] [CrossRef]
- Wang, T.; Jian, Z.; Baskys, A.; Yang, J.; Li, J.; Guo, H.; Hei, Y.; Xian, P.; He, Z.; Li, Z.; et al. MSC-Derived Exosomes Protect against Oxidative Stress-Induced Skin Injury via Adaptive Regulation of the NRF2 Defense System. Biomaterials 2020, 257, 120264. [Google Scholar] [CrossRef]
- Lei, X.; He, N.; Zhu, L.; Zhou, M.; Zhang, K.; Wang, C.; Huang, H.; Chen, S.; Li, Y.; Liu, Q.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles Attenuate Radiation-Induced Lung Injury via MiRNA-214-3p. Antioxid. Redox Signal. 2021, 35, 849–862. [Google Scholar] [CrossRef] [PubMed]
- Platas, J.; Guillén, M.I.; Pérez Del Caz, M.D.; Gomar, F.; Castejón, M.A.; Mirabet, V.; Alcaraz, M.J. Paracrine Effects of Human Adipose-Derived Mesenchymal Stem Cells in Inflammatory Stress-Induced Senescence Features of Osteoarthritic Chondrocytes. Aging 2016, 8, 1703–1717. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.O.; Reshi, Q.U.A.; Godakumara, K.; Kodithuwakku, S.; Fazeli, A. Extracellular Vesicles as Mediators of Stress Response in Embryo-Maternal Communication. Front. Cell Dev. Biol. 2024, 12, 1440849. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Zhu, L.; Qian, Z.-M.; Wu, X.-M.; Yung, W.-H.; Ke, Y. Hyperthermic Preconditioning Protects Astrocytes from Ischemia/Reperfusion Injury by up-Regulation of HIF-1 Alpha Expression and Binding Activity. Biochim. Biophys. Acta 2010, 1802, 1048–1053. [Google Scholar] [CrossRef]
- Fafián-Labora, J.; Morente-López, M.; Sánchez-Dopico, M.J.; Arntz, O.J.; van de Loo, F.A.J.; De Toro, J.; Arufe, M.C. Influence of Mesenchymal Stem Cell-Derived Extracellular Vesicles in Vitro and Their Role in Ageing. Stem Cell Res. Ther. 2020, 11, 13. [Google Scholar] [CrossRef]
- Jiang, L.; Zeng, H.; Ni, L.; Qi, L.; Xu, Y.; Xia, L.; Yu, Y.; Liu, B.; Yang, H.; Hao, H.; et al. HIF-1α Preconditioning Potentiates Antioxidant Activity in Ischemic Injury: The Role of Sequential Administration of Dihydrotanshinone I and Protocatechuic Aldehyde in Cardioprotection. Antioxid. Redox Signal. 2019, 31, 227–242. [Google Scholar] [CrossRef]
- Heyman, S.N.; Leibowitz, D.; Mor-Yosef Levi, I.; Liberman, A.; Eisenkraft, A.; Alcalai, R.; Khamaisi, M.; Rosenberger, C. Adaptive Response to Hypoxia and Remote Ischaemia Pre-Conditioning: A New Hypoxia-Inducible Factors Era in Clinical Medicine. Acta Physiol. 2016, 216, 395–406. [Google Scholar] [CrossRef]
- Muhandiram, S.; Dissanayake, K.; Orro, T.; Godakumara, K.; Kodithuwakku, S.; Fazeli, A. Secretory Proteomic Responses of Endometrial Epithelial Cells to Trophoblast-Derived Extracellular Vesicles. Int. J. Mol. Sci. 2023, 24, 11924. [Google Scholar] [CrossRef]
- Han, H.; Liu, Z.; Yin, J.; Gao, J.; He, L.; Wang, C.; Hou, R.; He, X.; Wang, G.; Li, T.; et al. D-Galactose Induces Chronic Oxidative Stress and Alters Gut Microbiota in Weaned Piglets. Front. Physiol. 2021, 12, 634283. [Google Scholar] [CrossRef]
- Wortzel, I.; Dror, S.; Kenific, C.M.; Lyden, D. Exosome-Mediated Metastasis: Communication from a Distance. Dev. Cell 2019, 49, 347–360. [Google Scholar] [CrossRef]
- Go, Y.Y.; Lee, C.M.; Ju, W.M.; Chae, S.-W.; Song, J.-J. Extracellular Vesicles (Secretomes) from Human Trophoblasts Promote the Regeneration of Skin Fibroblasts. Int. J. Mol. Sci. 2021, 22, 6959. [Google Scholar] [CrossRef]
- Wu, D.; Kang, L.; Tian, J.; Wu, Y.; Liu, J.; Li, Z.; Wu, X.; Huang, Y.; Gao, B.; Wang, H.; et al. Exosomes Derived from Bone Mesenchymal Stem Cells with the Stimulation of Fe3O4 Nanoparticles and Static Magnetic Field Enhance Wound Healing Through Upregulated MiR-21-5p. Int. J. Nanomed. 2020, 15, 7979–7993. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Ohkuchi, A.; Kuwata, T.; Usui, R.; Baba, Y.; Suzuki, H.; Chaw Kyi, T.T.; Matsubara, S.; Saito, S.; Takizawa, T. Endogenous and Exogenous MiR-520c-3p Modulates CD44-Mediated Extravillous Trophoblast Invasion. Placenta 2017, 50, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef] [PubMed]
- Rasekh, M.; Arshad, M.S.; Ahmad, Z. Advances in Drug Delivery Integrated with Regenerative Medicine: Innovations, Challenges, and Future Frontiers. Pharmaceutics 2025, 17, 456. [Google Scholar] [CrossRef]
- Gupta, D.; Zickler, A.M.; El Andaloussi, S. Dosing Extracellular Vesicles. Adv. Drug Deliv. Rev. 2021, 178, 113961. [Google Scholar] [CrossRef]
- Hildebrand, D.G.; Lehle, S.; Borst, A.; Haferkamp, S.; Essmann, F.; Schulze-Osthoff, K. α-Fucosidase as a Novel Convenient Biomarker for Cellular Senescence. Cell Cycle 2013, 12, 1922–1927. [Google Scholar] [CrossRef]
- Cruz, A.M.; Gonçalves, M.C.; Marques, M.S.; Veiga, F.; Paiva-Santos, A.C.; Pires, P.C. In Vitro Models for Anti-Aging Efficacy Assessment: A Critical Update in Dermocosmetic Research. Cosmetics 2023, 10, 66. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nacka-Aleksić, M.; Pirković, A.; Vilotić, A.; Kosanović, M.; Dekanski, D.; Legner, J.; Jovanović Krivokuća, M. Trophoblast Extracellular Vesicles as Modulators of Keratinocyte Stress Response and Senescence. Life 2025, 15, 918. https://doi.org/10.3390/life15060918
Nacka-Aleksić M, Pirković A, Vilotić A, Kosanović M, Dekanski D, Legner J, Jovanović Krivokuća M. Trophoblast Extracellular Vesicles as Modulators of Keratinocyte Stress Response and Senescence. Life. 2025; 15(6):918. https://doi.org/10.3390/life15060918
Chicago/Turabian StyleNacka-Aleksić, Mirjana, Andrea Pirković, Aleksandra Vilotić, Maja Kosanović, Dragana Dekanski, Janko Legner, and Milica Jovanović Krivokuća. 2025. "Trophoblast Extracellular Vesicles as Modulators of Keratinocyte Stress Response and Senescence" Life 15, no. 6: 918. https://doi.org/10.3390/life15060918
APA StyleNacka-Aleksić, M., Pirković, A., Vilotić, A., Kosanović, M., Dekanski, D., Legner, J., & Jovanović Krivokuća, M. (2025). Trophoblast Extracellular Vesicles as Modulators of Keratinocyte Stress Response and Senescence. Life, 15(6), 918. https://doi.org/10.3390/life15060918





