Effects of Neutral Postures on Mechanical Properties of Cervical Spine Under Different Gravitational Environments: A Musculoskeletal Model Study
Abstract
1. Introduction
2. Methods
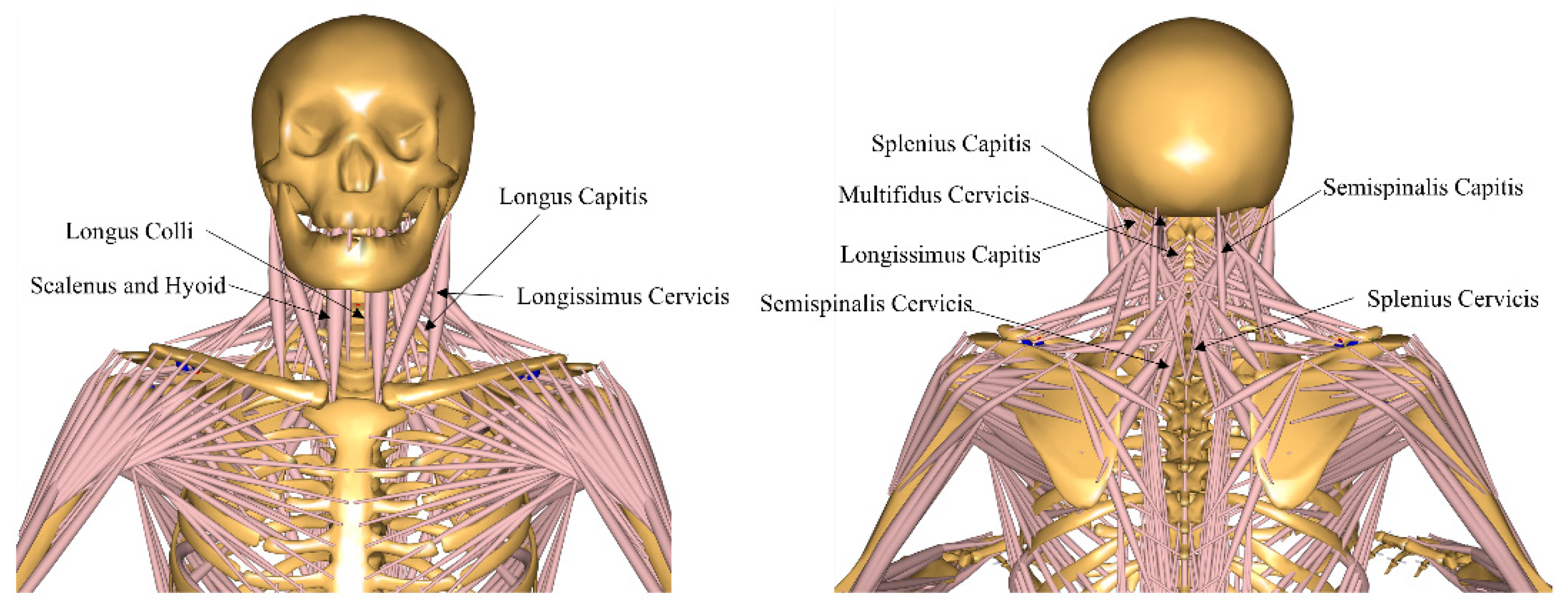
2.1. Musculoskeletal Model
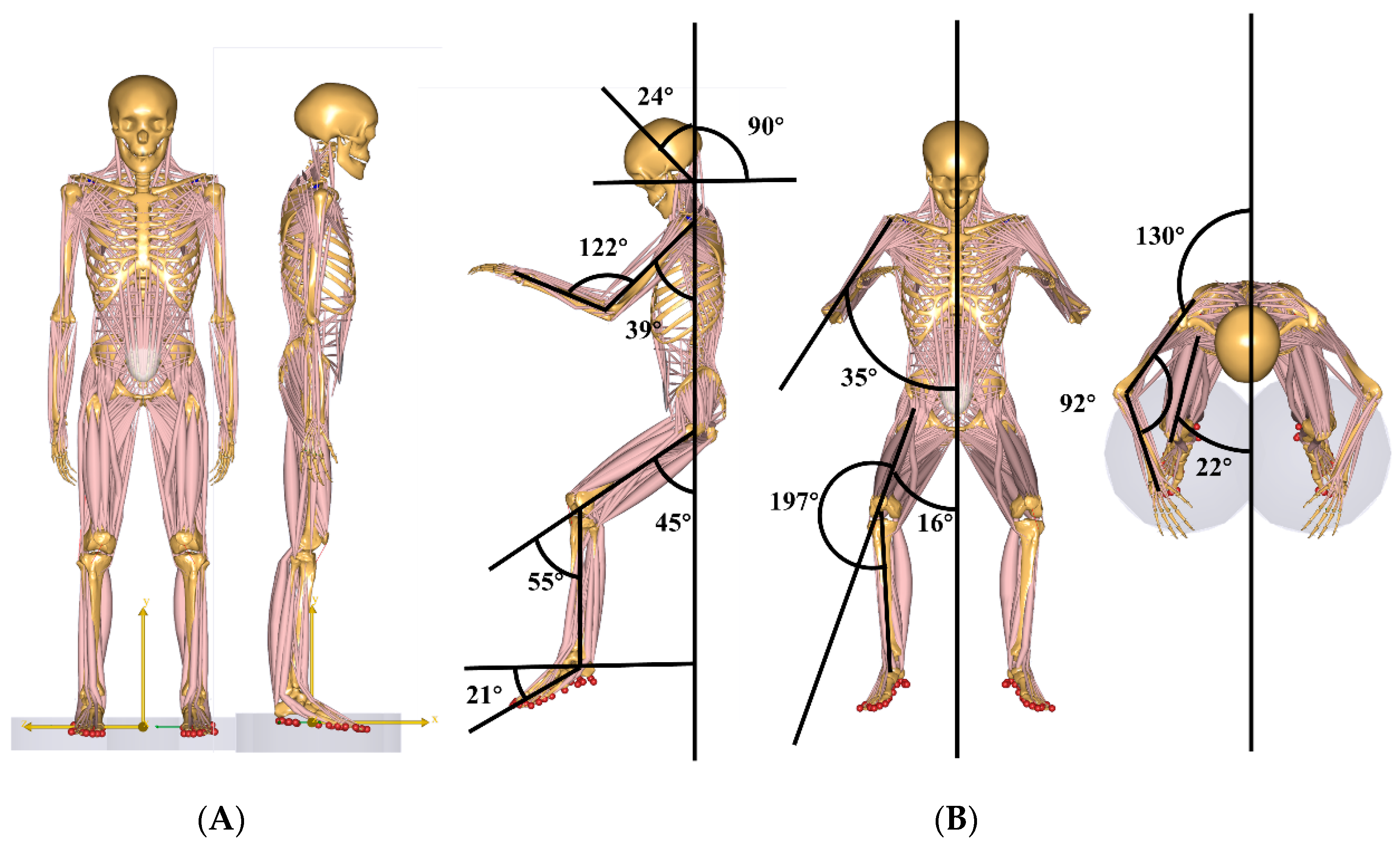
Research Posture
2.2. Research Variables
3. Results
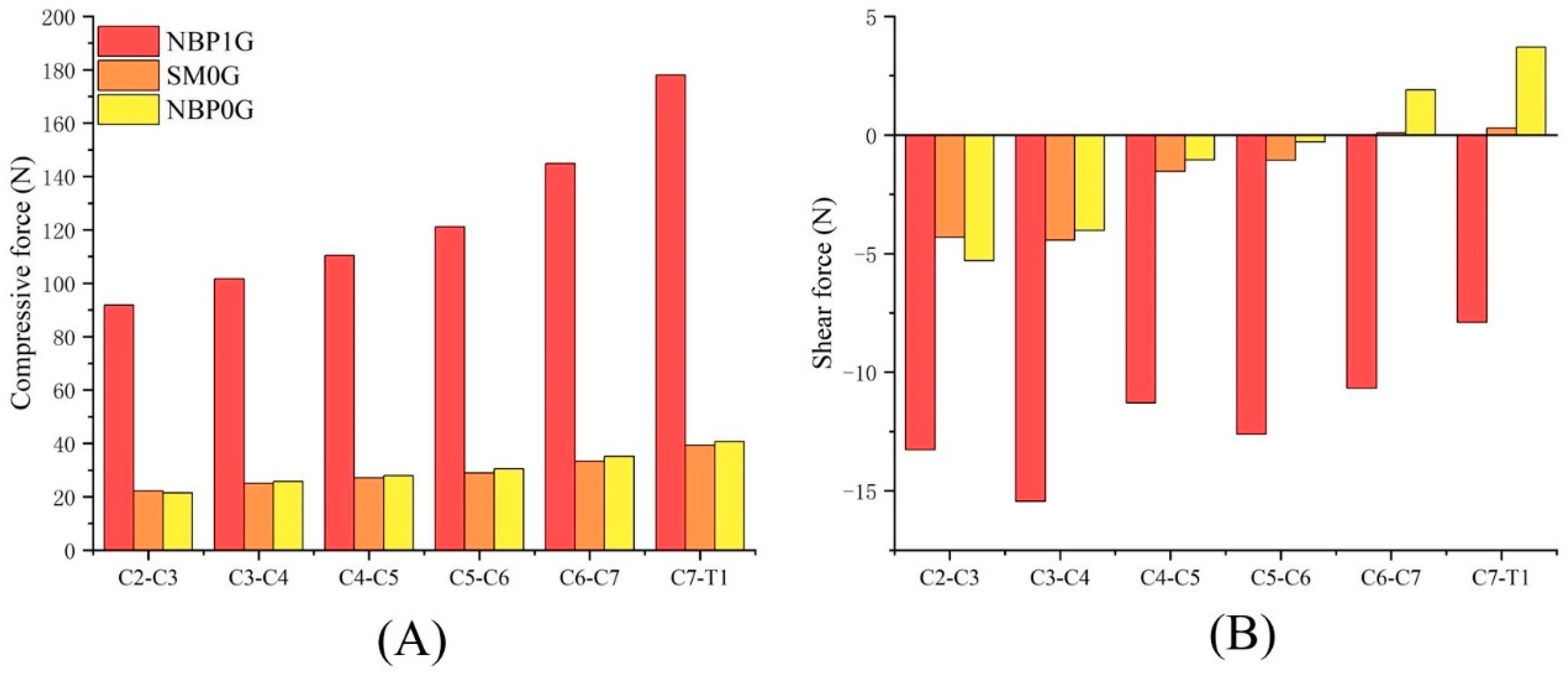
3.1. Cervical Disc Compressive Force and Shear Force
3.1.1. NBP 1G vs. SM 0G
3.1.2. NBP 1G vs. NBP 0G
3.1.3. NBP 0G vs. SM 0G
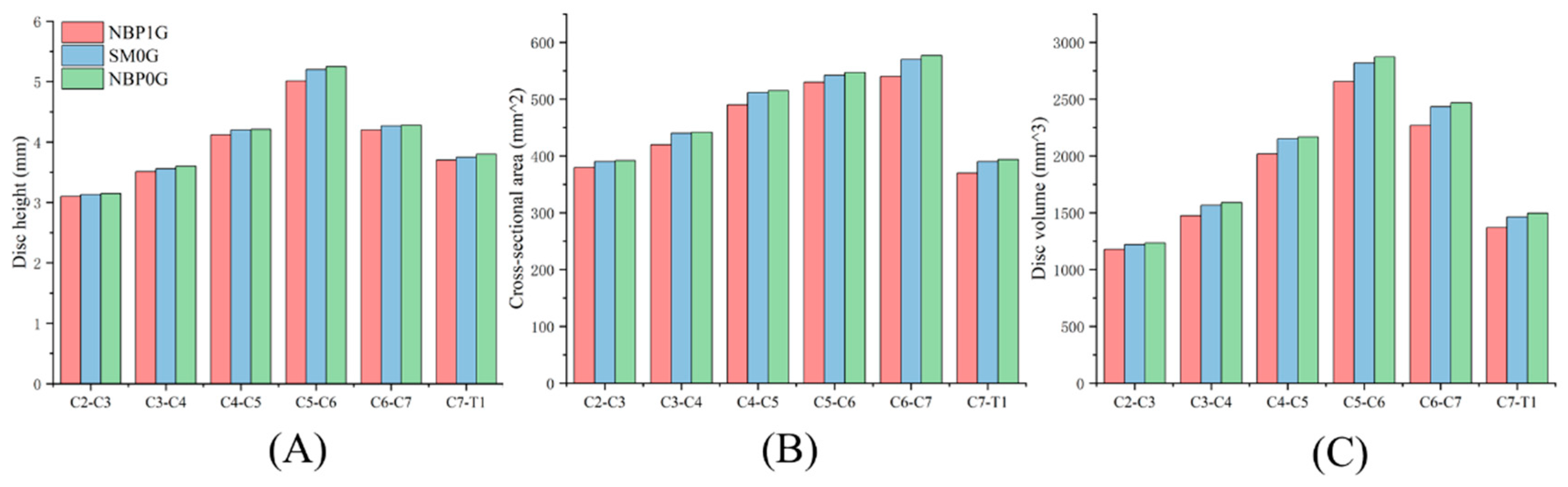
3.2. Cervical Disc Geometric
3.2.1. NBP 1G vs. SM 0G
3.2.2. NBP 1G vs. NBP 0G
3.2.3. NBP 0G vs. SM 0G
3.3. Cervical Joint Muscle Force
3.3.1. NBP 1G vs. SM 0G
3.3.2. NBP 1G vs. NBP 0G
3.3.3. NBP 0G vs. SM 0G
3.4. Intervertebral Disc Water Content
3.4.1. NBP 1G vs. SM 0G
3.4.2. NBP 1G vs. NBP 0G
3.4.3. NBP 0G vs. SM 0G
3.5. Ligament Forces
4. Discussion
Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scheuring, R.A.; Effenhauser, R.; Anderson, D.N.; Hei, M.V.; Holman, P.; Helgeson, M. Cervical Spine Intervertebral Disc Herniation on Board the International Space Station: Diagnosis, Treatment and Operational Mission Impact; National Aeronautics and Space Administration: Washington, DC, USA, 2024. [Google Scholar]
- Belavy, D.L.; Adams, M.; Brisby, H.; Cagnie, B.; Danneels, L.; Fairbank, J.; Hargens, A.R.; Judex, S.; Scheuring, R.A.; Sovelius, R.; et al. Disc herniations in astronauts: What causes them, and what does it tell us about herniation on earth? Eur. Spine J. 2016, 25, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Penchev, R.; Scheuring, R.A.; Soto, A.T.; Miletich, D.M.; Kerstman, E.; Cohen, S.P. Back pain in outer space. Anesthesiology 2021, 135, 384–395. [Google Scholar] [CrossRef]
- Johnston, S.L.; Campbell, M.; Scheuring, R.; Feiveson, A.H. Risk of herniated nucleus pulposus among US Astronauts. Aviat. Space Environ. Med. 2010, 81, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Gao, X.; Qin, B.; Baldoni, M.; Zhou, L.; Qian, Z.; Zhu, Q. Effect of microgravity on mechanical loadings in lumbar spine at various postures: A numerical study. npj Microgravity 2023, 9, 16. [Google Scholar] [CrossRef]
- Young, K.S.; Rajulu, S. The Effects of Microgravity on Seated Height (Spinal Elongation); National Aeronautics and Space Administration: Washington, DC, USA, 2011. [Google Scholar]
- Thornton, W.; Hoffler, G.W.; Rummel, J.A. Anthropometric Changes and Fluid Shifts; National Aeronautics and Space Administration: Washington, DC, USA, 1974. [Google Scholar]
- Marshburn, T.H.; Hadfield, C.A.; Sargsyan, A.E.; Garcia, K.; Ebert, D.; Dulchavsky, S.A. New heights in ultrasound: First report of spinal ultrasound from the international space station. J. Emerg. Med. 2014, 46, 61–70. [Google Scholar] [CrossRef]
- Flores-Hernandez, C.; Eskinazi, I.; Hoenecke, H.R.; D’Lima, D.D. Scapulothoracic rhythm affects glenohumeral joint force. JSES Open Access 2019, 3, 77–82. [Google Scholar] [CrossRef]
- Dzialo, C.M.; Pedersen, P.H.; Jensen, K.K.; de Zee, M.; Andersen, M.S. Evaluation of predicted patellofemoral joint kinematics with a moving-axis joint model. Med. Eng. Phys. 2019, 73, 85–91. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Liu, G.; Zhang, B.; Ye, B.; Zhu, H. Impact of specialized fatigue and backhand smash on the ankle biomechanics of female badminton players. Sci. Rep. 2024, 14, 10282. [Google Scholar] [CrossRef]
- Liu, G.; He, Z.; Ye, B.; Guo, H.; Pan, H.; Zhu, H.; Meng, G. Comparative analysis of the kinematic characteristics of lunge-style and squat-style jerk techniques in elite weightlifters. Life 2024, 14, 1086. [Google Scholar] [CrossRef]
- Ye, B.; Liu, G.; He, Z.; Xu, J.; Pan, H.; Zhu, H. Biomechanical mechanisms of anterior cruciate ligament injury in the jerk dip phase of clean and jerk: A case study of an injury event captured on-site. Heliyon 2024, 10, e31390. [Google Scholar] [CrossRef]
- Wilke, H.; Neef, P.; Hinz, B.; Seidel, H.; Claes, L. Intradiscal pressure together with anthropometric data–a data set for the validation of models. Clin. Biomech. 2001, 16, S111–S126. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Baldoni, M.; Wu, B.; Zhou, L.; Qian, Z.; Zhu, Q. Effect of lumbar muscle atrophy on the mechanical loading change on lumbar intervertebral discs. J. Biomech. 2022, 139, 111120. [Google Scholar] [CrossRef]
- Bhargava, A.; Scott, M.; Traylor, R.; Chung, R.; Mrozek, K.; Wolter, J.; Tan, H.Z. Effect of cognitive load on tactor location identification in zero-g. In Proceedings of the First Joint Eurohaptics Conference and Symposium on Haptic Interfaces for Virtual Environment and Teleoperator Systems. World Haptics Conference, Pisa, Italy, 18–20 March 2005. [Google Scholar]
- Brown, E.L. Human Performance and Behavior During Zero Gravity; Springer: Berlin/Heidelberg, Germany, 1961. [Google Scholar]
- Andreoni, G.; Rigotti, C.; Baroni, G.; Ferrigno, G.; Colford, N.; Pedotti, A. Quantitative analysis of neutral body posture in prolonged microgravity. Gait Posture 2000, 12, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Young, K.S.; Kim, K.H.; Rajulu, S. Anthropometric changes in spaceflight. Hum. Factors 2023, 65, 977–987. [Google Scholar] [CrossRef]
- Brooks, C.N.; Eskay-Auerbach, M.; Talmage, J.B.; Tencer, A.F. Cervical lordosis: The significance of decreased, straightened, and reversed curves. AMA Guides® Newsl. 2015, 20, 3–5. [Google Scholar] [CrossRef]
- Anderson, D.N.; Scheuring, R.A.; Effenhauser, R.K.; Helgeson, M.; Vandehei, M.; Holman, P.J. Cervical spine intervertebral disc herniation on board the international space station: Diagnosis, treatment and operational mission impact. In Proceedings of the NASA Spine Technical Interchange Meeting, Washington, DC, USA, 13–14 October 2021; National Aeronautics and Space Administration: Washington, DC, USA, 2023. [Google Scholar]
- De Zee, M.; Hansen, L.; Wong, C.; Rasmussen, J.; Simonsen, E.B. A generic detailed rigid-body lumbar spine model. J. Biomech. 2007, 40, 1219–1227. [Google Scholar] [CrossRef]
- Ignasiak, D.; Dendorfer, S.; Ferguson, S.J. Thoracolumbar spine model with articulated ribcage for the prediction of dynamic spinal loading. J. Biomech. 2016, 49, 959–966. [Google Scholar] [CrossRef]
- Maganaris, C.N. In vivo measurement-based estimations of the moment arm in the human tibialis anterior muscle-tendon unit. J. Biomech. 2000, 33, 375–379. [Google Scholar] [CrossRef]
- Baldoni, M.; Gu, W. Effect of fixed charge density on water content of ivd during bed rest: A numerical analysis. Med. Eng. Amp. Phys. 2019, 70, 72–77. [Google Scholar] [CrossRef]
- Yoganandan, N.; Kumaresan, S.; Pintar, F.A. Geometric and mechanical properties of human cervical spine ligaments. J. Biomech. Eng. 2000, 122, 623–629. [Google Scholar] [CrossRef]
- Lai, W.M.; Hou, J.S.; Mow, V.C. A triphasic theory for the swelling and deformation behaviors of articular cartilage. J. Biomech. Eng. 1991, 113, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.; Maroudas, A. The measurement of fixed charged density in the intervertebral disc. Biochim. Biophys. Acta (BBA) Gen. Subj. 1979, 586, 166–178. [Google Scholar] [CrossRef]
- Pooni, J.S.; Hukins, D.W.; Harris, P.F.; Hilton, R.C.; Davies, K.E. Comparison of the structure of human intervertebral discs in the cervical, thoracic and lumbar regions of the spine. Surg. Radiol. Anat. SRA 1986, 8, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Treffel, L.; Mkhitaryan, K.; Gellee, S.; Gauquelin-Koch, G.; Gharib, C.; Blanc, S.; Millet, C. Intervertebral disc swelling demonstrated by 3d and water content magnetic resonance analyses after a 3-day dry immersion simulating microgravity. Front. Physiol. 2016, 7, 605. [Google Scholar] [CrossRef]
- Young, K.S.; Rajulu, S. Changes in seated height in microgravity. Appl. Ergon. 2020, 83, 102995. [Google Scholar] [CrossRef] [PubMed]
- Kershner, D.; Binhammer, R. Intrathecal ligaments and nerve root tension: Possible sources of lumbar pain during spaceflight. Aviat. Space Environ. Med. 2004, 75, 354–358. [Google Scholar]
- Hutchinson, K.J.; Watenpaugh, D.E.; Murthy, G.; Convertino, V.A.; Hargens, A.R. Back pain during 6 degrees head-down tilt approximates that during actual microgravity. Aviat. Space Environ. Med. 1995, 66, 256–259. [Google Scholar]
- Gordon, S.J.; Yang, K.H.; Mayer, P.J.; Mace Jr, A.H.; Kish, V.L.; Radin, E.L. Mechanism of disc rupture: A preliminary report. Spine 1991, 16, 450–456. [Google Scholar] [CrossRef]
- Chan, S.C.; Ferguson, S.J.; Gantenbein-Ritter, B. The effects of dynamic loading on the intervertebral disc. Eur. Spine J. 2011, 20, 1796–1812. [Google Scholar] [CrossRef]
- Setton, L.A.; Chen, J. Cell mechanics and mechanobiology in the intervertebral disc. Spine 2004, 29, 2710–2723. [Google Scholar] [CrossRef]
- Guilak, F.; Ratcliffe, A.; Mow, V.C. Chondrocyte deformation and local tissue strain in articular cartilage: A confocal microscopy study. J. Orthop. Res. 1995, 13, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhu, Q.; Gu, W. Prediction of glycosaminoglycan synthesis in intervertebral disc under mechanical loading. J. Biomech. 2016, 49, 2655–2661. [Google Scholar] [CrossRef]
- Holm, S.; Maroudas, A.; Urban, J.; Selstam, G.; Nachemson, A. Nutrition of the intervertebral disc: Solute transport and metabolism. Connect. Tissue Res. 1981, 8, 101–119. [Google Scholar] [CrossRef]
- Adams, M.A.; Hutton, W.C. The effect of posture on the fluid content of lumbar intervertebral discs. Spine 1983, 8, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, J.; Steffen, T.; Nelson, F.; Winterbottom, N.; Hollander, A.P.; Poole, R.A.; Aebi, M.; Alini, M. The human lumbar intervertebral disc: Evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J. Clin. Investig. 1996, 98, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.A.; Dolan, P.; Hutton, W.C. Diurnal variations in the stresses on the lumbar spine. Spine 1987, 12, 130–137. [Google Scholar] [CrossRef]
- Adams, M.A.; Dolan, P. Time-dependent changes in the lumbar spine’s resistancc to bending. Clin. Biomech. 1996, 11, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Bass, C.R.; Lucas, S.R.; Salzar, R.S.; Oyen, M.L.; Planchak, C.; Shender, B.S.; Paskoff, G. Failure properties of cervical spinal ligaments under fast strain rate deformations. Spine 2007, 32, E7–E13. [Google Scholar] [CrossRef]
- Leblanc, A.; Lin, C.; Shackelford, L.; Sinitsyn, V.; Evans, H.; Belichenko, O.; Schenkman, B.; Kozlovskaya, I.; Oganov, V.; Bakulin, A. Muscle volume, mri relaxation times (t2), and body composition after spaceflight. J. Appl. Physiol. 2000, 89, 2158–2164. [Google Scholar] [CrossRef]


| Ligament Stiffness [N/mm] | Strain [%] | |
|---|---|---|
| ALL | 16 | 25.8–35.8 |
| 17.9 | 29.54–41.26 | |
| PLL | 25.4 | 14.99–21.41 |
| 23 | 25.33–42.87 | |
| LF | 25 | 64.1–89.9 |
| 21.6 | 101.5–75.3 |
| A0 [mm2] | h0 [mm] | |||
|---|---|---|---|---|
| NP | AF | |||
| C2–C3 | 380 | 0.80 | 0.75 | 3.1 |
| C3–C4 | 420 | 3.51 | ||
| C4–C5 | 490 | 4.12 | ||
| C5–C6 | 530 | 5.01 | ||
| C6–C7 | 540 | 4.2 | ||
| C7–T1 | 370 | 3.7 | ||
| Muscle (N) | NBP1G | SM0G | NBP0G |
|---|---|---|---|
| Scalenus And Hyoid | 8.41 | 9.55 × 10−6 | 1.14 × 10−5 |
| Longus Colli | 2.87 | 2.92 × 10−5 | 4.96 × 10−5 |
| Longus Capitis | 9.94 × 10−13 | 1.41 × 10−5 | 1.85 × 10−5 |
| Splenius Capitis | 9.44 × 10−14 | 6.27 × 10−8 | 0 |
| Splenius Cervicis | 2.78 × 10−13 | 1.40 × 10−6 | 4.19 × 10−7 |
| Semispinalis Capitis | 2.78 | 1.09 × 10−5 | 1.54 × 10−5 |
| Semispinalis Cervicis | 12.75 | 4.58 × 10−6 | 4.25 × 10−6 |
| Longissimus Capitis | 3.96 × 10−12 | 1.01 × 10−5 | 1.18 × 10−5 |
| Longissimus Cervicis | 0.69 | 1.92 × 10−5 | 1.98 × 10−5 |
| Multifidus Cervicis | 11.3 | 1.04 × 10−5 | 1.77 × 10−5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Zhang, B.; Ye, B.; Song, Z.; Mei, Q.; Xu, J.; Zhu, H. Effects of Neutral Postures on Mechanical Properties of Cervical Spine Under Different Gravitational Environments: A Musculoskeletal Model Study. Life 2025, 15, 447. https://doi.org/10.3390/life15030447
He Z, Zhang B, Ye B, Song Z, Mei Q, Xu J, Zhu H. Effects of Neutral Postures on Mechanical Properties of Cervical Spine Under Different Gravitational Environments: A Musculoskeletal Model Study. Life. 2025; 15(3):447. https://doi.org/10.3390/life15030447
Chicago/Turabian StyleHe, Zhanyang, Bin Zhang, Binyong Ye, Zhanbing Song, Qiang Mei, Jiahao Xu, and Houwei Zhu. 2025. "Effects of Neutral Postures on Mechanical Properties of Cervical Spine Under Different Gravitational Environments: A Musculoskeletal Model Study" Life 15, no. 3: 447. https://doi.org/10.3390/life15030447
APA StyleHe, Z., Zhang, B., Ye, B., Song, Z., Mei, Q., Xu, J., & Zhu, H. (2025). Effects of Neutral Postures on Mechanical Properties of Cervical Spine Under Different Gravitational Environments: A Musculoskeletal Model Study. Life, 15(3), 447. https://doi.org/10.3390/life15030447






