Renoprotection of Selected Antioxidant-Rich Foods (Water Spinach and Red Grape) and Probiotics in Gentamicin-Induced Nephrotoxicity and Oxidative Stress in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Preparation of Food Samples
2.2. Collection and Preparation of Probiotics
2.3. Animal Collection and Acclimatization
2.4. Drugs and Chemicals
2.5. Experimental Design
- Normal control (NC) group: This group of rats received no treatment/gentamicin and was given the standard laboratory diet.
- Gentamicin (GEN) group: Rats were provided with the standard laboratory diet.
- Water Spinach (GEN + WS) group: In this group, the laboratory diet was mixed with boiled water spinach in a 1:4 ratio (laboratory diet:WS). Each rat received about 20 g boiled water spinach per day for seven days.
- Red Grape (GEN + RG) group: Apart from the basal diet, each rat received 5 mL of red grape juice for the entire study period (7 days). The juice was provided through oral gavage feeding tubes to ensure each rat’s similar amount of intake.
- Probiotic 4B (GEN + P4) group: Throughout the entire study week, 4 × 109 CFUs were provided through oral gavage feeding tubes to each rat each day.
- Probiotic 8B (GEN + P8) group: For the whole study period, 8 × 109 CFUs were given through feeding tubes to each rat per day.
2.6. Blood Sampling
2.7. Tissue Sampling from Kidney
2.8. Estimation of Renal Function
2.9. Estimation of Stress Parameters
2.10. Preparation of Kidney Specimen for Histopathological Examination
2.11. Photography
2.12. Statistical Analysis
3. Result
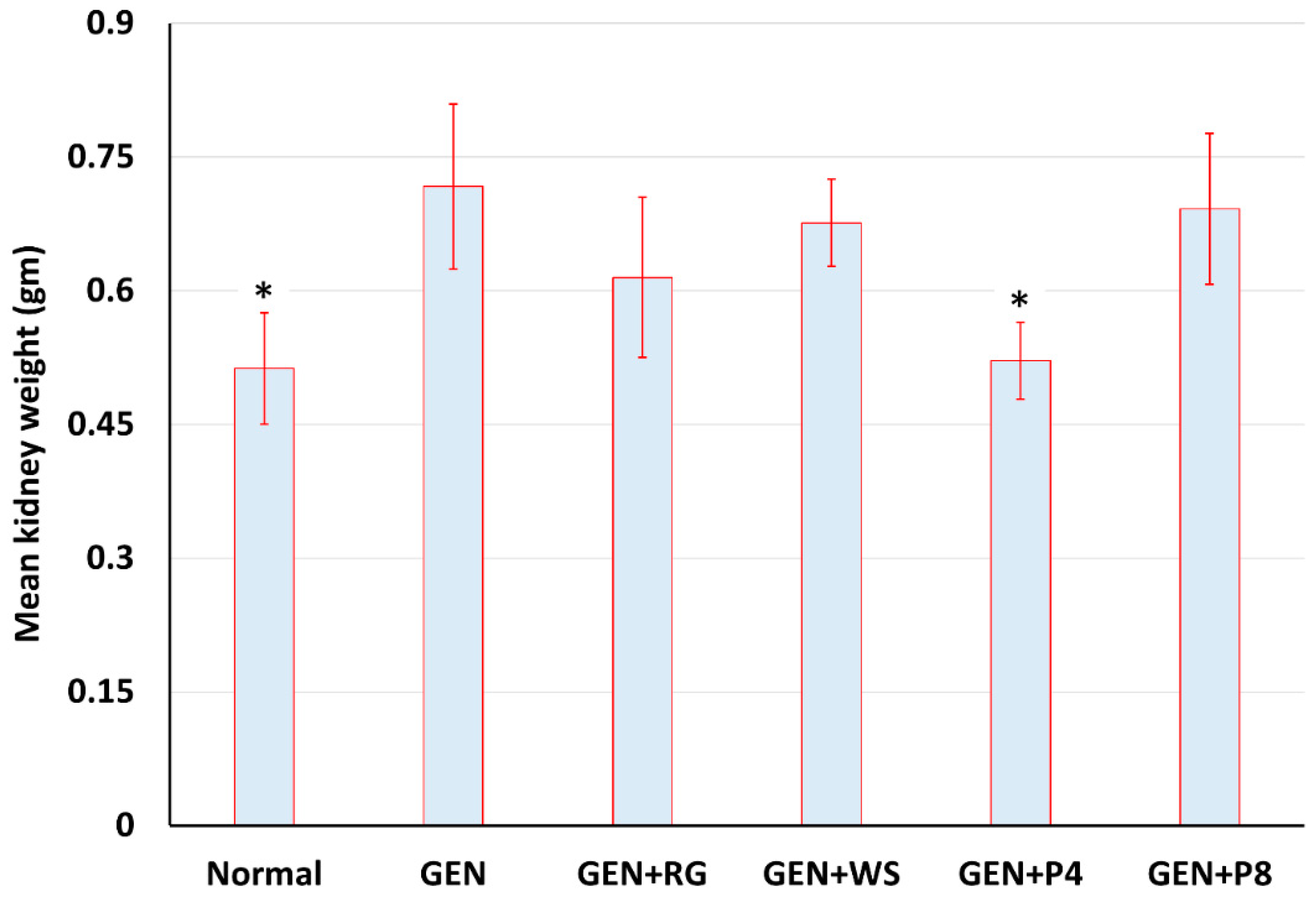
3.1. Effects of Gentamicin on Bodyweight and Kidney Weight
3.2. Effects of Gentamicin on Biochemical Parameters
3.3. Histopathology Analysis
4. Discussion
4.1. Strengths and Limitations
4.2. Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Awdishu, L. Drug-induced kidney disease in the ICU: Mechanisms, susceptibility, diagnosis and management strategies. Curr. Opin. Crit. Care. 2017, 23, 484–490. [Google Scholar] [CrossRef]
- Cheung, W.W.; Paik, K.H.; Mak, R.H. Inflammation and cachexia in chronic kidney disease. Pediatr. Nephrol. 2010, 25, 711–724. [Google Scholar] [CrossRef]
- Ince, S.; Kucukkurt, I.; Demirel, H.H.; Arslan-Acaroz, D.; Varol, N. Boron, a Trace Mineral, Alleviates Gentamicin-Induced Nephrotoxicity in Rats. Biol. Trace Element Res. 2020, 195, 515–524. [Google Scholar] [CrossRef]
- Shifow, A.A.; Kumar, K.V.; Naidu, M.U.R.; Ratnakar, K.S. Melatonin, a Pineal Hormone with Antioxidant Property, Protects against Gentamicin-Induced Nephrotoxicity in Rats. Nephron 2000, 85, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Nagai, J.; Takano, M. Molecular aspects of renal handling of aminoglycosides and strategies for preventing the nephrotoxicity. Drug Metab. Pharmacokinet. 2004, 19, 159–170. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Yang, C.-L.; Du, X.-H.; Han, Y.-X. Renal Cortical Mitochondria Are the Source of Oxygen Free Radicals Enhanced by Gentamicin. Ren. Fail. 1995, 17, 21–26. [Google Scholar] [CrossRef]
- Noiri, E.; Nakao, A.; Uchida, K.; Tsukahara, H.; Ohno, M.; Fujita, T.; Brodsky, S.; Goligorsky, M.S. Oxidative and nitrosative stress in acute renal ischemia. Am. J. Physiol. Renal Physiol. 2001, 281, F948–F957. [Google Scholar] [CrossRef]
- Awdishu, L.; Mehta, R.L. The 6R’s of drug induced nephrotoxicity. BMC Nephrol. 2017, 18, 124. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Yuan, J.; Rahimi, A.; Ni, Z.; Said, H.; Subramanian, V.S. Disintegration of colonic epithelial tight junction in uremia: A likely cause of CKD-associated inflammation. Nephrol. Dial. Transplant. 2012, 27, 2686–2693. [Google Scholar] [CrossRef] [PubMed]
- Koppe, L.; Mafra, D.; Fouque, D. Probiotics and chronic kidney disease. Kidney Int. 2015, 88, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Mitra, S.; Tareq, A.M.; Emran, T.B.; Hossain, M.J.; Alqahtani, A.M.; Alghazwani, Y.; Dhama, K.; Simal-Gandara, J. Medicinal plants used against hepatic disorders in Bangladesh: A comprehensive review. J. Ethnopharmacol. 2021, 282, 114588. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Liu, C.; Chen, Y.; Wang, Q.; Hao, Z. Protective effects of natural products against drug-induced nephrotoxicity: A review in recent years. Food Chem. Toxicol. 2021, 153, 112255. [Google Scholar] [CrossRef]
- Dua, T.K.; Dewanjee, S.; Gangopadhyay, M.; Khanra, R.; Zia-Ul-Haq, M.; De Feo, V. Ameliorative effect of water spinach, Ipomea aquatica (Convolvulaceae), against experimentally induced arsenic toxicity. J. Trans. Med. 2015, 13, 81. [Google Scholar] [CrossRef]
- Kalantari, H.; Rashidi, I. Protective Effects of Hydroalcoholic Extract of Red Grape Seed (Vitis Venifera) in Nephrotoxicity Induced by Amikacin in Mice. Jundishapur. J. Nat. Pharm. Prod. 2007, 2, 87–93. [Google Scholar]
- Safa, J.; Argani, H.; Bastani, B.; Nezami, N.; Rahimi Ardebili, B.; Ghorbanihaghjo, A.; Kalagheichi, H.; Amirfirouzi, A.; Mesgari, M. Protective effect of grape seed extract on gentamicin-induced acute kidney injury. Iran. J. Kidney Dis. 2010, 4, 285–291. [Google Scholar]
- Shahbazi, F.; Farvadi, F.; Dashti-Khavidaki, S.; Ataei, S.; Shojaei, L. Potential nephroprotective effects of resveratrol in drug induced nephrotoxicity: A narrative review of safety and efficacy data. Adv. Tradit. Med. 2020, 20, 529–544. [Google Scholar] [CrossRef]
- El-Mowafy, A.M.; Salem, H.; Al-Gayyar, M.; El-Mesery, M.E.; El-Azab, M. Evaluation of renal protective effects of the green-tea (EGCG) and red grape resveratrol: Role of oxidative stress and inflammatory cytokines. Nat. Prod. Res. 2011, 25, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Stephan, L.S.; Almeida, E.D.; Markoski, M.M.; Garavaglia, J.; Marcadenti, A. Red Wine, Resveratrol and Atrial Fibrillation. Nutrients 2017, 9, 1190. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant Properties of Probiotic Bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef]
- Hemarajata, P.; Versalovic, J. Effects of probiotics on gut microbiota: Mechanisms of intestinal immunomodulation and neuromodulation. Ther. Adv. Gastroenterol. 2013, 6, 39–51. [Google Scholar] [CrossRef]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef]
- Shaheen, N.; Rahim, A.T.M.A.; Mohiduzzaman, M.; Banu, C.P.; Bari, L.; Tukun, A.B.; Mannan, M.A.; Bhattacharjee, L.; Stadlmayr, B. Food Composition Table for Bangladesh. First Edition. June 2013. Available online: https://www.fao.org/fileadmin/templates/food_composition/documents/FCT_10_2_14_final_version.pdf (accessed on 10 November 2021).
- Longvah, T.; Ananthan, R.; Bhaskarachary, K.; Venkaiah, K. Indian Food Composition Tables 2017. Available online: https://www.academia.edu/31170922/Indian_Food_Composition_Tables (accessed on 10 November 2021).
- Hossain, A.; Khatun, M.A.; Islam, M.; Huque, R. Enhancement of Antioxidant Quality of Green Leafy Vegetables upon Different Cooking Method. Prev. Nutr. Food. Sci. 2017, 22, 216–222. [Google Scholar] [CrossRef]
- Parlakpinar, H.; Tasdemir, S.; Polat, A.; Bay-Karabulut, A.; Vardi, N.; Ucar, M.; Acet, A. Protective role of caffeic acid phenethyl ester (cape) on gentamicin-induced acute renal toxicity in rats. Toxicology 2005, 207, 169–177. [Google Scholar] [CrossRef]
- Fabiny, D.L.; Ertingshausen, G. Automated reaction-rate method for determination of serum creatinine with the CentrifiChem. Clin. Chem. 1971, 17, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Mostert, J.P.; Ramsaransing, G.S.; Heersema, D.J.; Heerings, M.; Wilczak, N.; De Keyser, J. Serum uric acid levels and leukocyte nitric oxide production in multiple sclerosis patients outside relapses. J. Neurol. Sci. 2005, 231, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Fehrman-Ekholm, I.; Skeppholm, L. Renal function in the elderly (>70 years old) measured by means of iohexol clearance, serum creatinine, serum urea and estimated clearance. Scand. J. Urol. Nephrol. 2004, 38, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Buege, J.A.; Aust, S.D. Microsomal Lipid Peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Magnani, L.; Gaydou, E.M.; Hubaud, J.C. Spectrophotometric measurement of antioxidant properties of flavones and flavonols against superoxide anion. Anal. Chim. Acta 2000, 411, 209–216. [Google Scholar] [CrossRef]
- Menaka, K.B.; Ramesh, A.; Thomas, B.; Kumari, N.S. Estimation of nitric oxide as an inflammatory marker in periodontitis. J. Ind. Soc. Periodontol. 2009, 13, 75–78. [Google Scholar] [CrossRef]
- Aiswarya, N.; Rashmi, R.R.; Preethi, S.J.; Chandran, V.; Teerthanath, S.; Sunil, P.B.; Rakesh, K.B.; Aiswarya, N.; Rashmi, R.R.; Preethi, S.J.; et al. Nephroprotective effect of aqueous extract of pimpinella anisum in gentamicin induced nephrotoxicity in wistar rats. Pharmacog. J. 2018, 10, 403–407. [Google Scholar] [CrossRef]
- Soares, S.; Souza, L.C.R.; Cronin, M.; Waaga-Gasser, A.M.; Grossi, M.F.; Franco, G.R.; A Tagliati, C. Biomarkers and in vitro strategies for nephrotoxicity and renal disease assessment. Nephrol. Ren. Dis. 2020, 5, 1–14. [Google Scholar] [CrossRef]
- Earnest Oghenesuvwe, E.; Daniel Lotanna, A. Guidelines on dosage calculation and stock solution preparation in experimental animals’ studies. J. Nat. Sci. Res. 2014, 4, 2225–2921. [Google Scholar]
- Sadeek, F. Effect of Arabic Gum as Prebiotics and Lactobacillus casei Shirota (LcS) as Probiotic on Oxidative Stress and Renal Function in Adenine–Induced Chronic Renal Failure in Rats. Eur. J. Nutr. Food Saf. 2018, 8, 29–46. [Google Scholar] [CrossRef]
- Gyurászová, M.; Gurecká, R.; Bábíčková, J.; Tóthová, Ľ. Oxidative Stress in the Pathophysiology of Kidney Disease: Implications for Noninvasive Monitoring and Identification of Biomarkers. Oxidative Med. Cell. Longev. 2020, 2020, 5478708. [Google Scholar] [CrossRef]
- Kao, M.P.C.; Ang, D.S.C.; Pall, A.; Struthers, A.D. Oxidative stress in renal dysfunction: Mechanisms, clinical sequelae and therapeutic options. J. Hum. Hypertens. 2009, 24, 1–8. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Naimi, M.S.; A Rasheed, H.; Hussien, N.R.; I Al-Gareeb, A. Nephrotoxicity: Role and significance of renal biomarkers in the early detection of acute renal injury. J. Adv. Pharm. Technol. Res. 2019, 10, 95–99. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; Mazzon, E.; Dugo, L.; Serraino, I.; di Paola, R.; Britti, D.; De Sarro, A.; Pierpaoli, S.; Caputi, A.P.; Masini, E.; et al. A role for superoxide in gentamicin-mediated nephropathy in rats. Eur. J. Pharmacol. 2002, 450, 67–76. [Google Scholar] [CrossRef]
- Basile, D.P.; Anderson, M.D.; Sutton, T.A. Pathophysiology of Acute Kidney Injury. Compr. Physiol. 2012, 2, 1303–1353. [Google Scholar] [CrossRef] [PubMed]
- Shifow, A.A.; Naidu, M.U.; Kumar, K.V.; Prayag, A.; Ratnakar, K.S. Effect of pentoxifylline on cyclosporine-induced nephrotoxicity in rats. Ind. J. Exp. Biol. 2000, 38, 347–352. [Google Scholar]
- Craig, E.A.; Yan, Z.; Zhao, Q.J. The relationship between chemical-induced kidney weight increases and kidney histopathology in rats. J. Appl. Toxicol. 2015, 35, 729–736. [Google Scholar] [CrossRef]
- Salem, N.A.; Salem, E.A. Renoprotective effect of grape seed extract against oxidative stress induced by gentamicin and hypercholesterolemia in rats. Ren. Fail. 2011, 33, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Xie, B.; Ma, S.; Zhu, X.; Fan, G.; Pan, S. Evaluation of antioxidant activities of principal carotenoids available in water spinach (Ipomoea aquatica). J. Food Comp. Anal. 2011, 24, 288–297. [Google Scholar] [CrossRef]
- Averilla, J.N.; Oh, J.; Kim, H.J.; Kim, J.S.; Kim, J.S. Potential health benefits of phenolic compounds in grape processing by-products. Food Sci. Biotechnol. 2019, 28, 1607–1615. [Google Scholar] [CrossRef]
- Shirwaikar, A.; Issac, D.; Malini, S. Effect of Aerva lanata on cisplatin and gentamicin models of acute renal failure. J. Ethnopharmacol. 2004, 90, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.S.; Xia, C.; Jiang, B.H.; Stinefelt, B.; Klandorf, H.; Harris, G.K.; Shi, X. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem. Biophys. Res. Commun. 2003, 309, 1017–1026. [Google Scholar] [CrossRef]
- Saldanha, J.F.; Leal, V.; Stenvinkel, P.; Carraro-Eduardo, J.C.; Mafra, D. Resveratrol: Why is it a promising therapy for chronic kidney disease patients? Oxid. Med. Cell. Longev. 2013, 2013, 963217. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Khatun, M.C.S.; Muhit, M.A.; Hossain, M.J.; Al-Mansur, M.A.; Rahman, S.A. Isolation of phytochemical constituents from Stevia rebaudiana (Bert.) and evaluation of their anticancer, antimicrobial and antioxidant properties via in vitro and in silico approaches. Heliyon 2021, 7, e08475. [Google Scholar] [CrossRef]
- Sharma, S.; Anjaneyulu, M.; Kulkarni, S.K.; Chopra, K. Resveratrol, a polyphenolic phytoalexin, attenuates diabetic nephropathy in rats. Pharmacology 2006, 76, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Sharmin, R.; Hossain, A.B.; Momtaz, A.; Sharmin, K.; Phil, M.; Mosaddek, A.S. Study on the effect of ethanol extract of Ipomoea aquatica (kalmi shak) leaves on gentamicin induced nephrotoxic rats. ARC J. Dent. Sci. 2016, 1, 9–14. [Google Scholar]
- Friedman, E.A. Can the bowel substitute for the kidney in advanced renal failure? Curr. Med. Res. Opin. 2009, 25, 1913–1918. [Google Scholar] [CrossRef][Green Version]
- Ranganathan, N.; Ranganathan, P.; Friedman, E.A.; Joseph, A.; Delano, B.; Goldfarb, D.S.; Tam, P.; Rao, A.V.; Anteyi, E.; Musso, C.G. Pilot study of probiotic dietary supplementation for promoting healthy kidney function in patients with chronic kidney disease. Adv. Ther. 2010, 27, 634–647. [Google Scholar] [CrossRef]
- Ranganathan, N.; Friedman, E.A.; Tam, P.; Rao, V.; Ranganathan, P.; Dheer, R. Probiotic dietary supplementation in patients with stage 3 and 4 chronic kidney disease: A 6-month pilot scale trial in Canada. Curr. Med. Res. Opin. 2009, 25, 1919–1930. [Google Scholar] [CrossRef]
- Ranganathan, N.; Patel, B.; Ranganathan, P.; Marczely, J.; Dheer, R.; Chordia, T.; Dunn, S.R.; Friedman, E.A. Probiotic amelioration of azotemia in 5/6th nephrectomized Sprague-Dawley rats. Sci. World J. 2005, 5, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Miranda Alatriste, P.V.; Urbina Arronte, R.; Gómez Espinosa, C.O.; Espinosa Cuevas Mde, L. Effect of probiotics on human blood urea levels in patients with chronic renal failure. Nutr. Hosp. 2014, 29, 582–590. [Google Scholar] [CrossRef]
- Mafra, D.; Borges, N.; Alvarenga, L.; Esgalhado, M.; Cardozo, L.; Lindholm, B.; Stenvinkel, P. Dietary Components That May Influence the Disturbed Gut Microbiota in Chronic Kidney Disease. Nutrients 2019, 11, 496. [Google Scholar] [CrossRef]
- De Faria Barros, A.; Borges, N.A.; Nakao, L.S.; Dolenga, C.J.; do Carmo, F.L.; de Carvalho Ferreira, D.; Stenvinkel, P.; Bergman, P.; Lindholm, B.; Mafra, D. Effects of probiotic supplementation on inflammatory biomarkers and uremic toxins in non-dialysis chronic kidney patients: A double-blind, randomized, placebo-controlled trial. J. Funct. Foods. 2018, 46, 378–383. [Google Scholar] [CrossRef]
- Tsao, S.P.; Nurrahma, B.A.; Kumar, R.; Wu, C.H.; Yeh, T.H.; Chiu, C.C.; Lee, Y.P.; Liao, Y.C.; Huang, C.H.; Yeh, Y.T.; et al. Probiotic Enhancement of Antioxidant Capacity and Alterations of Gut Microbiota Composition in 6-Hydroxydopamin-Induced Parkinson’s Disease Rats. Antioxidants 2021, 10, 1823. [Google Scholar] [CrossRef]
- Kullisaar, T.; Zilmer, M.; Mikelsaar, M.; Vihalemm, T.; Annuk, H.; Kairane, C.; Kilk, A. Two antioxidative lactobacilli strains as promising probiotics. Int. J. Food Microbiol. 2002, 72, 215–224. [Google Scholar] [CrossRef]
- Stecchini, M.L.; Del Torre, M.; Munari, M. Determination of peroxy radical-scavenging of lactic acid bacteria. Int. J. Food Microbiol. 2001, 64, 183–188. [Google Scholar] [CrossRef]

| Ingredients | Percentage (%) |
|---|---|
| Rice polish and ground maize (w/w) | 55.7 |
| Ground whole grain wheat (w/w) | 30.9 |
| Dried fish meal (w/w) | 5.4 |
| Soybean oil (v/w) | 3.2 |
| Salt (NaCl) (w/w) | 2.4 |
| Vitamin mixture (Vitamin B12 and Vitamin C) (w/w) | 0.4 |
| Distilled water for mixing | 2.0 |
| Groups | Initial Weight (g) Mean ± SD | Final Weight (g) Mean ± SD | Mean Difference (Final-Initial) | Paired t-Test | p-Value |
|---|---|---|---|---|---|
| Normal control (NC) | 196.4 ± 52.22 | 209.80 ± 54.21 | 13.4 | −11.49 | <0.001 |
| Gentamicin (GEN) | 196.8 ± 37.11 | 181.98 ± 35.69 | −14.42 | 6.158 | 0.004 |
| Water spinach (GEN + WS) | 196.4 ± 16.82 | 203.21 ± 12.05 | 6.81 | −2.369 | 0.077 |
| Red grape (GEN + RG) | 196.0 ± 22.67 | 201.24 ± 21.57 | 5.24 | −1.414 | 0.230 |
| Probiotic (4B) (GEN + P4) | 196.0 ± 12.20 | 207.0 ± 13.30 | 11.0 | 19.038 | <0.001 |
| Probiotic (8B) (GEN + P8) | 196.0 ± 12.25 | 174.80 ± 13.72 | −21.2 | −5.982 | 0.004 |
| Biochemical Parameters | Groups | |||||
|---|---|---|---|---|---|---|
| Normal Control (NC) | Gentamicin Control (GEN) | Water Spinach (GEN + WS) | Red Grape (GEN + RG) | Probiotic (4B) (GEN + P4) | Probiotic (8B) (GEN + P8) | |
| Creatinine (mg/dL) | 0.67 ± 0.15 * | 1.64 ± 0.55 | 0.82 ± 0.28 * | 1.16 ± 0.44 | 1.48 ± 0.144 | 1.51 ± 0.31 |
| Urea (mg/dL) | 17.94 ± 5.40 * | 82.57 ± 44.28 | 52.35 ± 16.22 | 81.08 ± 5.04 | 17.94 ±3.01 * | 116.74 ± 11.76 * |
| Uric acid (mg/dL) | 7.31 ± 4.12 * | 17.77 ± 5.70 | 10.22 ± 1.919 * | 7.47 ± 3.46 * | 5.42± 1.52 * | 7.43 ± 1.27 * |
| MDA (nmol/L) | 3.86 ± 1.34 * | 12.76 ± 1.52 | 4.03 ± 0.15 * | 5.46 ± 2.84 * | 4.54 ± 1.61 * | 2.50 ± 0.402 * |
| NO (mmol/mL) | 1.82 ± 0.33 * | 3.42 ± 1.03 | 1.24 ± 0.21 * | 1.50 ± 0.43 * | 1.08 ± 0.21 * | 1.36 ± 0.07 * |
| SOD (U/mL) | 3.22 ± 1.73 | 1.68 ± 0.84 | 2.73 ± 0.79 | 2.98 ± 1.08 | 3.23 ± 0.485 | 3.25 ± 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarwar, S.; Hossain, M.J.; Irfan, N.M.; Ahsan, T.; Arefin, M.S.; Rahman, A.; Alsubaie, A.; Alharthi, B.; Khandaker, M.U.; Bradley, D.A.; et al. Renoprotection of Selected Antioxidant-Rich Foods (Water Spinach and Red Grape) and Probiotics in Gentamicin-Induced Nephrotoxicity and Oxidative Stress in Rats. Life 2022, 12, 60. https://doi.org/10.3390/life12010060
Sarwar S, Hossain MJ, Irfan NM, Ahsan T, Arefin MS, Rahman A, Alsubaie A, Alharthi B, Khandaker MU, Bradley DA, et al. Renoprotection of Selected Antioxidant-Rich Foods (Water Spinach and Red Grape) and Probiotics in Gentamicin-Induced Nephrotoxicity and Oxidative Stress in Rats. Life. 2022; 12(1):60. https://doi.org/10.3390/life12010060
Chicago/Turabian StyleSarwar, Sneha, Md. Jamal Hossain, Nafis Md. Irfan, Tamima Ahsan, Md. Saidul Arefin, Arebia Rahman, Abdullah Alsubaie, Badr Alharthi, Mayeen Uddin Khandaker, David A. Bradley, and et al. 2022. "Renoprotection of Selected Antioxidant-Rich Foods (Water Spinach and Red Grape) and Probiotics in Gentamicin-Induced Nephrotoxicity and Oxidative Stress in Rats" Life 12, no. 1: 60. https://doi.org/10.3390/life12010060
APA StyleSarwar, S., Hossain, M. J., Irfan, N. M., Ahsan, T., Arefin, M. S., Rahman, A., Alsubaie, A., Alharthi, B., Khandaker, M. U., Bradley, D. A., Emran, T. B., & Islam, S. N. (2022). Renoprotection of Selected Antioxidant-Rich Foods (Water Spinach and Red Grape) and Probiotics in Gentamicin-Induced Nephrotoxicity and Oxidative Stress in Rats. Life, 12(1), 60. https://doi.org/10.3390/life12010060







