Prospective (Bio)leaching of Historical Copper Slags as an Alternative to Their Disposal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedures
2.3. Analytical Measurements
2.3.1. Solution Chemistry
2.3.2. Mineralogical Analyses
3. Results and Discussion
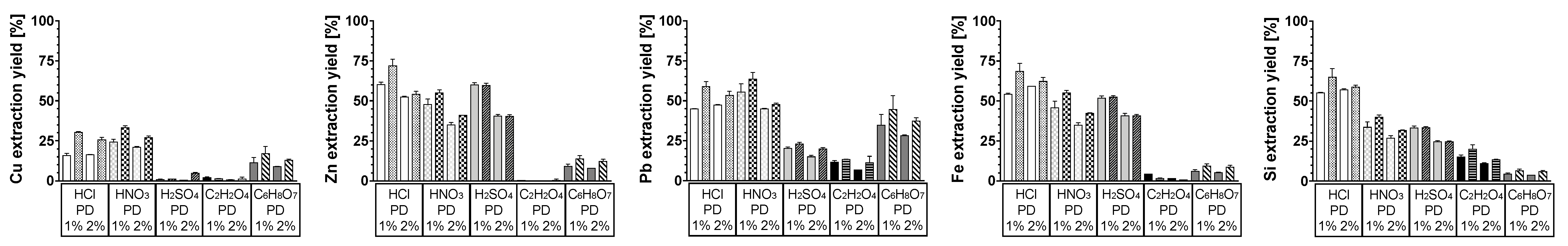
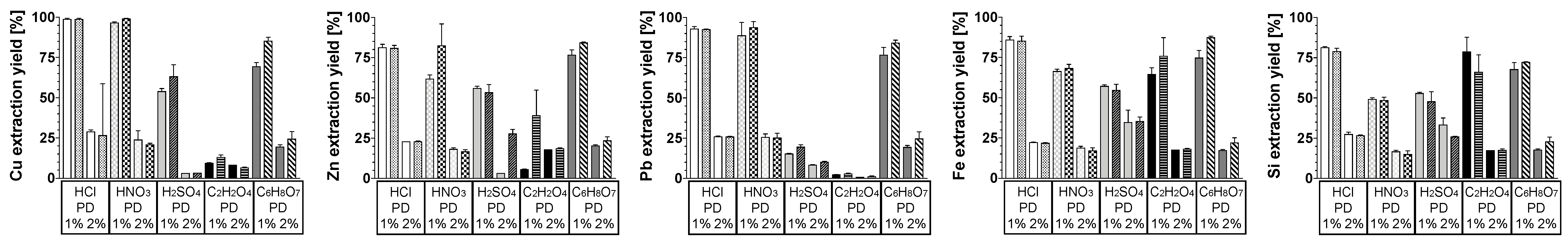
3.1. Effect of Sulfuric Acid Concentration
3.1.1. Crystalline Slag
3.1.2. Amorphous Slag
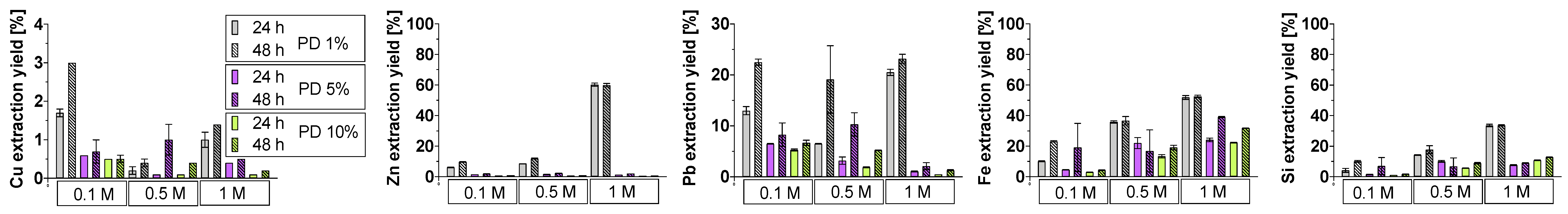
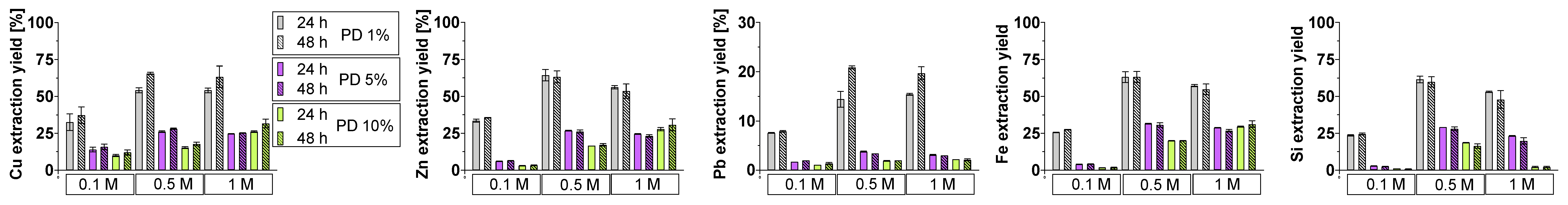
3.2. Effect of Pulp Density
3.3. Silica Gel Formation
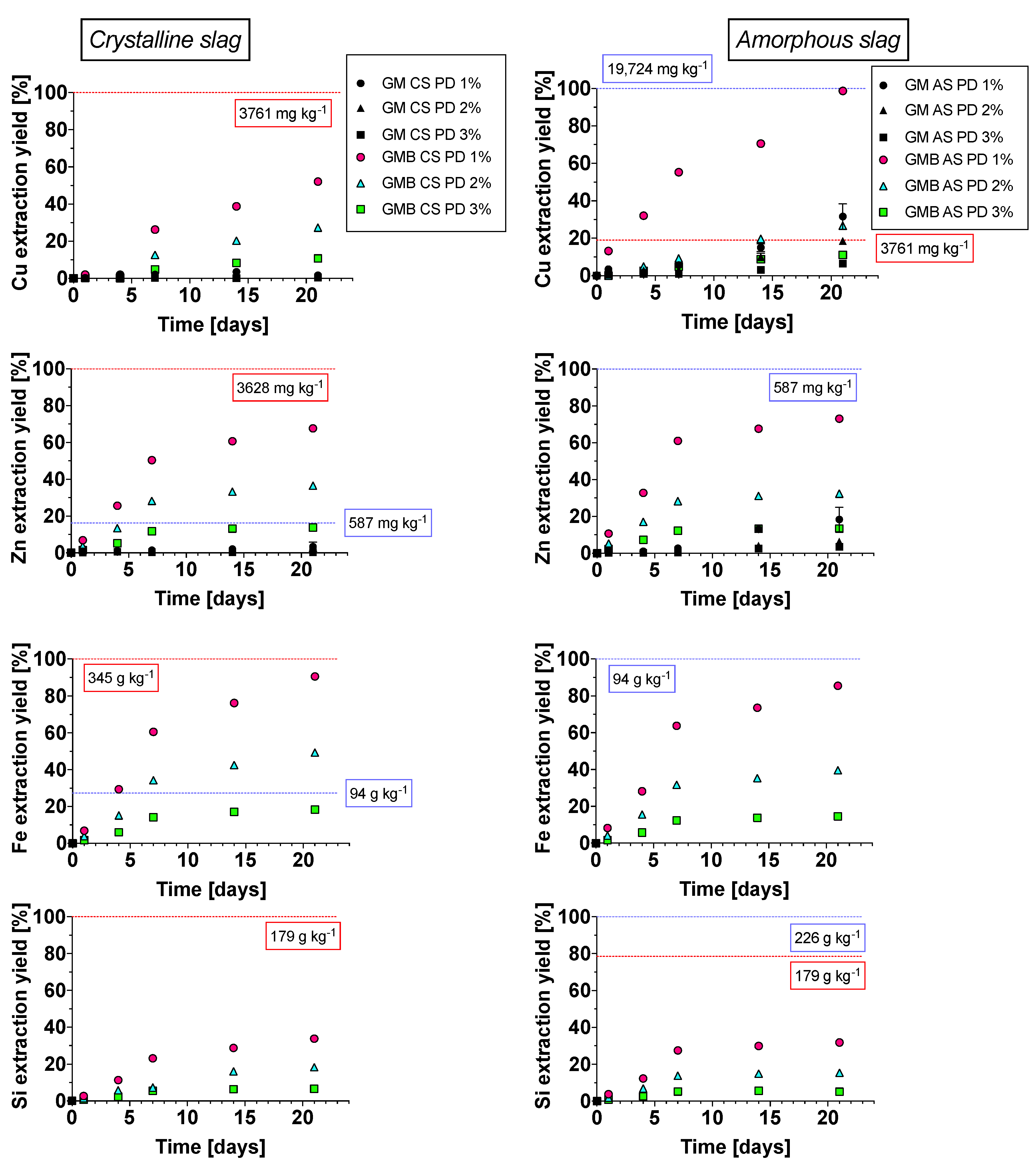
3.4. Bioleaching Efficiency
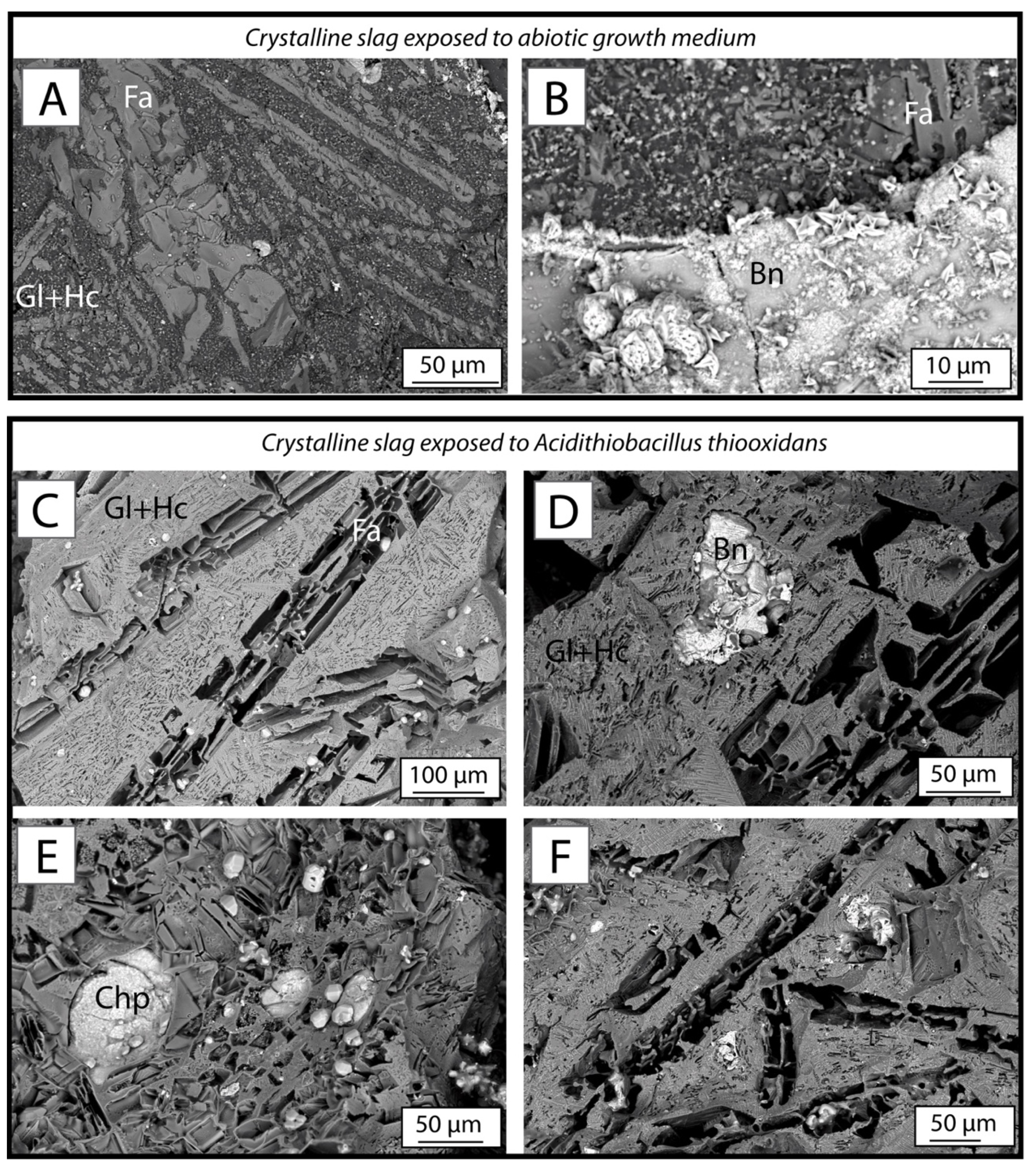
3.4.1. Crystalline Slag
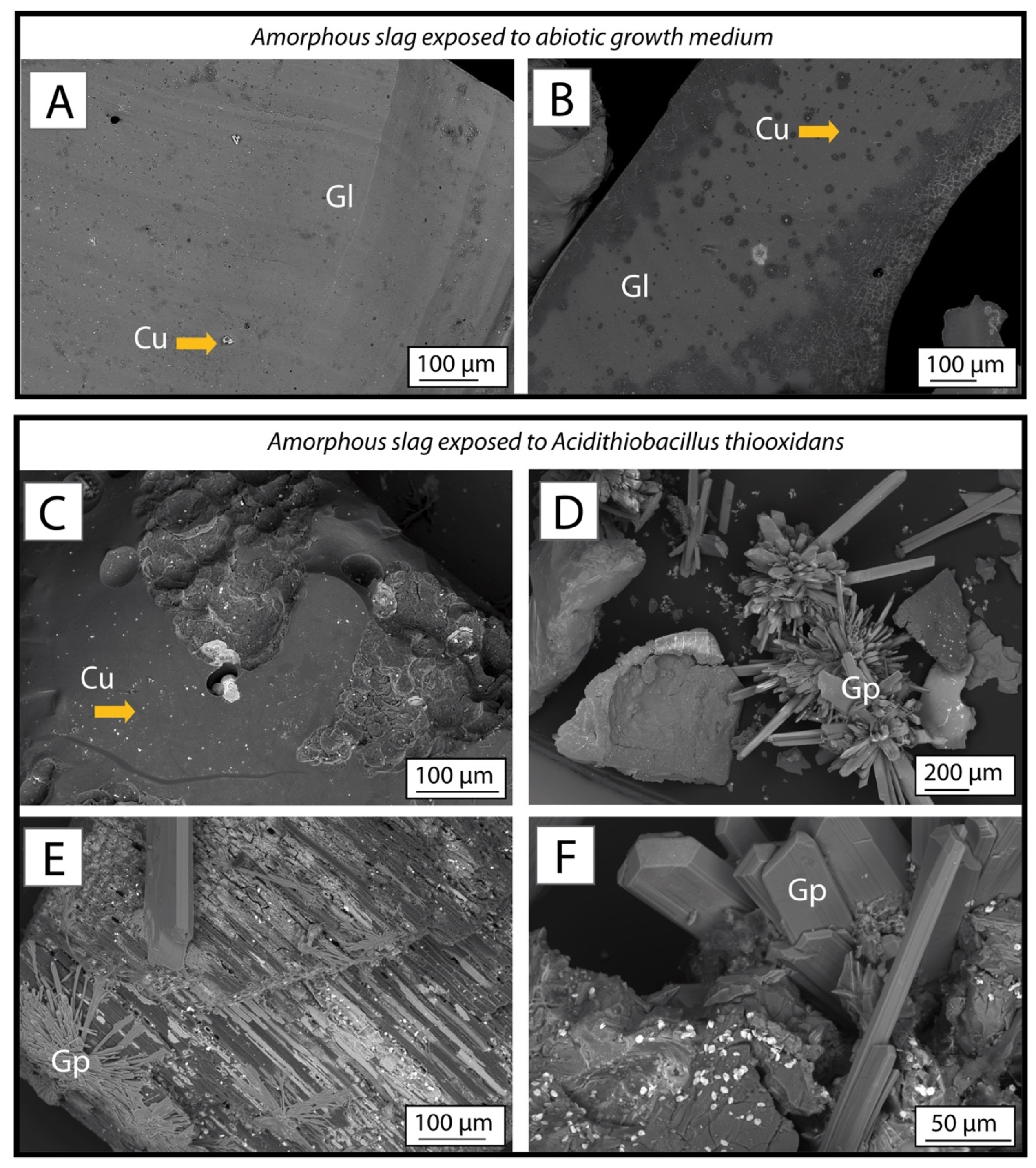
3.4.2. Amorphous Slag
3.4.3. Comparison of Slag Behavior
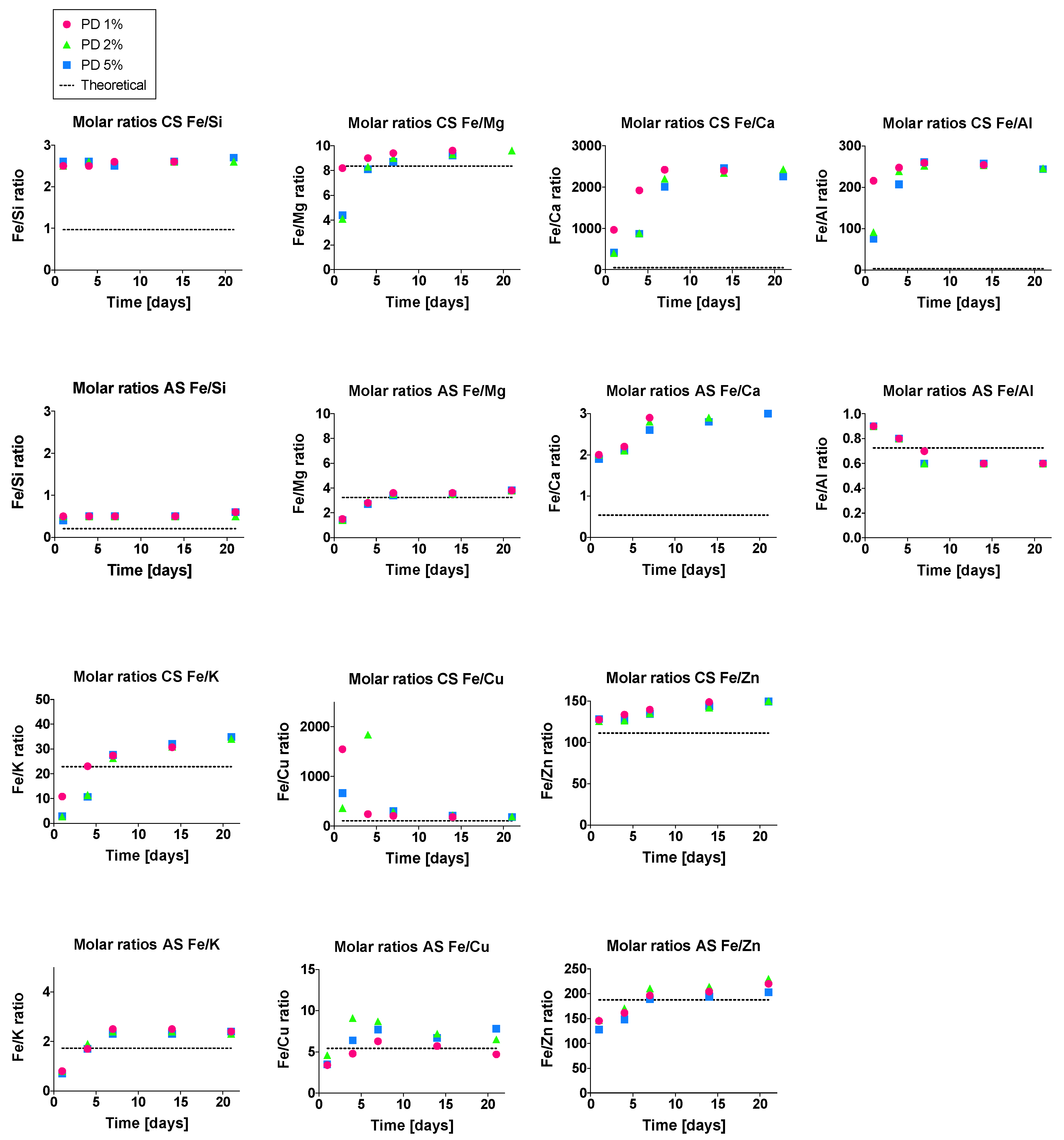
3.5. Phase Dissolution in Bioleaching System
3.6. Economic Potential for Treatment of Historical Slags
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Piatak, N.M.; Parsons, M.B.; Seal, R.R. Characteristics and environmental aspects of slag: A review. Appl. Geochem. 2015, 57, 236–266. [Google Scholar] [CrossRef]
- Gorai, B.; Jana, R.K. Characteristics and utilisation of copper slag–A review. Resour. Conserv. Recycl. 2003, 39, 299–313. [Google Scholar] [CrossRef]
- Ettler, V. Soil contamination near non-ferrous metal smelters: A review. Appl. Geochem. 2015, 64, 56–74. [Google Scholar] [CrossRef]
- Kasemodel, M.C.; Papa, T.B.R.; Sígolo, J.B.; Rodrigues, V.G.S. Assessment of the mobility, bioaccessibility, and ecological risk of Pb and Zn on a dirt road located in a former mining area–Ribeira Valley–Brazil. Environ. Monit. Assess. 2019, 191, 101. [Google Scholar] [CrossRef] [PubMed]
- Forghani, G.; Kelm, U.; Mazinani, V. Spatial distribution and chemical partitioning of potentially toxic elements in soils around Khatoon-Abad Cu Smelter, SE Iran. J. Geochem. Explor. 2019, 196, 66–80. [Google Scholar] [CrossRef]
- Moura, W.A.; Gonçalves, J.P.; Lima, M.B.L. Copper slag waste as a supplementary cementing material to concrete. J. Mater. Sci. 2007, 42, 2226–2230. [Google Scholar] [CrossRef]
- Shi, C.; Meyer, C.; Behnood, A. Utilization of copper slag in cement and concrete. Resour. Conserv. Recycl. 2008, 52, 1115–1120. [Google Scholar] [CrossRef]
- Schmukat, A.; Duester, L.; Ecker, D.; Schmid, H.; Heil, C.; Heininger, P.; Ternes, T.A. Leaching of metal(loid)s from a construction material: Influence of the particle size, specific surface area and ionic strength. J. Hazard. Mater. 2012, 227, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Schmukat, A.; Duester, L.; Ecker, D.; Heininger, P.; Ternes, T.A. Determination of the long-term release of metal(loid)s from construction materials using DGTs. J. Hazard. Mater. 2013, 260, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Potysz, A.; van Hullebusch, E.D.; Kierczak, J. Perspectives regarding the use of metallurgical slags as secondary metal resources—A review of bioleaching approaches. J. Environ. Manag. 2018, 219, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Kaksonen, A.H.; Särkijärvi, S.; Peuraniemi, E.; Junnikkala, S.; Puhakka, J.A.; Tuovinen, O.H. Metal biorecovery in acid solutions from a copper smelter slag. Hydrometallurgy 2017, 168, 135–140. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Lavonen, L.; Kuusenaho, M.; Kolli, A.; Närhi, H.; Vestola, E.; Puhakka, J.A.; Tuovinen, O.H. Bioleaching and recovery of metals from final slag waste of the copper smelting industry. Miner. Eng. 2011, 24, 1113–1121. [Google Scholar] [CrossRef]
- Panda, S.; Mishra, S.; Rao, D.S.; Pradhan, N.; Mohapatra, U.; Angadi, S.; Mishra, B.K. Extraction of copper from copper slag: Mineralogical insights, physical beneficiation and bioleaching studies. Korean J. Chem. Eng. 2015, 32, 667–676. [Google Scholar] [CrossRef]
- Potysz, A.; Lens, P.N.L.; van de Vossenberg, J.; Rene, E.R.; Grybos, M.; Guibaud, G.; Kierczak, J.; van Hullebusch, E.D. Comparison of Cu, Zn and Fe bioleaching from Cu-metallurgical slags in the presence of Pseudomonas fluorescens and Acidithiobacillus thiooxidans. Appl. Geochem. 2016, 68, 39–52. [Google Scholar] [CrossRef]
- Pollmann, K.; Kutschke, S.; Matys, S.; Raff, J.; Hlawacek, G.; Lederer, F.L. Bio-recycling of metals: Recycling of technical products using biological applications. Biotechnol. Adv. 2018, 36, 1048–1062. [Google Scholar] [CrossRef] [PubMed]
- Hennebel, T.; Boon, N.; Maes, S.; Lenz, M. Biotechnologies for critical raw material recovery from primary and secondary sources: R&D priorities and future perspectives. New Biotechnol. 2015, 32, 121–127. [Google Scholar]
- Prior, T.; Giurco, D.; Mudd, G.; Mason, L.; Behrisch, J. Resource depletion, peak minerals and the implications for sustainable resource management. Glob. Environ. Chang. 2012, 22, 577–587. [Google Scholar] [CrossRef]
- Radetzki, M. Seven thousand years in the service of humanity-the history of copper, the red metal. Resour. Policy 2009, 34, 176–184. [Google Scholar] [CrossRef]
- Themelis, N.J. Pyrometallurgy near the end of the 20th century. JOM 1994, 46, 51–57. [Google Scholar] [CrossRef]
- Potysz, A.; van Hullebusch, E.D.; Kierczak, J.; Grybos, M.; Lens, P.N.L.; Guibaud, G. Copper metallurgical slags-current knowledge and fate: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2424–2488. [Google Scholar] [CrossRef]
- Banza, A.N.; Gock, E.; Kongolo, K. Base metals recovery from copper smelter slag by oxidising leaching and solvent extraction. Hydrometallurgy 2002, 67, 63–69. [Google Scholar] [CrossRef]
- Carranza, F.; Romero, R.; Mazuelos, A.; Iglesias, N.; Forcat, O. Biorecovery of copper from converter slags: Slags characterization and exploratory ferric leaching tests. Hydrometallurgy 2009, 97, 39–45. [Google Scholar] [CrossRef]
- Tong, D.; Yunhan, L. Processing of copper converter slag for metal reclamation. Part I: Extraction and recovery of copper and cobalt. Waste Manag. Res. 2007, 25, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Giller, K.E.; Witter, E.; Mcgrath, S.P. Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: A review. Soil Biol. Biochem. 1998, 30, 1389–1414. [Google Scholar] [CrossRef]
- Sahni, A.; Kumar, A.; Kumar, S. Chemo-biohydrometallurgy—A hybrid technology to recover metals from obsolete mobile SIM cards. Environ. Nanotechnol. Monit. Manag. 2016, 6, 130–133. [Google Scholar] [CrossRef]
- Sukla, L.B.; Kar, R.N.; Panchanadikar, V. Leaching of copper converter slag with Aspergillus niger culture filtrate. Biometals 1992, 5, 169–172. [Google Scholar] [CrossRef]
- Gadd, G.M. Metals, minerals and microbes: Geomicrobiology and bioremediation. Microbiology 2010, 156, 609–643. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Sarveswara Rao, K.; Jena, P.K. Pressure leaching of copper converter slag using dilute sulphuric acid for the extraction of cobalt, nickel and copper values. Hydrometallurgy 1983, 10, 305–312. [Google Scholar] [CrossRef]
- Altundoǧan, H.S.; Tümen, F. Metal recovery from copper converter slag by roasting with ferric sulphate. Hydrometallurgy 1997, 44, 261–267. [Google Scholar] [CrossRef]
- Beşe, A.V.; Ata, O.N.; Çelik, C.; Çolak, S. Determination of the optimum conditions of dissolution of copper in converter slag with chlorine gas in aqueous media. Chem. Eng. Process. 2003, 42, 291–298. [Google Scholar] [CrossRef]
- Sukla, L.B.; Panda, S.C.; Jena, P.K. Recovery of cobalt, nickel and copper from converter slag through roasting with ammonium sulphate and sulphuric acid. Hydrometallurgy 1986, 16, 153–165. [Google Scholar] [CrossRef]
- Nadirov, R.K.; Syzdykova, L.I.; Zhussupova, A.K.; Usserbaev, M.T. Recovery of value metals from copper smelter slag by ammonium chloride treatment. Int. J. Miner. Process. 2013, 124, 145–149. [Google Scholar] [CrossRef]
- Herreros, O.; Quiroz, R.; Manzano, E.; Bou, C.; Viñals, J. Copper extraction from reverberatory and flash furnace slags by chlorine leaching. Hydrometallurgy 1998, 49, 87–101. [Google Scholar] [CrossRef]
- Basir, S.M.; Rabah, M.A. Hydrometallurgical recovery of metal values from brass melting slag. Hydrometallurgy 1999, 53, 31–44. [Google Scholar] [CrossRef]
- Yang, Z.; Rui-Lin, M.; Wang-Dong, N.; Hui, W. Selective leaching of base metals from copper smelter slag. Hydrometallurgy 2010, 103, 25–29. [Google Scholar] [CrossRef]
- Ahmed, I.M.; Nayl, A.A.; Daoud, J.A. Leaching and recovery of zinc and copper from brass slag by sulfuric acid. J. Saudi Chem. Soc. 2016, 20, 280–285. [Google Scholar] [CrossRef]
- Arslan, C.; Arslan, F. Recovery of copper, cobalt, and zinc from copper smelter and converter slags. Hydrometallurgy 2002, 67, 1–7. [Google Scholar] [CrossRef]
- Altundogan, H.S.; Boyrazli, M.; Tumen, F. A study on the sulphuric acid leaching of copper converter slag in the presence of dichromate. Miner. Eng. 2004, 17, 465–467. [Google Scholar] [CrossRef]
- Baghalha, M.; Papangelakis, V.G.; Curlook, W. Factors affecting the leachability of Ni/Co/Cu slags at high temperature. Hydrometallurgy 2007, 85, 42–52. [Google Scholar] [CrossRef]
- Potysz, A.; Grybos, M.; Kierczak, J.; Guibaud, G.; Lens, P.N.L.; van Hullebusch, E.D. Bacterially-mediated weathering of crystalline and amorphous Cu-slags. Appl. Geochem. 2015, 64, 92–106. [Google Scholar] [CrossRef]
- Kierczak, J.; Potysz, A.; Pietranik, A.; Tyszka, R.; Modelska, M.; Néel, C.; Ettler, V.; Mihaljevič, M. Environmental impact of the historical Cu smelting in the Rudawy Janowickie Mountains (south-western Poland). J. Geochem. Explor. 2013, 124, 183–194. [Google Scholar] [CrossRef]
- Potysz, A.; Kierczak, J.; Fuchs, Y.; Grybos, M.; Guibaud, G.; Lens, P.N.L.; van Hullebusch, E.D. Characterization and pH-dependent leaching behaviour of historical and modern copper slags. J. Geochem. Explor. 2016, 160, 1–15. [Google Scholar] [CrossRef]
- Potysz, A.; Kierczak, J.; Pietranik, A.; Kądziołka, K. Mineralogical, geochemical, and leaching study of historical Cu-slags issued from processing of the Zechstein formation (Old Copper Basin, southwestern Poland). Appl. Geochemistry 2018, 98, 22–35. [Google Scholar] [CrossRef]
- Mikoda, B.; Potysz, A.; Kmiecik, E. Bacterial leaching of critical metal values from Polish copper metallurgical slags using Acidithiobacillus thiooxidans. J. Environ. Manag. 2019, 236, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Stuurman, S.; Ndlovu, S.; Sibanda, V. Comparing the extent of the dissolution of copper-cobalt ores from the DRC region. J. S. Afr. Inst. Min. Metall. 2014, 114, 347–349. [Google Scholar]
- Crundwell, F.K. The mechanism of dissolution of minerals in acidic and alkaline solutions: Part III. Application to oxide, hydroxide and sulfide minerals. Hydrometallurgy 2014, 149, 71–81. [Google Scholar] [CrossRef]
- Watling, H.R. The bioleaching of sulphide minerals with emphasis on copper sulphides-A review. Hydrometallurgy 2006, 84, 81–108. [Google Scholar] [CrossRef]
- Beşe, A.V. Effect of ultrasound on the dissolution of copper from copper converter slag by acid leaching. Ultrason. Sonochem. 2007, 14, 790–796. [Google Scholar] [CrossRef]
- Dopson, M.; Halinen, A.K.; Rahunen, N.; Boström, D.; Sundkvist, J.E.; Riekkola-Vanhanen, M.; Kaksonen, A.H.; Puhakka, J.A. Silicate mineral dissolution during heap bioleaching. Biotechnol. Bioeng. 2008, 96, 288–293. [Google Scholar] [CrossRef]
- Potysz, A.; Kierczak, J.; Grybos, M.; Pędziwiatr, A.; van Hullebusch, E.D. Weathering of historical copper slags in dynamic experimental system with rhizosphere-like organic acids. J. Environ. Manag. 2018, 222, 325–337. [Google Scholar] [CrossRef]
- Urosevic, D.M.; Dimitrijevic, M.D.; Jankovic, Z.D.; Antic, D.V. Recovery of copper from copper slag and copper slag flotation tailings by oxidative leaching. Physicochem. Probl. Miner. Process. 2015, 51, 73–82. [Google Scholar]
- Lewis, A.E. Review of metal sulphide precipitation. Hydrometallurgy 2010, 104, 222–234. [Google Scholar] [CrossRef]
- Sethurajan, M.; Huguenot, D.; Lens, P.N.L.; Horn, H.A.; Figueiredo, L.H.A.; Van Hullebusch, E.D. Leaching and selective copper recovery from acidic leachates of Três Marias zinc plant (MG, Brazil) metallurgical purification residues. J. Environ. Manag. 2016, 177, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, C.N.; Kamali, M.; Gibbs, B.F. Bioleaching of heavy metals from a low-grade mining ore using Aspergillus niger. J. Hazard. Mater. 2004, 110, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Zou, Y.; Xin, B.; Chen, S.; Liu, C.; Li, Y. Process controls for improving bioleaching performance of both Li and Co from spent lithium ion batteries at high pulp density and its thermodynamics and kinetics exploration. Chemosphere 2014, 109, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.M.; Wang, J.W.; Wang, C.T.; Tsai, C.H. Effect of water quenching and SiO2 addition during vitrification of fly ash. Part 1: On the crystalline characteristics of slags. J. Hazard. Mater. 2008, 152, 994–1001. [Google Scholar] [CrossRef]
- Mohanty, U.; Rintala, L.; Halli, P.; Taskinen, P.; Lundström, M. Hydrometallurgical approach for leaching of metals from copper rich side stream originating from base metal production. Metals 2018, 8, 40. [Google Scholar] [CrossRef]
- Khodadoust, A.P.; Reddy, K.R.; Maturi, K. Effect of different extraction agents on metal and organic contaminant removal from a field soil. J. Hazard. Mater. 2005, 117, 15–24. [Google Scholar] [CrossRef]
- Astuti, W.; Hirajima, T.; Sasaki, K.; Okibe, N. Comparison of effectiveness of citric acid and other acids in leaching of low-grade Indonesian saprolitic ores. Miner. Eng. 2016, 85, 1–16. [Google Scholar] [CrossRef]
- Ujaczki, É.; Zimmermann, Y.S.; Gasser, C.A.; Molnár, M.; Feigl, V.; Lenz, M. Red mud as secondary source for critical raw materials—Extraction study. J. Chem. Technol. Biotechnol. 2017, 92, 2835–2844. [Google Scholar] [CrossRef]
- Najafi, M.; Rostamian, R.; Rafati, A.A. Chemically modified silica gel with thiol group as an adsorbent for retention of some toxic soft metal ions from water and industrial effluent. Chem. Eng. J. 2011, 168, 426–432. [Google Scholar] [CrossRef]
- Terry, B. The acid decomposition of silicate minerals part I. Reactivities and modes of dissolution of silicates. Hydrometallurgy 1983, 10, 135–150. [Google Scholar] [CrossRef]
- Shahid, M.; Dumat, C.; Pourrut, B.; Silvestre, J.; Laplanche, C.; Pinelli, E. Influence of EDTA and citric acid on lead-induced oxidative stress to Vicia faba roots. J. Soils Sediments 2014, 14, 835–843. [Google Scholar] [CrossRef]
- Davris, P.; Stopic, S.; Balomenos, E.; Panias, D.; Paspaliaris, I.; Friedrich, B. Leaching of rare earth elements from eudialyte concentrate by suppressing silica gel formation. Miner. Eng. 2017, 108, 115–122. [Google Scholar] [CrossRef]
- Kierczak, J.; Pietranik, A. Mineralogy and composition of historical Cu slags from the Rudawy Janowickie mountains, Southwestern Poland. Can. Miner. 2011, 49, 1281–1296. [Google Scholar] [CrossRef]
- Kobayashi, M.; Sawada, A.; Tani, Y.; Soma, M.; Tanaka, A.; Honma, T.; Seyama, H.; Theng, B.K.G. Acid dissolution of olivines, feldspars and dunite. Water. Air. Soil Pollut. 2001, 130, 757–762. [Google Scholar] [CrossRef]
- Wang, H.; Bigham, J.M.; Tuovinen, O.H. Formation of schwertmannite and its transformation to jarosite in the presence of acidophilic iron-oxidizing microorganisms. Mater. Sci. Eng. C 2006, 26, 588–592. [Google Scholar] [CrossRef]
- De Michelis, I.; Ferella, F.; Karakaya, E.; Beolchini, F.; Vegliò, F. Recovery of zinc and manganese from alkaline and zinc-carbon spent batteries. J. Power Sour. 2007, 172, 975–983. [Google Scholar] [CrossRef]
- Schippers, A. Bioleaching of copper slag material. Solid State Phenom. 2017, 262, 61–64. [Google Scholar] [CrossRef]
- Wang, R.; Lin, J.Q.; Liu, X.M.; Pang, X.; Zhang, C.J.; Yang, C.L.; Gao, X.Y.; Lin, C.M.; Li, Y.Q.; Li, Y.; et al. Sulfur oxidation in the acidophilic autotrophic Acidithiobacillus spp. Front. Microbiol. 2019, 9, 1–20. [Google Scholar] [CrossRef]
- Suzuki, I.; Silver, M. The initial product and properties of the sulfur-oxidizing enzyme of thiobacilli. BBA Enzymol. Biol. Oxid. 1966, 122, 22–33. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Särkijärvi, S.; Puhakka, J.A.; Peuraniemi, E.; Junnikkala, S.; Tuovinen, O.H. Chemical and bacterial leaching of metals from a smelter slag in acid solutions. Hydrometallurgy 2016, 159, 46–53. [Google Scholar] [CrossRef]
- Yin, H.; Zhang, X.; Li, X.; He, Z.; Liang, Y.; Guo, X.; Hu, Q.; Xiao, Y.; Cong, J.; Ma, L.; et al. Whole-genome sequencing reveals novel insights into sulfur oxidation in the extremophile Acidithiobacillus thiooxidans. BMC Microbiol. 2014, 179, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Barreira, R.P.R.; Villar, L.D.; Garcia, O. Tolerance to copper and zinc of Acidithiobacillus thiooxidans isolated from sewage sludge. World J. Microbiol. Biotechnol. 2005, 21, 89–91. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Särkijärvi, S.; Puhakka, J.A.; Peuraniemi, E.; Junnikkala, S.; Tuovinen, O.H. Solid phase changes in chemically and biologically leached copper smelter slag. Miner. Eng. 2017, 106, 97–101. [Google Scholar] [CrossRef]
- Bevilaqua, D.; Garcia, O.; Tuovinen, O.H. Oxidative dissolution of bornite by Acidithiobacillus ferrooxidans. Process Biochem. 2010, 45, 101–106. [Google Scholar] [CrossRef]
- Pathak, A.; Dastidar, M.G.; Sreekrishnan, T.R. Bioleaching of heavy metals from sewage sludge: A review. J. Environ. Manag. 2009, 90, 2343–2353. [Google Scholar] [CrossRef] [PubMed]
- Ettler, V.; Mihaljevic, M.; Touray, J.C.; Piantone, P. Leaching of polished sections: An integrated approach for studying the liberation of heavy metals from lead-zinc metallurgical slags. Bull. Soc. Geol. Fr. 2002, 173, 161–169. [Google Scholar] [CrossRef]
- Vítková, M.; Ettler, V.; Johan, Z.; Kříbek, B.; Šebek, O.; Mihaljevič, M. Primary and secondary phases in copper-cobalt smelting slags from the Copperbelt Province, Zambia. Miner. Mag. 2010, 74, 581–600. [Google Scholar] [CrossRef]
- Seyama, H.; Soma, M.; Tanaka, A. Surface characterization of acid-leached olivines by X-ray photoelectron spectroscopy. Chem. Geol. 1996, 129, 209–216. [Google Scholar] [CrossRef]
- Thomassin, J.H.; Touray, J.C. Etude des premiers stades de l’interaction eau-verre basaltique: Données de la spectrométrie de photoélectrons (XPS) et de la microscopie électronique à balayage. Bull. Mineral. 2019, 102, 594–599. [Google Scholar] [CrossRef]
- Makoi, J.H.J.R.; Verplancke, H. Effect of gypsum placement on the physical chemical properties of a saline sandy loam soil. Aust. J. Crop Sci. 2010, 4, 556–563. [Google Scholar]
- Pathak, A.; Morrison, L.; Healy, M.G. Catalytic potential of selected metal ions for bioleaching, and potential techno-economic and environmental issues: A critical review. Bioresour. Technol. 2017, 229, 211–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
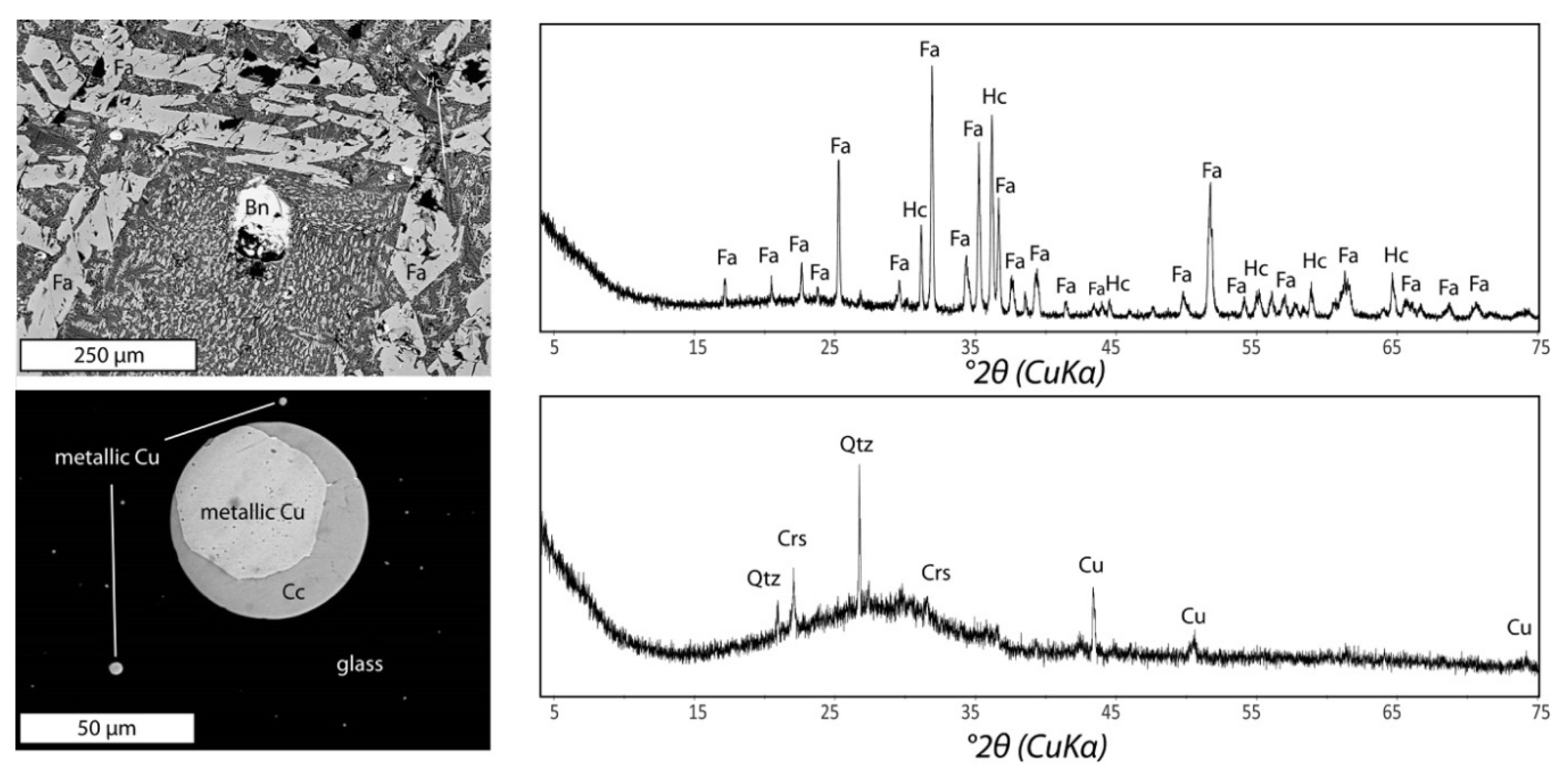
| Sample ID | CS | AS |
|---|---|---|
| Major and minor elements (wt. %) | ||
| SiO2 | 38.26 | 48.33 |
| TiO2 | 0.58 | 0.57 |
| Al2O3 | 9.29 | 11.81 |
| FeO | 44.33 | 12.11 |
| MnO | 0.25 | 0.49 |
| MgO | 2.97 | 2.10 |
| CaO | 0.68 | 17.47 |
| Na2O | 0.19 | 0.21 |
| K2O | 1.27 | 4.59 |
| Minor and trace elements (mg kg−1) | ||
| Cu | 3761 | 19,724 |
| Pb | 55 | 53 |
| Zn | 3628 | 587 |
| Phase composition | ||
| Fayalite (Fe2+2SiO4) ++ | Glass ++ | |
| Glass ++ | Chalcocite (Cu2S) + | |
| Hercynite (FeAl2O4) + | Quartz (SiO2) + | |
| Bornite (Cu5FeS4) + | Cristobalite (SiO2) + | |
| Pyrrhotite (Fe(x−1)S) + | Metallic Cu + | |
| Chalcopyrite (CuFeS2) (+) | ||
| Final pH Values in the Leachates | |||
|---|---|---|---|
| Solution Used as Extracting Agent | Operating Parameters | Crystalline Slag | Amorphous Slag |
| Sulfuric Acid (0.1–1 M) | |||
| Sulfuric acid | 0.1 M, PD: 1%, T: 48 h | 1.22 | 1.45 |
| 0.1 M, PD: 5%, T: 48 h | 1.31 | 1.60 | |
| 0.1 M, PD: 10%, T: 48 h | 1.36 | 1.68 | |
| Sulfuric acid | 0.5 M, PD: 1%, T: 48 h | 0.51 | 0.56 |
| 0.5 M, PD: 5%, T: 48 h | 0.52 | 0.62 | |
| 0.5 M, PD: 10%, T: 48 h | 0.60 | 0.70 | |
| Sulfuric acid | 1 M, PD: 1%, T: 48 h | 0.38 | 0.31 |
| 1 M, PD: 5%, T: 48 h | 0.40 | 0.35 | |
| 1 M, PD: 10%, T: 48 h | 0.39 | 0.36 | |
| Normality Equivalent Acids (2N) | |||
| Hydrochloric acid | PD: 1%, T: 48 h | 0.04 | 0.05 |
| PD: 2%, T: 48 h | 0.08 | 0.17 | |
| Sulfuric acid | PD: 1%, T: 48 h | 0.38 | 0.31 |
| PD: 2%, T: 48 h | 0.41 | 0.40 | |
| Nitric acid | PD: 1%, T: 48 h | 0.12 | 0.05 |
| PD: 2%, T: 48 h | 0.11 | 0.11 | |
| Citric acid | PD: 1%, T: 48 h | 1.99 | 2.03 |
| PD: 2%, T: 48 h | 2.08 | 2.29 | |
| Oxalic acid | PD: 1%, T: 48 h | 0.77 | 1.48 |
| PD: 2%, T: 48 h | 0.80 | 2.19 | |
| (Bio)leaching | |||
| Growth medium (sterile) | PD: 1%, T: 21 days | 3.98 | 4.83 |
| PD: 2%, T: 21 days | 3.88 | 4.65 | |
| PD: 3%, T: 21 days | 3.85 | 4.60 | |
| Growth medium + bacteria | PD: 1%, T: 21 days | 0.90 | 0.62 |
| PD: 2%, T: 21 days | 1.15 | 0.71 | |
| PD: 3%, T: 21 days | 1.43 | 0.98 | |
| Crystalline Slag | Amorphous Slag | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Solution Used as Extracting Agent | Treatment with the Best Operating Parameters | Fe 0.09 $ kg−1 | Cu 6.5 $ kg−1 | Zn 2.8 $ kg−1 | Pb 2.1 $ kg−1 | Sum | Fe 0.09 $ kg−1 | Cu 6.5 $ kg−1 | Zn 2.8 $ kg−1 | Pb 2.1 $ kg−1 | Fe 0.09 $ kg−1 |
| Economic Profit Dollars [$] Per Ton of Slag Treated | |||||||||||
| Hydrochloric acid | PD: 1%, T: 48 h | 20.23 | 7.47 | 7.33 | 0.07 | 35.10 | 6.88 | 118.48 | 1.33 | 0.10 | 126.80 |
| Sulfuric acid | PD: 1%, T: 48 h | 15.49 | 0.35 | 6.09 | 0.03 | 21.95 | 4.42 | 81.19 | 0.88 | 0.02 | 86.51 |
| Nitric acid | PD: 1%, T: 48 h | 16.28 | 8.19 | 5.62 | 0.07 | 30.16 | 5.51 | 101.68 | 1.36 | 0.10 | 108.66 |
| Citric acid | PD: 1%, T: 48 h | 2.76 | 4.20 | 1.41 | 0.05 | 8.42 | 7.04 | 109.51 | 1.39 | 0.11 | 118.05 |
| Oxalic acid | PD: 1%, T: 48 h | 0.51 | 0.36 | 0.01 | 0.02 | 0.89 | 6.12 | 16.75 | 0.64 | 0.00 | 23.52 |
| Growth medium (sterile) | PD: 1%, T: 21 days | 0.00 | 0.42 | 0.35 | 0.00 | 0.77 | 0.00 | 40.58 | 0.30 | 0.00 | 40.89 |
| Growth medium + bacteria | PD: 1%, T: 21 days | 27.00 | 12.88 | 6.95 | 0.00 | 46.84 | 6.89 | 126.57 | 1.20 | 0.01 | 134.67 |
| Evaluated Parameter | Chemical Treatment | Biological Treatment |
|---|---|---|
| Chemicals consumption during extraction | high | low |
| Processing time | short | long |
| Metal yield | comparable | |
| Environmental impact | high | low |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potysz, A.; Kierczak, J. Prospective (Bio)leaching of Historical Copper Slags as an Alternative to Their Disposal. Minerals 2019, 9, 542. https://doi.org/10.3390/min9090542
Potysz A, Kierczak J. Prospective (Bio)leaching of Historical Copper Slags as an Alternative to Their Disposal. Minerals. 2019; 9(9):542. https://doi.org/10.3390/min9090542
Chicago/Turabian StylePotysz, Anna, and Jakub Kierczak. 2019. "Prospective (Bio)leaching of Historical Copper Slags as an Alternative to Their Disposal" Minerals 9, no. 9: 542. https://doi.org/10.3390/min9090542





