Characterization, Comparison of Four New Mitogenomes of Centrotinae (Hemiptera: Membracidae) and Phylogenetic Implications Supports New Synonymy
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Acquisition
2.2. DNA Extraction, Mitogenome Sequencing, Assembly and Annotation
2.3. Bioinformatic Analysis
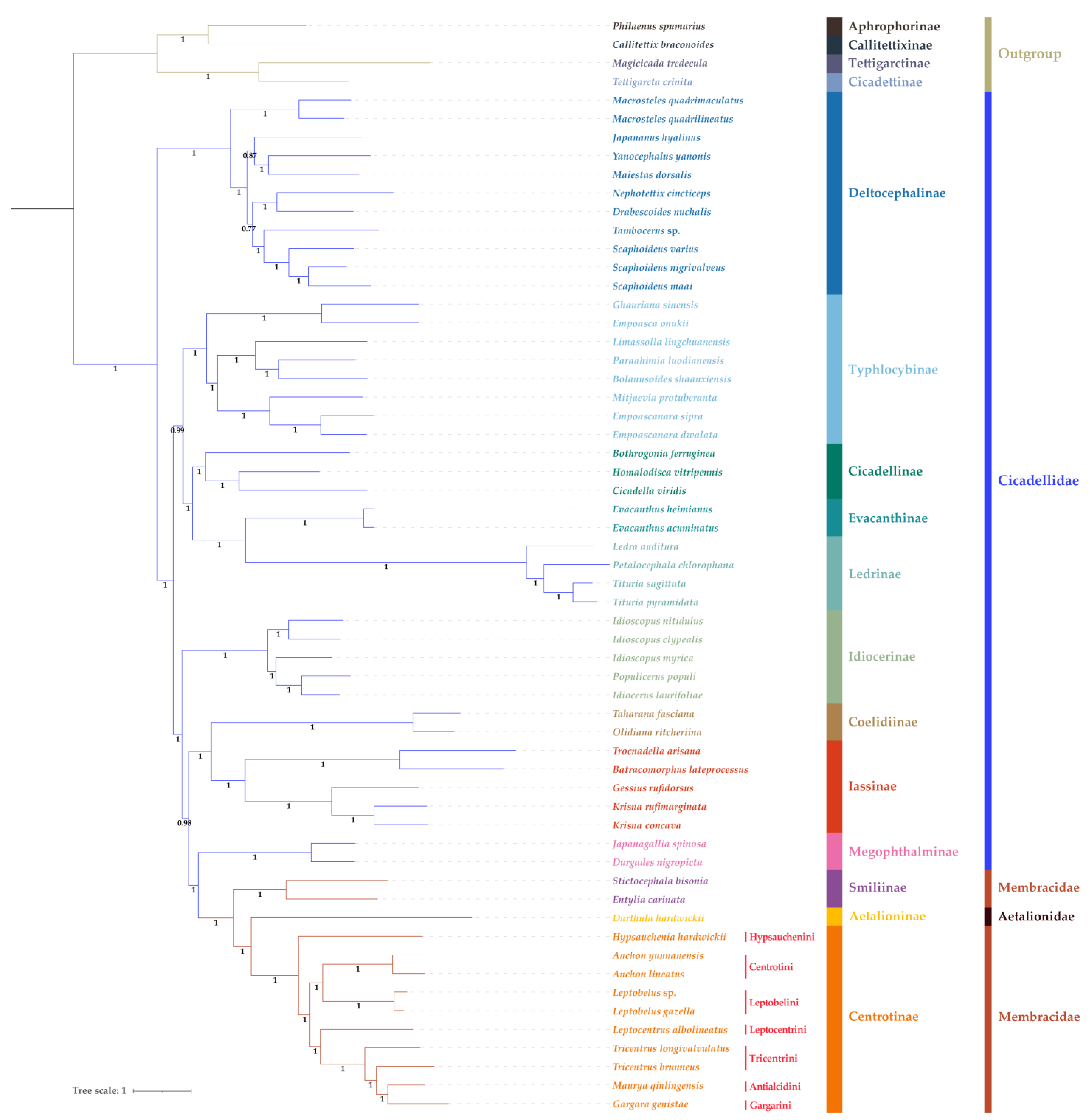
2.4. Phylogenetic Analysis
| Superfamily | Family/Subfamily | Species | Accession Number | Reference |
|---|---|---|---|---|
| Outgroup | ||||
| Cercopoidea | Aphrophoridae/Aphrophorinae | Philaenus spumarius | NC_005944 | [38] |
| Cercopidae/Callitettixinae | Callitettix braconoides | NC_025497 | [39] | |
| Cicadoidea | Cicadidae/Cicadettinae | Magicicada tredecula | MH937705 | [40] |
| Tettigarctidae/Tettigarctinae | Tettigarcta crinita | MG737758 | [41] | |
| Ingroup | ||||
| Membracoidea | Cicadellidae/Cicadellinae | Bothrogonia ferruginea | KU167550 | Unpublished |
| Cicadella viridis | KY752061 | Unpublished | ||
| Homalodisca vitripennis | NC_006899 | Unpublished | ||
| Olidiana ritcheriina | MK738125 | Unpublished | ||
| Taharana fasciana | NC_036015 | [42] | ||
| Cicadellidae/Deltocephalinae | Drabescoides nuchalis | NC_028154 | [43] | |
| Japananus hyalinus | NC_036298 | [44] | ||
| Macrosteles quadrilineatus | NC_034781 | [45] | ||
| Macrosteles quadrimaculatus | NC_039560 | [46] | ||
| Maiestas dorsalis | NC_036296 | [44] | ||
| Nephotettix cincticeps | NC_026977 | Unpublished | ||
| Scaphoideus maai | KY817243 | [47] | ||
| Scaphoideus nigrivalveus | KY817244 | [47] | ||
| Scaphoideus varius | KY817245 | [47] | ||
| Tambocerus sp. | KT827824 | [48] | ||
| Yanocephalus yanonis | NC_036131 | [47] | ||
| Cicadellidae/Evacanthinae | Evacanthus acuminatus | MK948205 | [49] | |
| Evacanthus heimianus | MG813486 | [50] | ||
| Cicadellidae/Iassinae | Batracomorphus lateprocessus | MG813489 | [51] | |
| Gessius rufidorsus | MN577633 | [51] | ||
| Krisna concava | MN577635 | [51] | ||
| Krisna rufimarginata | NC_046068 | [51] | ||
| Trocnadella arisana | NC_036480 | [51] | ||
| Cicadellidae/Idiocerinae | Idiocerus laurifoliae | NC_039741 | [52] | |
| Idioscopus clypealis | NC_039642 | [53] | ||
| Idioscopus myrica | MH492317 | [52] | ||
| Idioscopus nitidulus | NC_029203 | [54] | ||
| Populicerus populi | NC_039427 | [52] | ||
| Cicadellidae/Ledrinae | Ledra auditura | MK387845 | [55] | |
| Petalocephala chlorophana | MT610899 | [56] | ||
| Tituria pyramidata | MN920440 | Unpublished | ||
| Tituria sagittata | MT610900 | [56] | ||
| Cicadellidae/Megophthalminae | Durgades nigropicta | NC_035684 | [57] | |
| Japanagallia spinosa | NC_035685 | [57] | ||
| Cicadellidae/Typhlocybinae | Bolanusoides shaanxiensis | MN661136 | Unpublished | |
| Empoascanara dwalata | MT350235 | Unpublished | ||
| Empoasca onukii | NC_037210 | [58] | ||
| Empoascanara sipra | MN604278 | [59] | ||
| Ghauriana sinensis | MN699874 | [60] | ||
| Limassolla lingchuanensis | NC_046037 | [61] | ||
| Mitjaevia protuberanta | NC_047465 | [62] | ||
| Paraahimia luodianensis | NC_047464 | [63] | ||
| Aetalionidae/Aetalioninae | Darthula hardwickii | NC_026699 | [64] | |
| Membracidae/Smiliinae | Entylia carinata | NC_033539 | [65] | |
| Stictophala bisonia | MW342606 | [66] | ||
| Membracidae/Centrotinae | Anchon lineatus | MZ504904 | This study | |
| Anchon yunnanensis | MZ504905 | This study | ||
| Gargara genistae | MZ504906 | This study | ||
| Hypsauchenia hardwichii | MK746135 | [2] | ||
| Leptobelus gazella | JF801955 | [67] | ||
| Leptobelus sp. | JQ910984 | [68] | ||
| Leptocentrus albolineatus | MK746137 | [2] | ||
| Maurya qinlingensis | MK746136 | [2] | ||
| Tricentrus longivalvulatus | MZ504907 | This study | ||
| Tricentrus brunneus | MK746138 | [2] |
3. Results
3.1. Genome Organization and Base Composition
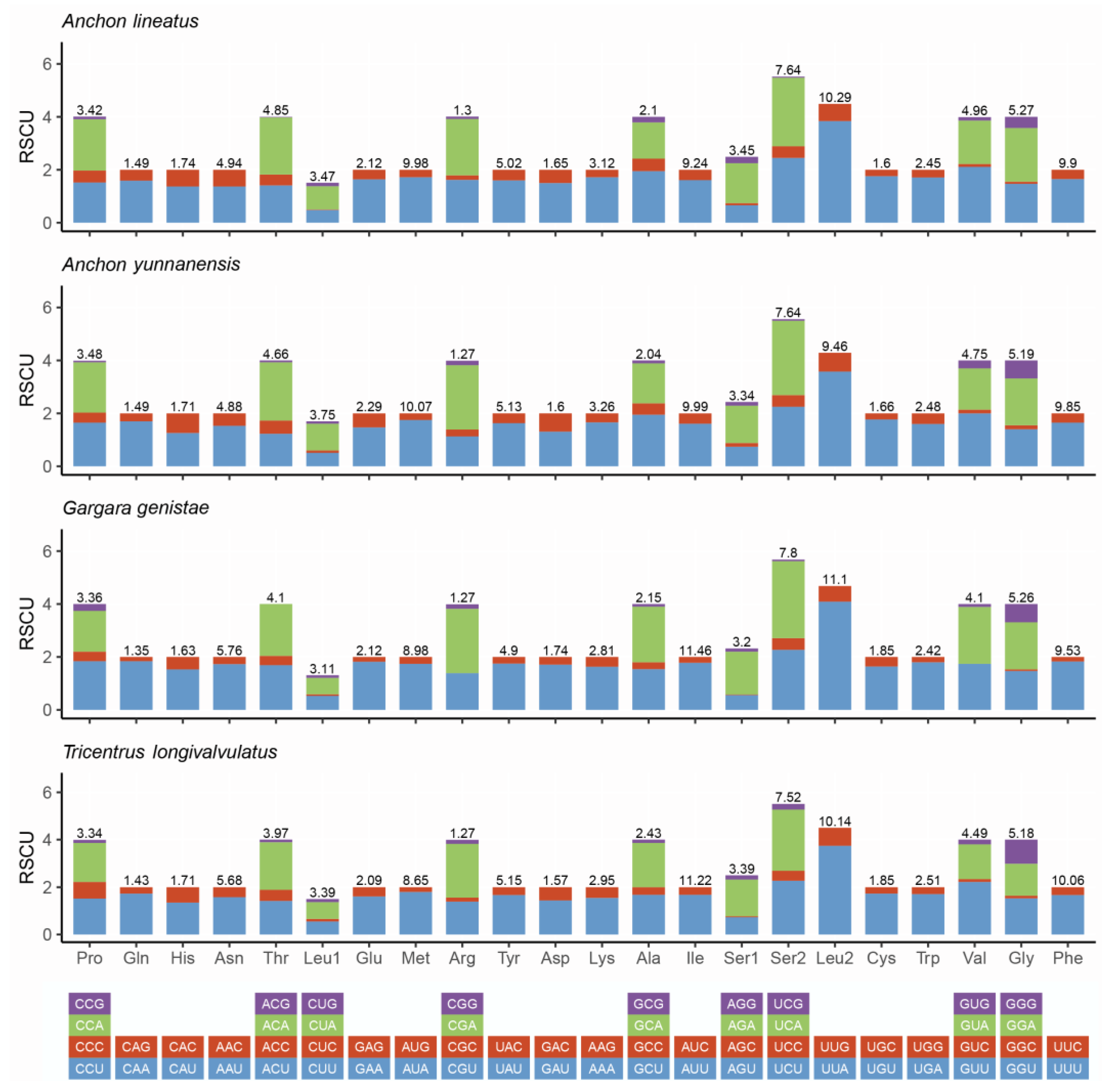
3.2. Protein-Coding Genes and Codon Usage
3.3. Transfer and Ribosomal RNA Genes
3.4. Gene Overlaps
3.5. Non-Coding Regions
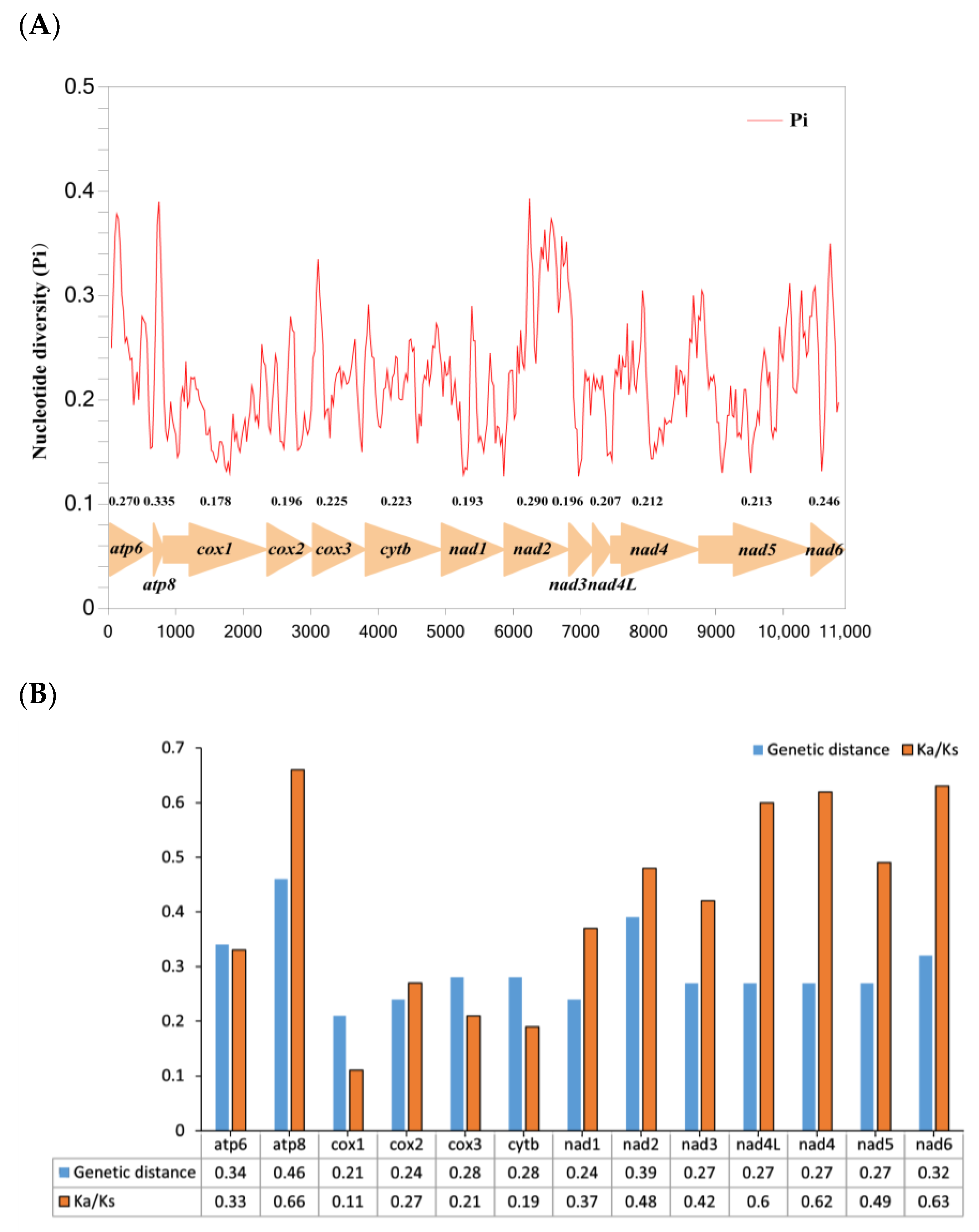
3.6. Nucleotide Diversity and Evolutionary Rate Analysis
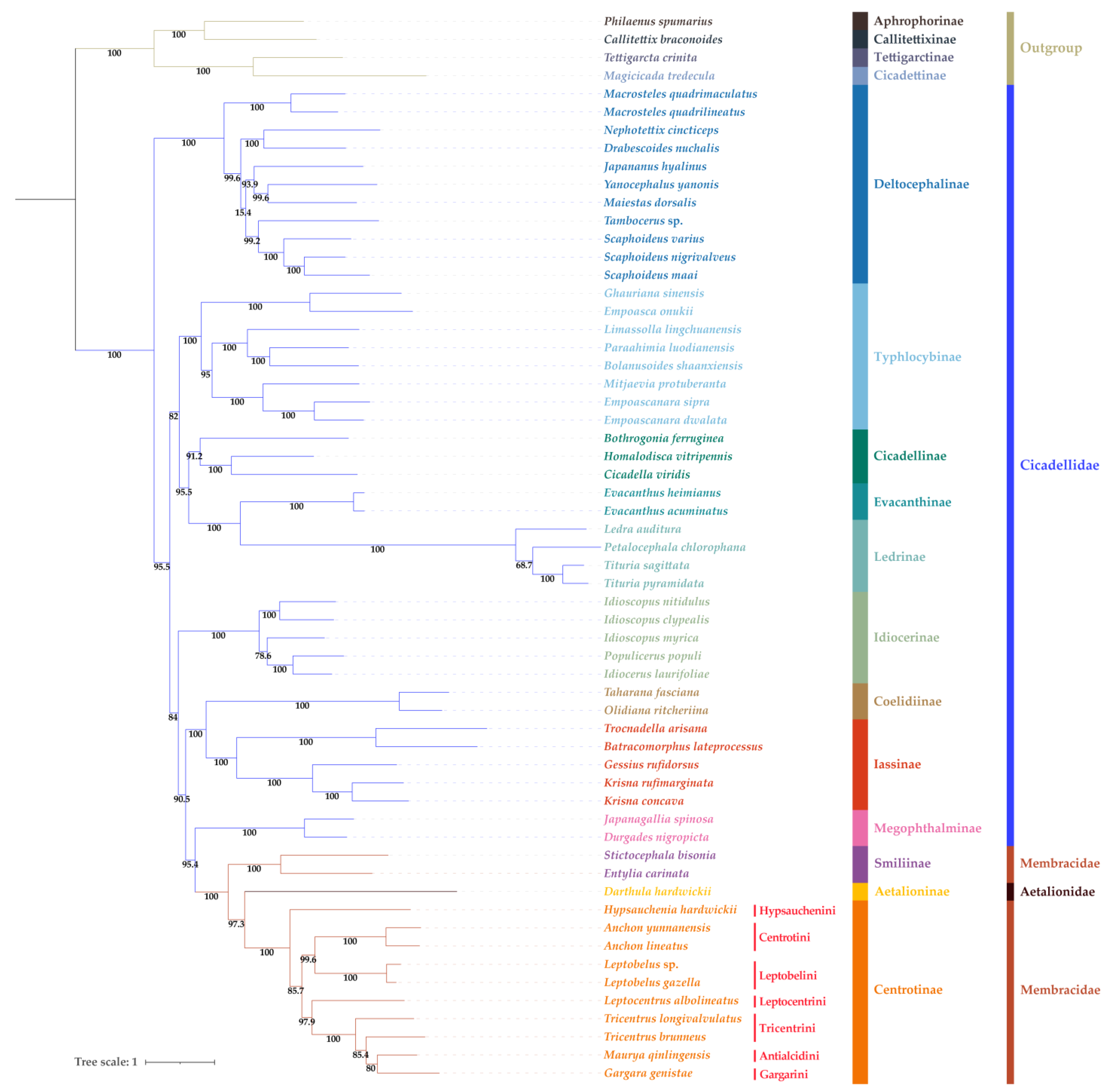
3.7. Phylogenetic Relationships
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dietz, L.L.; Wallace, M.S. Treehoppers: Aetalionidae, Melizoderidae, and Membracidae (Hemiptera). Available online: http://treehoppers.insectmuseum.org/public/site/treehoppers/home (accessed on 30 July 2021).
- Hu, K.; Yuan, F.; Dietrich, C.H.; Yuan, X.Q. Structural features and phylogenetic implications of four new mitogenomes of Centrotinae (Hemiptera: Membracidae). Int. J. Biol. Macromol. 2019, 139, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.S.; Deitz, L.L. Phylogeny and systematics of the treehopper subfamily Centrotinae (Hemiptera: Membracidae); Associated Publishers: Washington, DC, USA, 2004; pp. 1–377. [Google Scholar]
- Deitz, L.L. Bibliography of the Membracoidea (Homoptera: Aetalionidae, Biturritiidae, Membracidae, and Nicomiidae) 1981–1987; North Carolina Agricultural Research Service, North Carolina State University: Raleigh, NC, USA, 1989. [Google Scholar]
- McKamey, S.H.; Deitz, L.L. Revision of the Neotropical Treehopper Genus Metcalfiella (Homoptera: Membracidae); Department of Agricultural Communications, North Carolina State University: Raleigh, NC, USA, 1991. [Google Scholar]
- Evans, J.W. A Natural Classification of Leaf-Hoppers (Jassoidea, Homoptera) Part 1. External Morphology and Systematic Position. Trans. R. Entomol. Soc. Lond. 1946, 96, 47–60. [Google Scholar] [CrossRef]
- Evans, J.W. A Natural Classification of Leaf-Hoppers (Homoptera, Jassoidea). Part 2: Aetalionidae, Hylicidae, Eurymelidae. Trans. R. Entomol. Soc. Lond. 1946, 97, 39–54. [Google Scholar] [CrossRef]
- Evans, J.W. The leafhoppers and froghoppers of Australia and New Zealand (Homoptera: Cicadelloidea and Cercopoidea). Part 2. Rec. Aust. Mus. 1977, 31, 83–129. [Google Scholar] [CrossRef]
- Dietrich, C.H.; Deitz, L.L. Superfamily Membracoidea (Homoptera: Auchenorrhyncha). II. Cladistic analysis and conclusions. Syst. Entomol. 1993, 18, 297–311. [Google Scholar] [CrossRef]
- Dietrich, C.H.; Rakitov, R.A.; Holmes, J.L.; Black, W.C., IV. Phylogeny of the major lineages of Membracoidea (Insecta: Hemiptera: Cicadomorpha) based on 28S rDNA sequences. Mol. Phylogenet. Evol. 2001, 18, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.R. Molecular phylogeny of Cicadomorpha (Insecta: Hemiptera: Cicadoidea, Cercopoidea and Membracoidea): Adding evidence to the controversy. Syst. Entomol. 2005, 30, 563–574. [Google Scholar] [CrossRef]
- Dietrich, C.H.; Allen, J.M.; Lemmon, A.R.; Lemmon, E.M.; Takiya, D.M.; Evangelista, O.; Walden, K.K.O.; Grady, P.G.S.; Johnson, K.P. Anchored hybrid enrichment-based phylogenomics of leafhoppers and treehoppers (Hemiptera: Cicadomorpha: Membracoidea). Insect Syst. Divers. 2017, 1, 57–72. [Google Scholar] [CrossRef]
- Skinner, R.K.; Dietrich, C.H.; Walden, K.K.O.; Gordon, E.; Sweet, A.D.; Podsiadlowski, L.; Petersen, M.; Simon, C.; Takiya, D.M.; Johnson, K.P. Phylogenomics of Auchenorrhyncha (Insecta: Hemiptera) using transcriptomes: Examining controversial relationships via degeneracy coding and interrogation of gene conflict. Syst. Entomol. 2020, 45, 85–113. [Google Scholar] [CrossRef]
- Cryan, J.R.; Wiegmann, B.M.; Deitz, L.L.; Dietrich, C.H. Phylogeny of the treehoppers (Insecta: Hemiptera: Membracidae): Evidence from two nuclear genes. Mol. Phylogenet. Evol. 2000, 17, 317–334. [Google Scholar] [CrossRef]
- Cryan, J.R.; Wiegmann, B.M.; Deitz, L.L.; Dietrich, C.H.; Whiting, M.F. Treehopper trees: Phylogeny of Membracidae (Hemiptera: Cicadomorpha: Membracoidea) based on molecules and morphology. Syst. Entomol. 2004, 29, 441–454. [Google Scholar] [CrossRef]
- McKamey, S.H. Taxonomic Catalogue of the Membracoidea (Exclusive of Leafhoppers): Second Supplement to Fascicle 1, Membracidae, of the General Catalogue of the Hemiptera; Contributions of the American Entomological Institute; American Entomological Institute: Ann Arbor, MI, USA, 1998; ISBN 9781887988049. [Google Scholar]
- Cameron, S.L. Insect mitochondrial genomics: Implications for evolution and phylogeny. Annu. Rev. Entomol. 2014, 59, 95–117. [Google Scholar] [CrossRef]
- Galtier, N.; Nabholz, B.; Glémin, S.; Hurst, G.D.D. Mitochondrial DNA as a marker of molecular diversity: A reappraisal. Mol. Ecol. 2009, 18, 4541–4550. [Google Scholar] [CrossRef] [PubMed]
- Fenn, J.D.; Song, H.; Cameron, S.L.; Whiting, M.F. A preliminary mitochondrial genome phylogeny of Orthoptera (Insecta) and approaches to maximizing phylogenetic signal found within mitochondrial genome data. Mol. Phylogenet. Evol. 2008, 49, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Cai, W.; Li, H. Insufficient power of mitogenomic data in resolving the auchenorrhynchan monophyly. Zool. J. Linn. Soc. 2018, 183, 776–790. [Google Scholar] [CrossRef]
- Song, N.; An, S.; Yin, X.; Zhao, T.; Wang, X. Insufficient resolving power of mitogenome data in deciphering deep phylogeny of Holometabola. J. Syst. Evol. 2016, 54, 545–559. [Google Scholar] [CrossRef]
- Talavera, G.; Vila, R. What is the phylogenetic signal limit from mitogenomes? The reconciliation between mitochondrial and nuclear data in the Insecta class phylogeny. BMC Evol. Biol. 2011, 11, 315. [Google Scholar] [CrossRef]
- Yuan, F.; Chou, I. Homoptera Membracoidea Aetalionidae Membracidae. Fauna Sin. Insecta 2002, 28, 1–590. [Google Scholar]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Bernt, M.; Donath, A.; Jühling, F.; Externbrink, F.; Florentz, C.; Fritzsch, G.; Pütz, J.; Middendorf, M.; Stadler, P.F. MITOS: Improved de novo metazoan mitochondrial genome annotation. Mol. Phylogenet. Evol. 2013, 69, 313–319. [Google Scholar] [CrossRef]
- Grant, J.R.; Stothard, P. The CGView Server: A comparative genomics tool for circular genomes. Nucleic Acids Res. 2008, 36, 181–184. [Google Scholar] [CrossRef] [PubMed]
- RStudio Team. RStudio: Integrated development environment for R (Version 0.97.311). J. Wildl. Manag. 2011, 75, 1753–1766. [Google Scholar]
- Zhang, D.; Gao, F.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Rozas, J.; Ferrer-Mata, A.; Sanchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sanchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Talavera, G.; Castresana, J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 2007, 56, 564–577. [Google Scholar] [CrossRef]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. Partitionfinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2017, 34, 772–773. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Minh, B.Q.; Nguyen, M.A.T.; Von Haeseler, A. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. Mrbayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the 2010 Gateway Computing Environments Workshop (GCE 2010), New Orleans, LA, USA, 14 November 2010. [Google Scholar]
- Stewart, J.B.; Beckenbach, A.T. Insect mitochondrial genomics: The complete mitochondrial genome sequence of the meadow spittlebug Philaenus spumarius (Hemiptera: Auchenorrhyncha: Cercopoidae). Genome 2005, 48, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Bu, C.; Wipfler, B.; Liang, A. Comparative analysis of the mitochondrial genomes of Callitettixini spittlebugs (Hemiptera: Cercopidae) confirms the overall high evolutionary speed of the at-rich region but reveals the presence of short conservative elements at the tribal level. PLoS ONE 2014, 9, e109140. [Google Scholar] [CrossRef]
- Du, Z.; Hasegawa, H.; Cooley, J.R.; Simon, C.; Yoshimura, J.; Cai, W.; Sota, T.; Li, H. Mitochondrial genomics reveals shared phylogeographic patterns and demographic history among three periodical cicada species groups. Mol. Biol. Evol. 2019, 36, 1187–1200. [Google Scholar] [CrossRef] [PubMed]
- Łukasik, P.; Chong, R.A.; Nazario, K.; Matsuura, Y.; Bublitz, D.A.C.; Campbell, M.A.; Meyer, M.C.; Van Leuven, J.T.; Pessacq, P.; Veloso, C.; et al. One hundred mitochondrial genomes of cicadas. J. Hered. 2019, 110, 247–256. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Dai, R. Complete mitochondrial genome of Taharana fasciana (Insecta, Hemiptera: Cicadellidae) and comparison with other Cicadellidae insects. Genetica 2017, 145, 593–602. [Google Scholar] [CrossRef]
- Wu, Y.; Dai, R.; Zhan, H.; Qu, L. Complete mitochondrial genome of Drabescoides nuchalis (Hemiptera: Cicadellidae). Mitochondrial DNA 2016, 27, 3626–3627. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhang, C.; Dietrich, C.H.; Zhang, Y.; Dai, W. Characterization of the complete mitochondrial genomes of Maiestas dorsalis and Japananus hyalinus (Hemiptera: Cicadellidae) and comparison with other Membracoidea. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Mao, M.; Yang, X.; Bennett, G. The complete mitochondrial genome of Macrosteles quadrilineatus (Hemiptera: Cicadellidae). Mitochondrial DNA Part B Resour. 2017, 2, 173–175. [Google Scholar] [CrossRef]
- Du, Y.; Dietrich, C.H.; Dai, W. Complete mitochondrial genome of Macrosteles quadrimaculatus (Matsumura) (Hemiptera: Cicadellidae: Deltocephalinae) with a shared tRNA rearrangement and its phylogenetic implications. Int. J. Biol. Macromol. 2019, 122, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Dai, W.; Dietrich, C.H. Mitochondrial genomic variation and phylogenetic relationships of three groups in the genus Scaphoideus (Hemiptera: Cicadellidae: Deltocephalinae). Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef][Green Version]
- Yu, P.; Wang, M.; Cui, L.; Chen, X.; Han, B. The complete mitochondrial genome of Tambocerus sp. (Hemiptera: Cicadellidae). Mitochondrial DNA Part A DNA Mapping Seq. Anal. 2017, 28, 133–134. [Google Scholar] [CrossRef]
- Yuan, Z.; Yang, X.; Li, C.; Song, Y. The complete mitochondrial genome of the leafhopper Evacanthus acuminatus (Hemiptera: Cicadellidae: Evacanthinae). Mitochondrial DNA Part B 2019, 4, 3866–3867. [Google Scholar] [CrossRef]
- Xing, J.C.; Wang, J.J. Complete mitochondrial genome of Paralaevicephalus gracilipenis (Hemiptera: Cicadellidae: Deltocephalinae) from China. Mitochondrial DNA Part B Resour. 2019, 4, 1372–1373. [Google Scholar] [CrossRef]
- Wang, J.J.; Wu, Y.; Dai, R.; Yang, M.F. Comparative mitogenomes of six species in the subfamily Iassinae (Hemiptera: Cicadellidae) and phylogenetic analysis. Int. J. Biol. Macromol. 2020, 149, 1294–1303. [Google Scholar] [CrossRef]
- Wang, J.J.; Yang, M.F.; Dai, R.H.; Li, H.; Wang, X.Y. Characterization and phylogenetic implications of the complete mitochondrial genome of Idiocerinae (Hemiptera: Cicadellidae). Int. J. Biol. Macromol. 2018, 120, 2366–2372. [Google Scholar] [CrossRef]
- Dai, R.H.; Wang, J.J.; Yang, M.F. The complete mitochondrial genome of the leafhopper Idioscopus clypealis (Hemiptera: Cicadellidae: Idiocerinae). Mitochondrial DNA Part B 2018, 3, 32–33. [Google Scholar]
- Choudhary, J.S.; Naaz, N.; Das, B.; Bhatt, B.P.; Prabhakar, C.S. Complete mitochondrial genome of Idioscopus nitidulus (Hemiptera: Cicadellidae). Mitochondrial DNA Part B 2018, 3, 191–192. [Google Scholar] [CrossRef]
- Wang, J.J.; Li, D.F.; Li, H.; Yang, M.F.; Dai, R.H. Structural and phylogenetic implications of the complete mitochondrial genome of Ledra auditura. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, Y. Characterization of two complete mitochondrial genomes of Ledrinae (Hemiptera: Cicadellidae) and phylogenetic analysis. Insects 2020, 11, 609. [Google Scholar] [CrossRef]
- Wang, J.; Dai, R.; Li, H.; Zhan, H. Characterization of the complete mitochondrial genome of Japanagallia spinosa and Durgades nigropicta (Hemiptera: Cicadellidae: Megophthalminae). Biochem. Syst. Ecol. 2017, 74, 33–41. [Google Scholar] [CrossRef]
- Liu, J.H.; Sun, C.Y.; Long, J.; Guo, J.J. Complete mitogenome of tea green leafhopper Empoasca onukii (Hemiptera: Cicadellidae) from Anshun, Guizhou Province in China. Mitochondrial DNA Part B 2017, 2, 808–809. [Google Scholar] [CrossRef]
- Tan, C.; Chen, X.; Li, C.; Song, Y. The complete mitochondrial genome of Empoascanara sipra (Hemiptera: Cicadellidae: Typhlocybinae) with phylogenetic consideration. Mitochondrial DNA Part B 2020, 5, 260–261. [Google Scholar] [CrossRef]
- Shi, R.; Yu, X.F.; Yang, M.F. Complete mitochondrial genome of Ghauriana sinensis (Hemiptera: Cicadellidae: Typhlocybinae). Mitochondrial DNA Part B 2020, 5, 1367–1368. [Google Scholar] [CrossRef]
- Yuan, X.; Xiong, K.; Li, C.; Song, Y. The complete mitochondrial genome of Limassolla lingchuanensis (Hemiptera: Cicadellidae: Typhlocybinae). Mitochondrial DNA Part B 2020, 5, 229–230. [Google Scholar] [CrossRef]
- Yuan, X.; Li, C.; Song, Y. Characterization of the complete mitochondrial genome of Mitjaevia protuberanta (Hemiptera: Cicadellidae: Typhlocybinae). Mitochondrial DNA Part B 2020, 5, 601–602. [Google Scholar] [CrossRef]
- Song, Y.; Yuan, X.; Li, C. The mitochondrial genome of Paraahimia luodianensis (Hemiptera: Cicadellidae: Typhlocybinae), a new genus and species from China. Mitochondrial DNA Part B 2020, 5, 1351–1352. [Google Scholar] [CrossRef]
- Liang, A.P.; Gao, J.; Zhao, X. Characterization of the complete mitochondrial genome of the treehopper Darthula hardwickii (Hemiptera: Aetalionidae). Mitochondrial DNA Part A 2016, 27, 3291–3292. [Google Scholar] [CrossRef]
- Mao, M.; Yang, X.; Bennett, G. The complete mitochondrial genome of Entylia carinata (Hemiptera: Membracidae). Mitochondrial DNA Part B 2016, 1, 662–663. [Google Scholar] [CrossRef]
- Yu, R.; Feng, L.; Yuan, X. Complete mitochondrial genome sequence of the global invasive species Stictocephala bisonia (Hemiptera: Membracidae: Smiliinae). Mitochondrial DNA Part B Resour. 2021, 6, 1601–1602. [Google Scholar] [CrossRef]
- Zhao, X.; Liang, A.P. Complete DNA sequence of the mitochondrial genome of the treehopper Leptobelus gazella (Membracoidea: Hemiptera). Mitochondrial DNA 2016, 27, 3318–3319. [Google Scholar] [CrossRef]
- Li, H.; Leavengood, J.M., Jr.; Chapman, E.G.; Burkhardt, D.; Song, F.; Jiang, P.; Liu, J.; Zhou, X.; Cai, W. Mitochondrial phylogenomics of Hemiptera reveals adaptive innovations driving the diversification of true bugs. Proc. R. Soc. B Biol. Sci. 2017, 284, 20171223. [Google Scholar] [CrossRef]
- Garey, J.R.; Wolstenholme, D.R. Platyhelminth mitochondrial DNA: Evidence for early evolutionary origin of a tRNA ser AGN that contains a dihydrouridine arm replacement loop, and of serine-specifying AGA and AGG codons. J. Mol. Evol. 1989, 28, 374–387. [Google Scholar] [CrossRef]
- Xu, D.; Yu, T.; Zhang, Y. Characterization of the complete mitochondrial genome of Drabescus ineffectus and Roxasellana stellata (Hemiptera: Cicadellidae: Deltocephalinae: Drabescini) and their phylogenetic implications. Insects 2020, 11, 534. [Google Scholar] [CrossRef]
- Yu, T.; Zhang, Y. Two complete mitochondrial genomes of Mileewinae (Hemiptera: Cicadellidae) and a Phylogenetic Analysis. Insects 2021, 12, 668. [Google Scholar] [CrossRef]
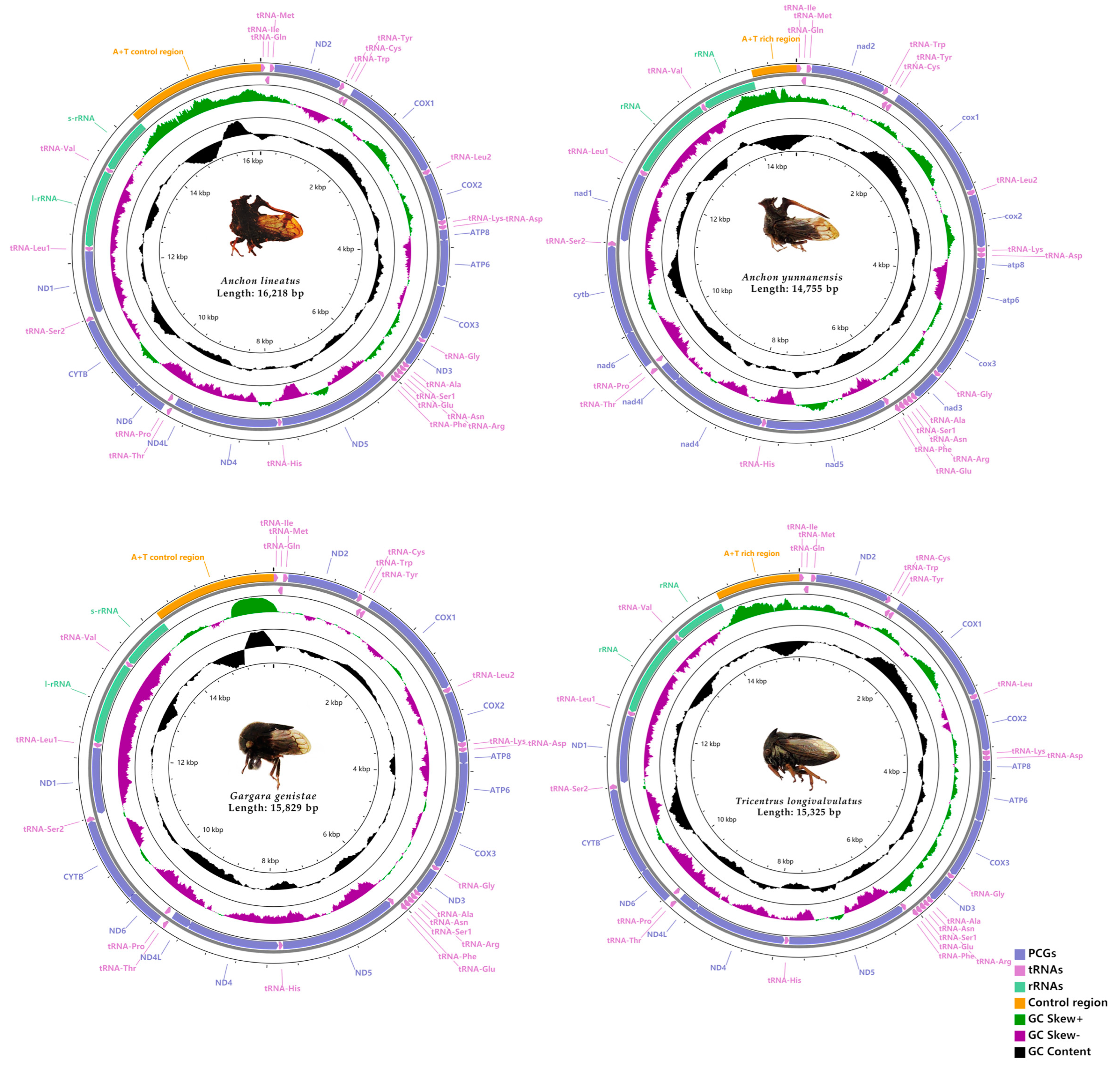
| Organism | Locality | Time | Collector |
|---|---|---|---|
| Anchon lineatus | Jinghong, Yunnan | 8 July 2017 | Hu-Kai |
| Anchon yunnanensis | Jinghong, Yunnan | 9 July 2017 | Hu-Kai |
| Gargara genistae | Northwest A&F University, Yangling, Shaanxi | 13 June 2018 | Hu-Kai |
| Tricentrus longivalvulatus | Ruyuan, Guangdong | 24 July 2020 | Yu-Ruitao |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, R.; Feng, L.; Dietrich, C.H.; Yuan, X. Characterization, Comparison of Four New Mitogenomes of Centrotinae (Hemiptera: Membracidae) and Phylogenetic Implications Supports New Synonymy. Life 2022, 12, 61. https://doi.org/10.3390/life12010061
Yu R, Feng L, Dietrich CH, Yuan X. Characterization, Comparison of Four New Mitogenomes of Centrotinae (Hemiptera: Membracidae) and Phylogenetic Implications Supports New Synonymy. Life. 2022; 12(1):61. https://doi.org/10.3390/life12010061
Chicago/Turabian StyleYu, Ruitao, Leining Feng, Christopher H. Dietrich, and Xiangqun Yuan. 2022. "Characterization, Comparison of Four New Mitogenomes of Centrotinae (Hemiptera: Membracidae) and Phylogenetic Implications Supports New Synonymy" Life 12, no. 1: 61. https://doi.org/10.3390/life12010061
APA StyleYu, R., Feng, L., Dietrich, C. H., & Yuan, X. (2022). Characterization, Comparison of Four New Mitogenomes of Centrotinae (Hemiptera: Membracidae) and Phylogenetic Implications Supports New Synonymy. Life, 12(1), 61. https://doi.org/10.3390/life12010061






