Properties of Inorganic Polymers Produced from Brick Waste and Metallurgical Slag
Abstract
1. Introduction
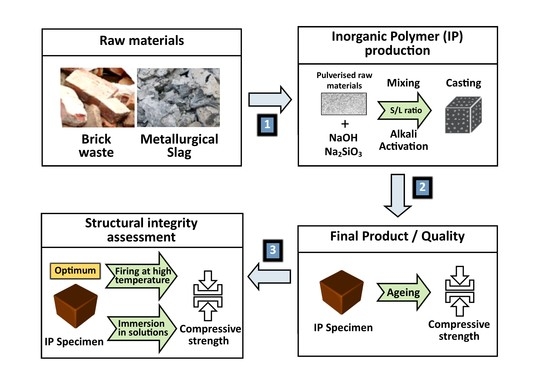
2. Materials and Methods
3. Results
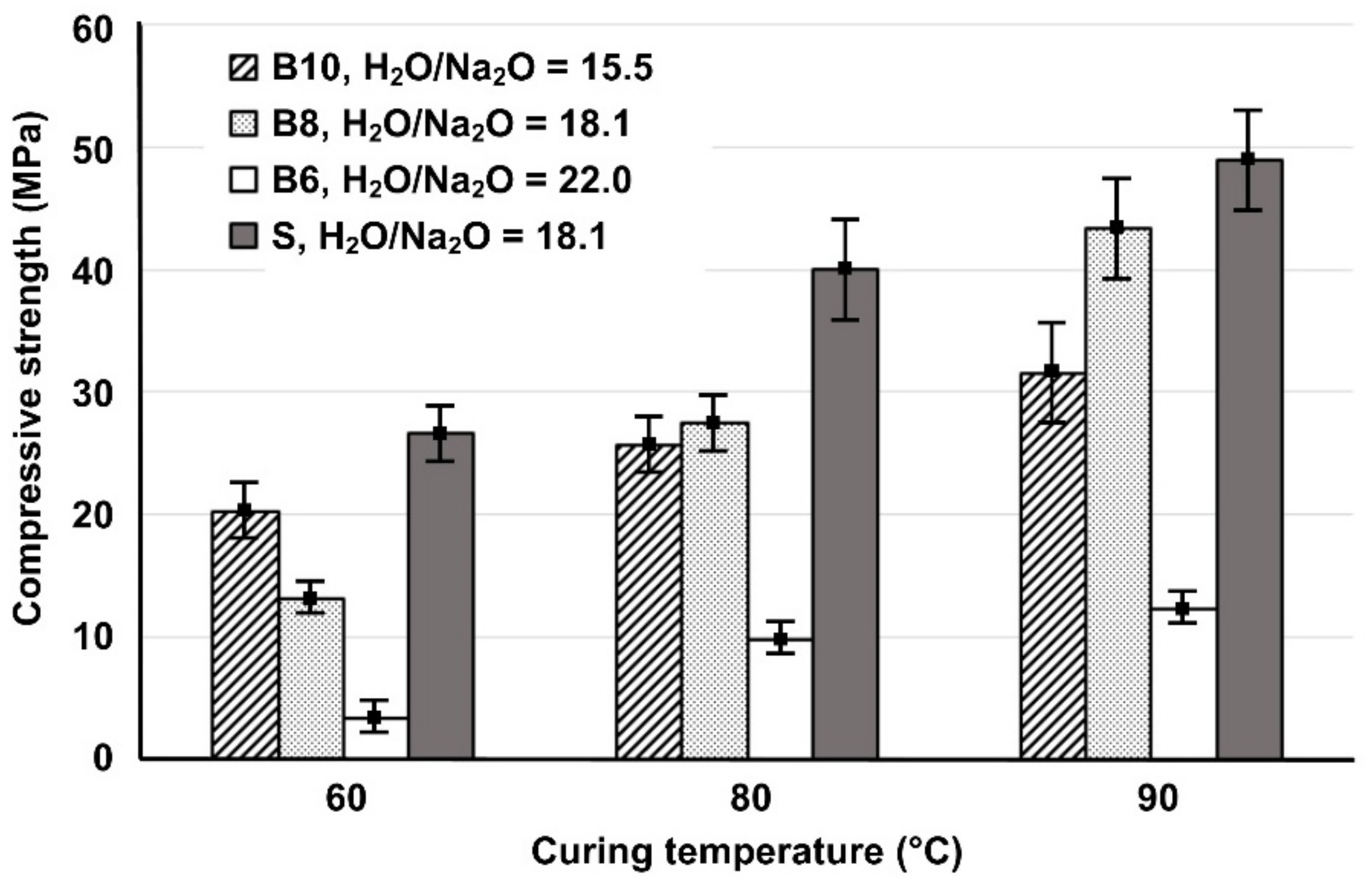
3.1. Effect of H2O/Na2O Molar Ratio in the Activating Solutions and Curing Temperatures on the Compressive Strength
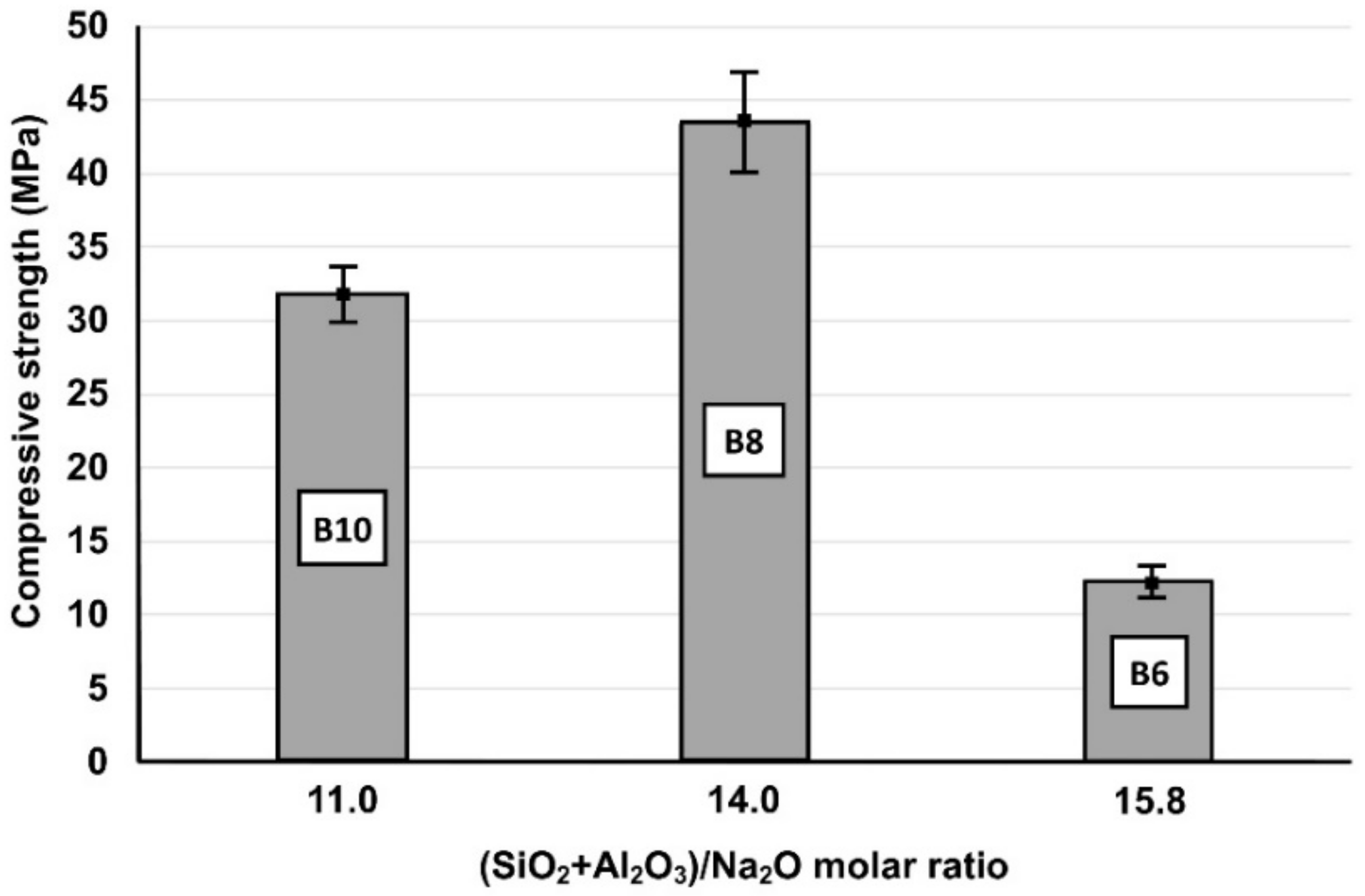
3.2. The Effects of Molar Ratios of Oxides in the Initial Paste
3.3. Morphology of Selected IPs
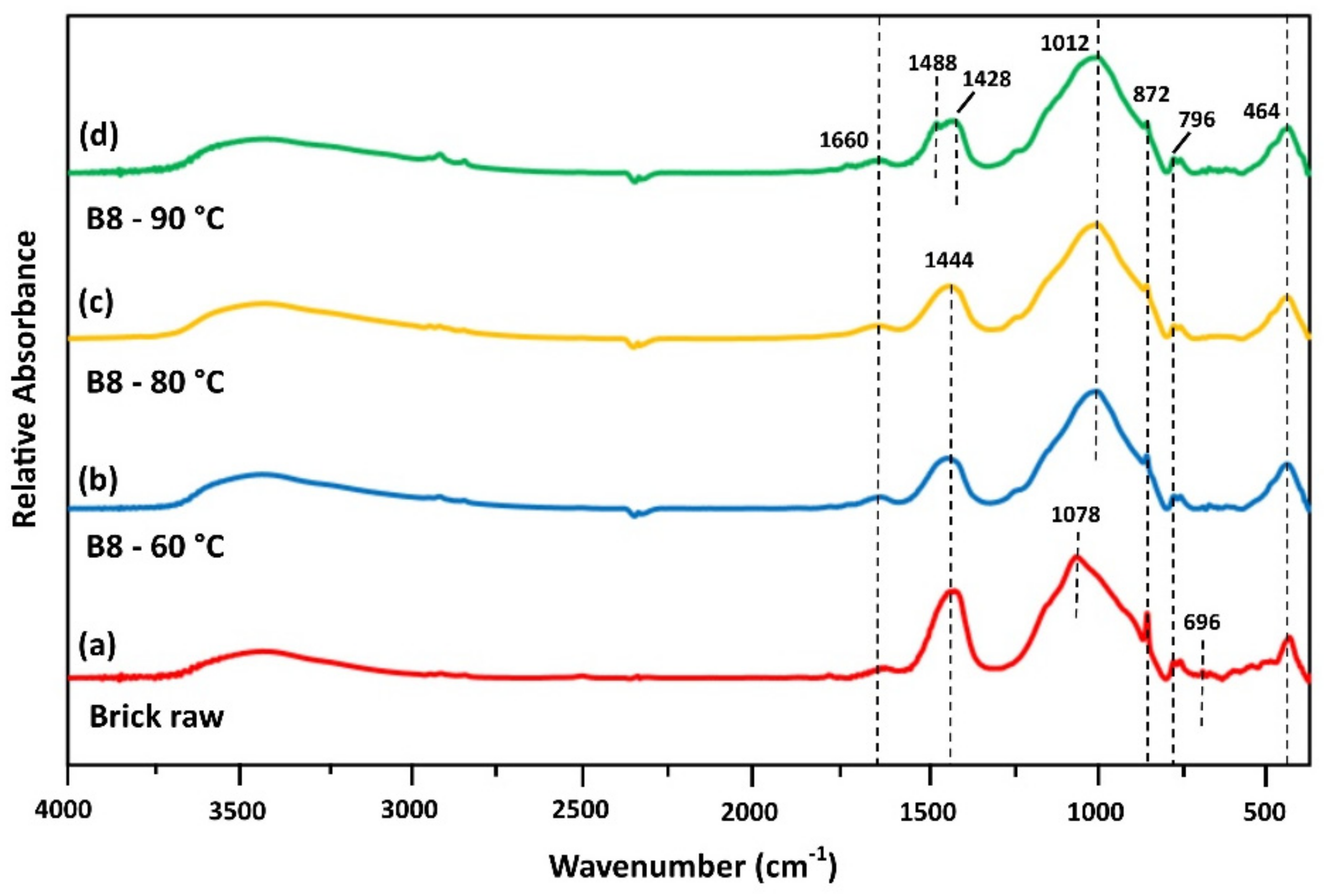
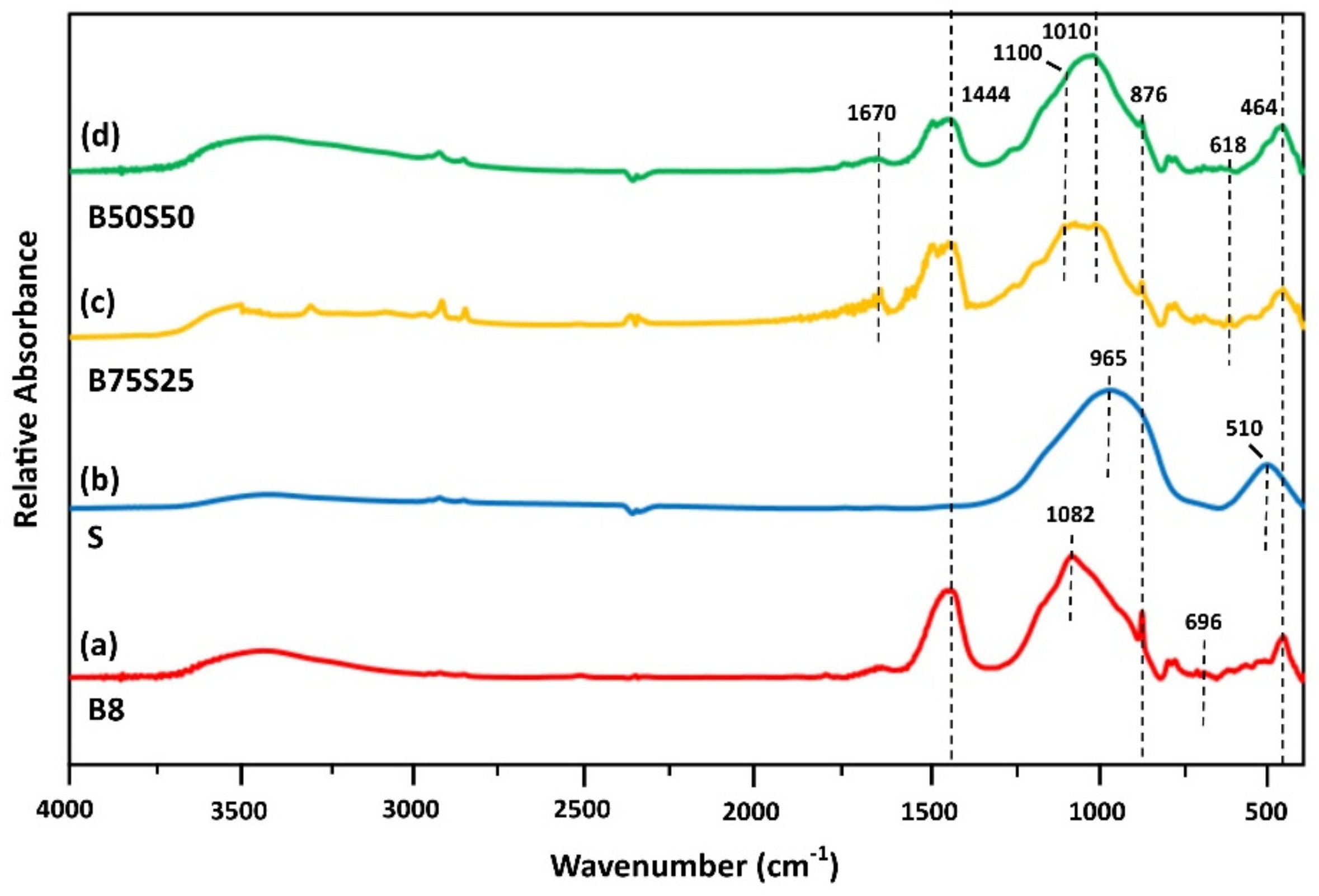
3.3.1. FTIR Analysis
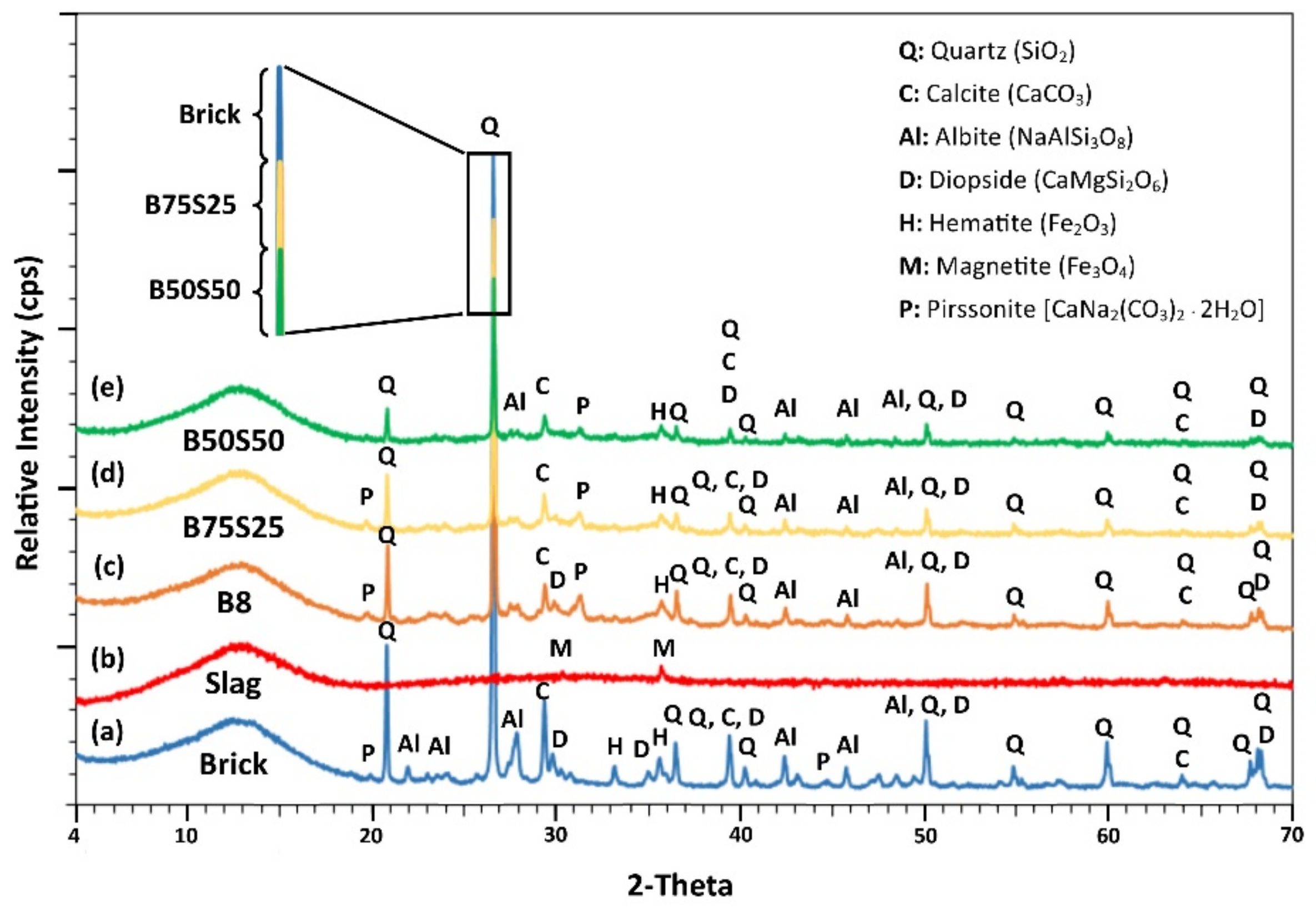
3.3.2. Mineralogical Studies
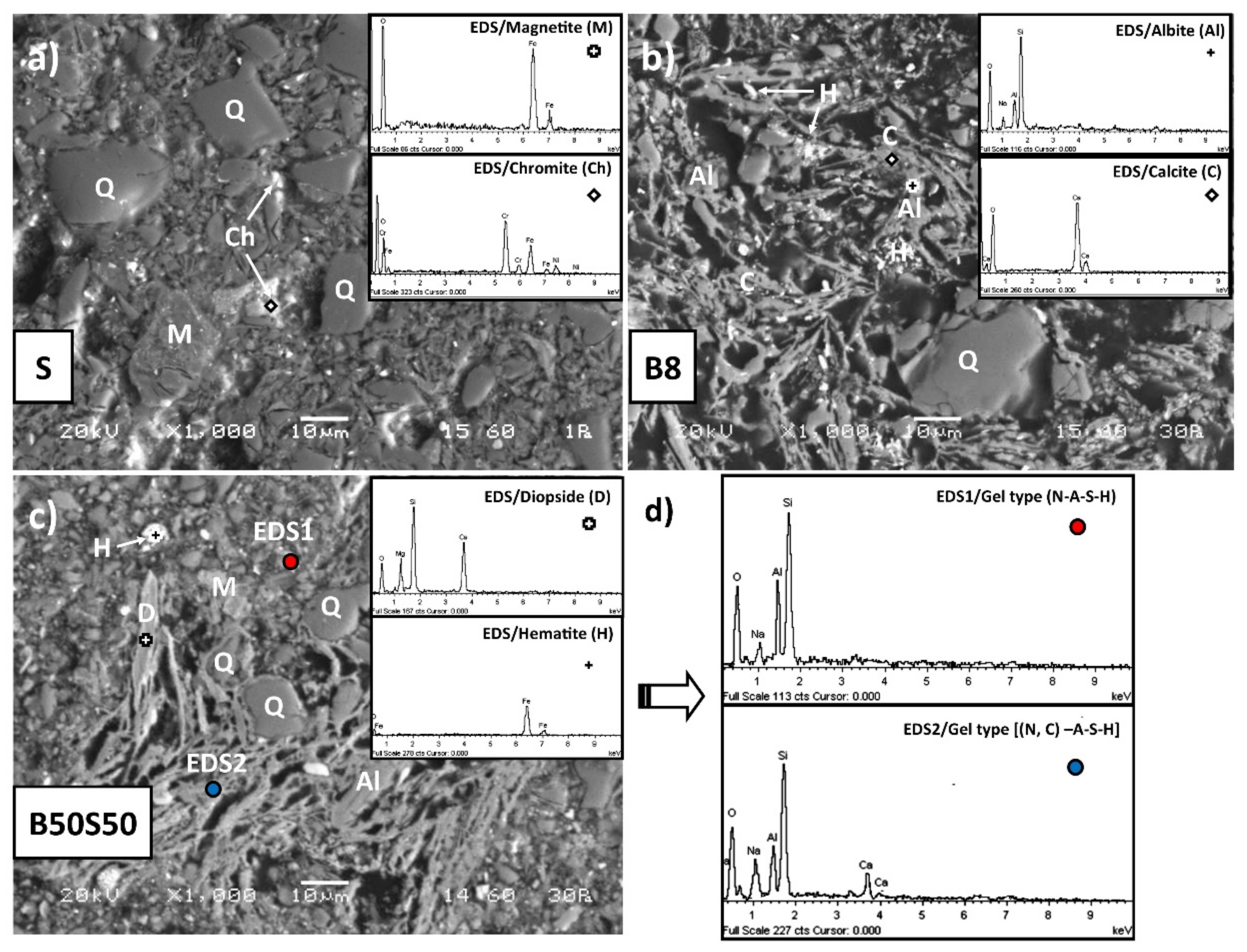
3.3.3. SEM Analysis
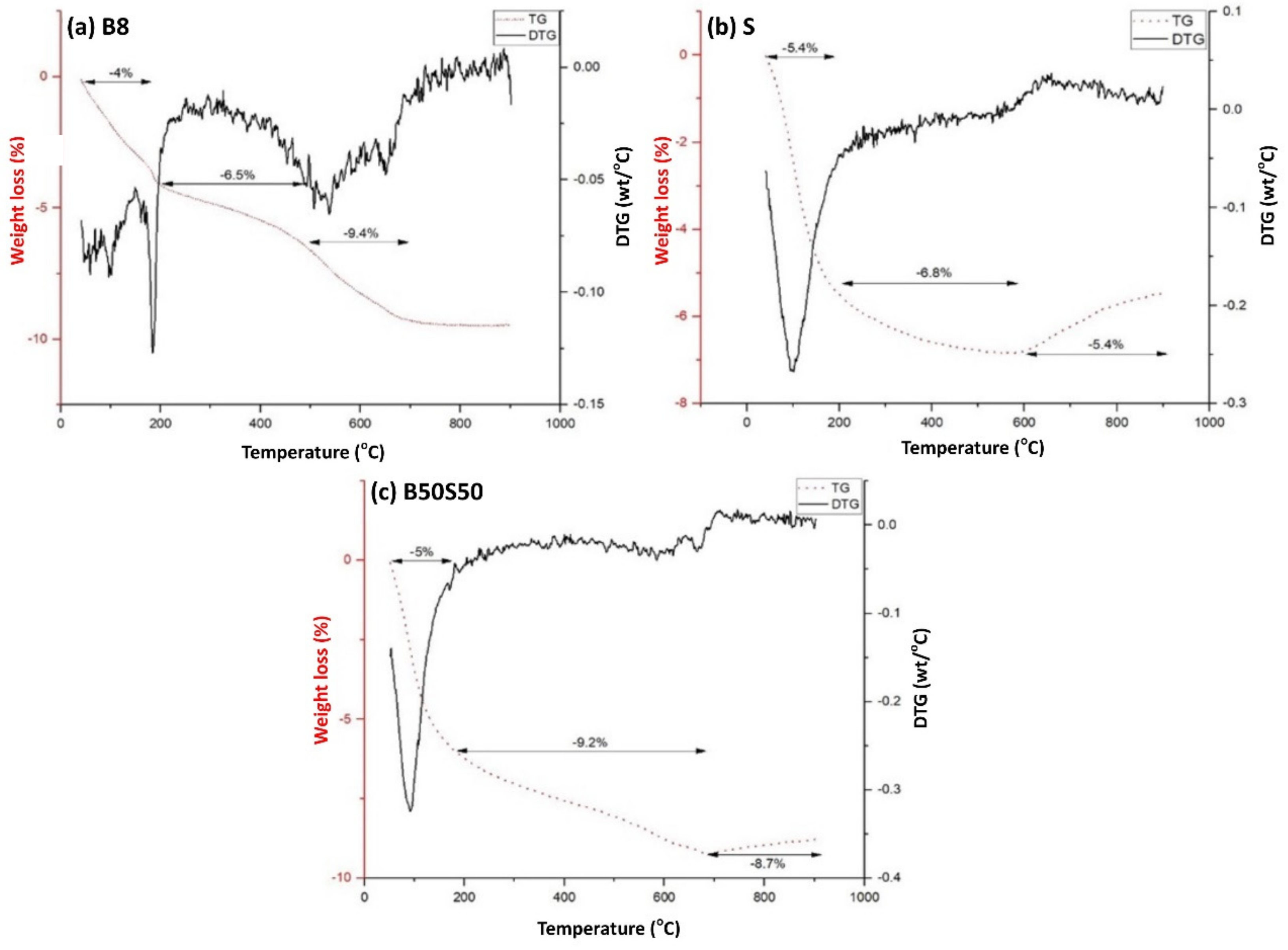
3.3.4. TG analysis
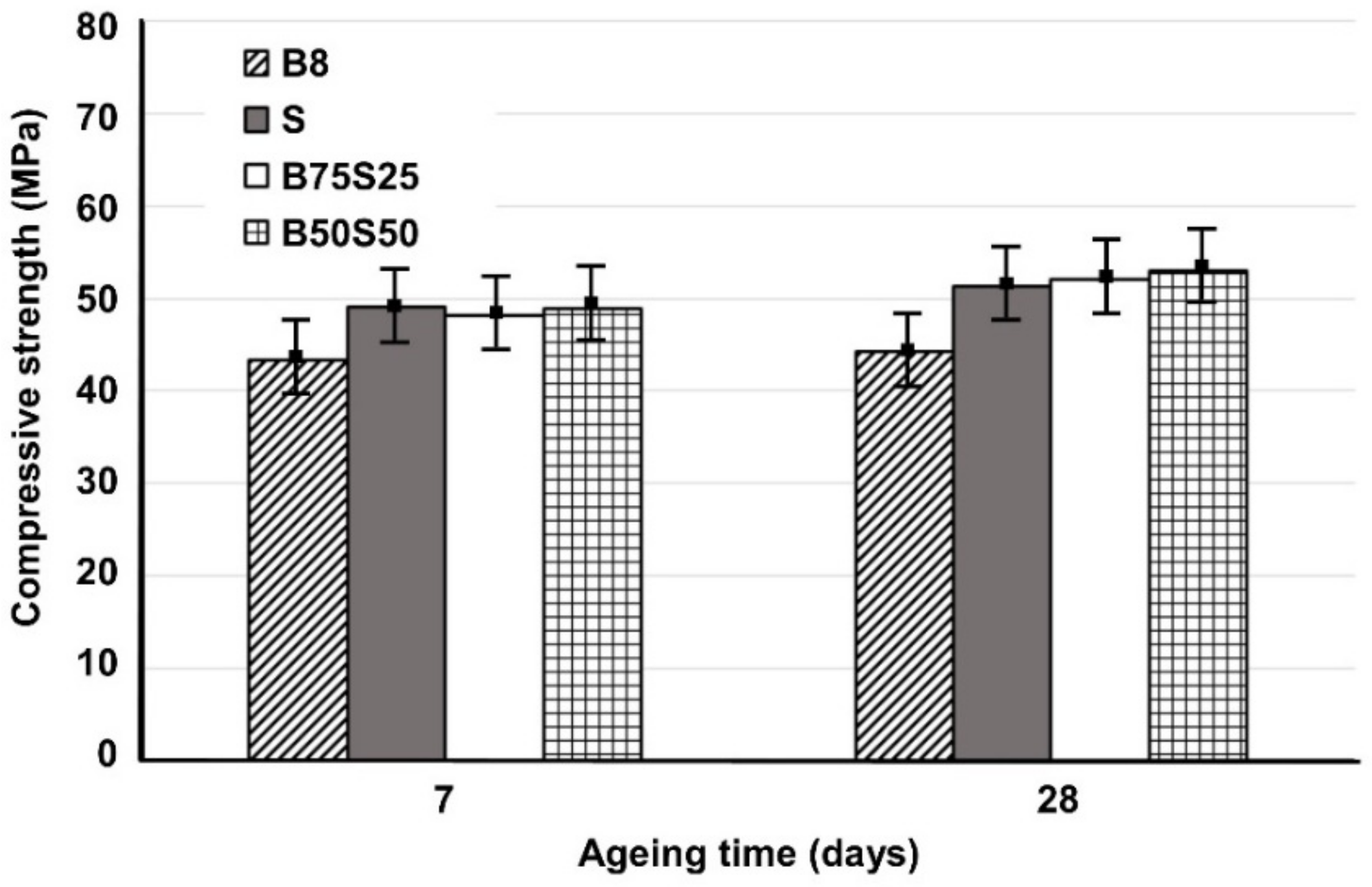
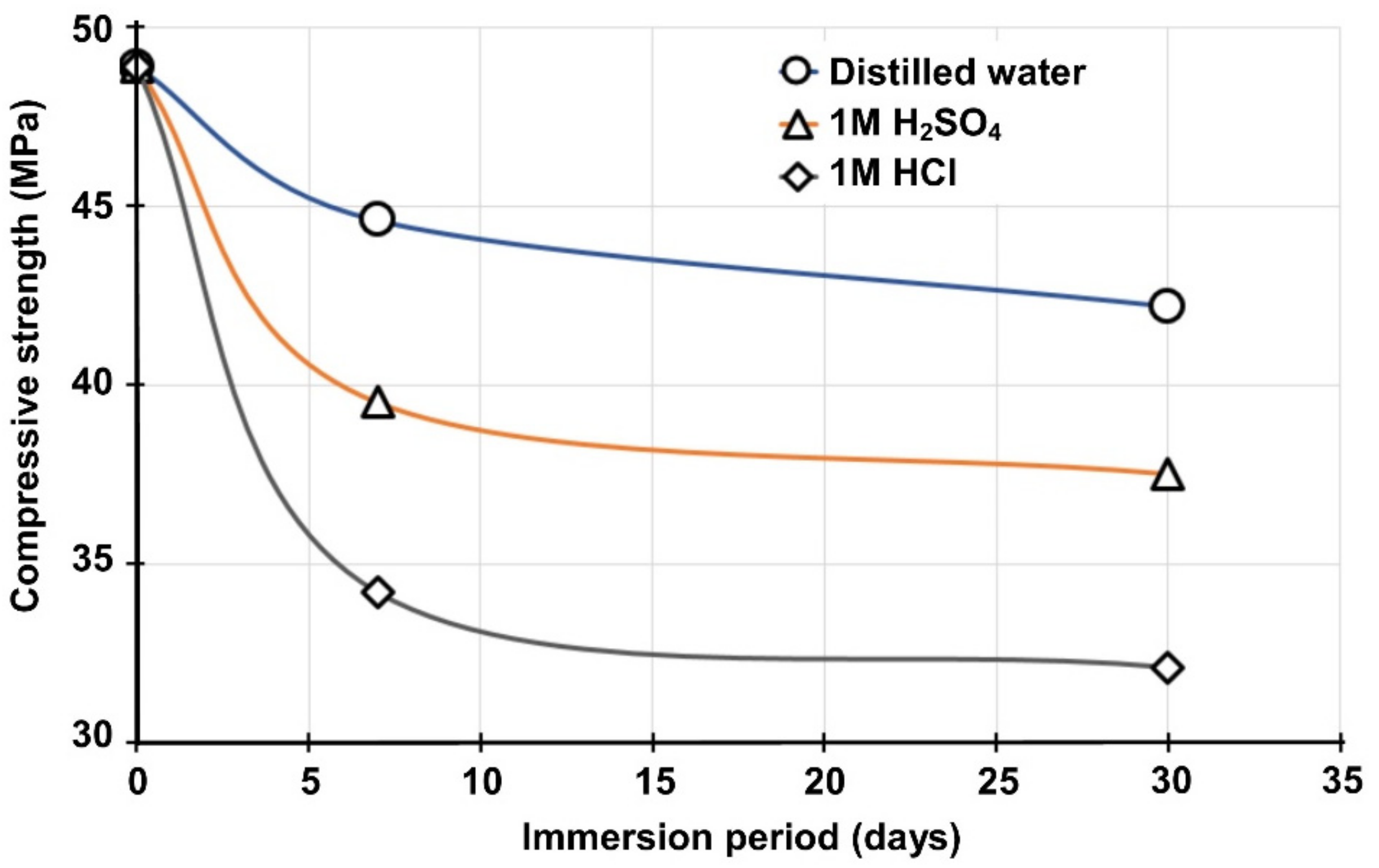
3.4. Durability Performance of Selected IPs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Panizza, M.; Natali, M.; Garbin, E.; Tamburini, S.; Secco, M. Assessment of geopolymers with Construction and Demolition Waste (CDW) aggregates as a building material. Constr. Build. Mater. 2018, 181, 119–133. [Google Scholar] [CrossRef]
- Wong, C.L.; Mo, K.H.; Yap, S.P.; Alengaram, U.J.; Ling, T.C. Potential use of brick waste as alternate concrete-making materials: A review. J. Clean. Prod. 2018, 195, 226–239. [Google Scholar] [CrossRef]
- Robayo-Salazar, R.A.; Rivera, J.F.; de Gutiérrez, R.M. Alkali-activated building materials made with recycled construction and demolition wastes. Constr. Build. Mater. 2017, 149, 130–138. [Google Scholar] [CrossRef]
- Kioupis, D.; Kavakakis, C.; Tsivilis, S.; Kakali, G. Synthesis and characterization of porous fly ash-based geopolymers using Si as foaming agent. Adv. Mater. Sci. Eng. 2018. [Google Scholar] [CrossRef]
- Komnitsas, K. Potential of geopolymer technology towards green buildings and sustainable cities. Procedia Eng. 2011, 21, 1023–1032. [Google Scholar] [CrossRef]
- Keawpapasson, P.; Tippayasam, C.; Ruangjan, S.; Thavorniti, P.; Panyathanmaporn, T.; Fontaine, A.; Leonelli, C.; Chaysuwan, D. Metakaolin-Based Porous Geopolymer with Aluminium Powder. Key Eng. Mater. 2014, 608, 132–138. [Google Scholar] [CrossRef]
- Fořt, J.; Vejmelková, E.; Koňáková, D.; Alblová, N.; Čáchová, M.; Keppert, M.; Rovnaníková, P.; Černý, R. Application of waste brick powder in alkali-activated aluminosilicates: Functional and environmental aspects. J. Clean. Prod. 2018, 194, 714–725. [Google Scholar] [CrossRef]
- Ghanbari, M.; Hadiana, A.M.; Nourbakhsh, A.A. Effect of processing parameters on compressive strength of metakaolinite based geopolymers: Using DOE approach. Procedia Mater. Sci. 2015, 11, 711–716. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Perdikatsis, V. Geopolymerisation of low calcium ferronickel slags. J. Mater. Sci. 2007, 42, 3073–3082. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D. Geopolymerisation: A review and prospects for the minerals industry. Miner. Eng. 2007, 20, 1261–1277. [Google Scholar] [CrossRef]
- Hamaideh, A.; Komnitsas, Κ.; Esaifan, Μ.; Al-Kafawein, J.K.; Rahier, H.; Alshaaer, Μ. Advantages of applying a steam curing cycle for the production of kaolinite-based geopolymers. Arab. J. Sci. Eng. 2014, 39, 7591–7597. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers and geopolymeric materials. J. Therm. Anal. 1991, 35, 429–441. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Vlachou, A.; Bartzas, G.; Galetakis, M. Effect of synthesis parameters on the quality of construction and demolition wastes (CDW) geopolymers. Adv. Powder Technol. 2015, 26, 368–376. [Google Scholar] [CrossRef]
- Alshaaer, M.; Zaharaki, D.; Komnitsas, K. Microstructural characteristics and adsorption potential of zeolitic tuff-metakaolin geopolymers. Desal. Water Treat. 2015, 56, 338–345. [Google Scholar] [CrossRef]
- Arnold, M.C.; de Vargas, A.S.; Bianchini, L. Study of electric-arc furnace dust (EAFD) in fly ash and rice husk ash-based geopolymers. Adv. Powder Technol. 2017, 28, 2023–2034. [Google Scholar] [CrossRef]
- Xia, M.; Muhammad, F.; Zeng, L.; Li, S.; Huang, X. Solidification/stabilization of lead-zinc smelting slag in composite based geopolymer. J. Clean. Prod. 2019, 209, 1206–1215. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Bartzas, G. Effect of sulphate and nitrate anions on heavy metal immobilisation in ferronickel slag geopolymers. Appl. Clay Sci. 2013, 73, 103–109. [Google Scholar] [CrossRef]
- Zaharaki, D.; Komnitsas, K.; Perdikatsis, V. Use of analytical techniques for identification of inorganic polymer gel composition. J. Mater. Sci. 2010, 45, 2715–2724. [Google Scholar] [CrossRef]
- Bernal, S.A.; Rodríguez, E.D.; Kirchheim, A.P.; Provis, J.L. Management and valorisation of wastes through use in producing alkali-activated cement materials. J. Chem. Technol. Biotechnol. 2016, 91, 2365–2388. [Google Scholar] [CrossRef]
- Zaharaki, D.; Galetakis, M.; Komnitsas, K. Valorization of construction and demolition (C&D) and industrial wastes through alkali activation. Constr. Build. Mater. 2016, 121, 686–693. [Google Scholar] [CrossRef]
- Rakhimova, N.R.; Rakhimov, R.Z. Alkali-activated cements and mortars based on blast furnace slag and red clay brick waste. Mater. Des. 2015, 85, 324–331. [Google Scholar] [CrossRef]
- Komnitsas, K. Co-valorization of marine sediments and construction & demolition wastes through alkali activation. J. Environ. Chem. Eng. 2016, 4, 4661–4669. [Google Scholar] [CrossRef]
- Xu, H.; Van Deventer, J.S.J. The geopolymerisation of alumino-silicate minerals. Int. J. Miner. Process. 2000, 59, 247–266. [Google Scholar] [CrossRef]
- Liu, Ζ.; El-Tawil, S.; Hansen, W.; Wang, F. Effect of slag cement on the properties of ultra-high performance concrete. Constr. Build. Mater. 2018, 190, 830–837. [Google Scholar] [CrossRef]
- Bougara, A.; Lynsdale, C.; Neil, B.; Milestone, N.B. The influence of slag properties, mix parameters and curing temperature on hydration and strength development of slag/cement blends. Constr. Build. Mater. 2018, 187, 339–347. [Google Scholar] [CrossRef]
- Zaharaki, D.; Komnitsas, K. Effect of additives on the compressive strength of slag-based inorganic polymers. Glob. Nest J. 2009, 11, 137–146. [Google Scholar] [CrossRef]
- Perera, D.S.; Cashion, J.D.; Blackford, G.M.; Zhang, Z.; Vance, E.R. Fe speciation in geoplymers with Si/Al molar ratio of ~2. J. Eur. Ceram. Soc. 2007, 27, 2697–2703. [Google Scholar] [CrossRef]
- Lemougna, P.N.; MacKenzie, K.J.D.; Jameson, G.N.L.; Rahier, H.; Chinje Melo, U.F. The role of iron in the formation of inorganic polymers (geopolymers) from volcanic ash: A 57Fe Mössbauer spectroscopy study. Int. J. Mater. Sci. 2013, 48, 5280–5286. [Google Scholar] [CrossRef]
- Peys, A.; White, C.E.; Rahier, H.; Blanpain, B.; Pontikes, Y. Alkali-activation of CaO-FeOx-SiO2 slag. Formation mechanism from in-situ X-ray total scattering. Cem. Concr. Res. 2019, 122, 179–188. [Google Scholar] [CrossRef]
- Akcil, A.; Agcasulu, Ι.; Swain, Β. Valorization of waste LCD and recovery of critical raw material for circular economy: A review. Resour. Conserv. Recy. 2019, 149, 622–637. [Google Scholar] [CrossRef]
- Baldassarre, B.; Schepers, M.; Bocken, N.; Cuppen, E.; Korevaar, G.; Calabretta, G. Industrial Symbiosis: Towards a design process for eco-industrial clusters by integrating Circular Economy and Industrial Ecology perspectives. J. Clean. Prod. 2019, 216, 446–460. [Google Scholar] [CrossRef]
- Komnitsas, K.; Bartzas, G.; Karmali, V.; Petrakis, E.; Kurylak, W.; Pietek, G.; Kanasiewicz, J. Assessment of Alkali Activation Potential of a Polish Ferronickel Slag. Sustainability 2019, 11, 1863. [Google Scholar] [CrossRef]
- EN 13755, Natural Stone Test Methods—Determination of Water Absorption at Atmospheric Pressure; British Standards Institution: London, UK, 2008.
- Tuyan, M.; Andiç-Çakir, Ö.; Ramyar, K. Effect of alkali activator concentration and curing condition on strength and microstructure of waste clay brick powder-based geopolymer. Compos. Part B Eng. 2018, 135, 242–252. [Google Scholar] [CrossRef]
- Vásquez, A.; Cárdenas, V.; Robayo, R.A.; de Gutiérrez, R.M. Geopolymer based on concrete demolition waste. Adv. Powder Technol. 2016, 27, 1173–1179. [Google Scholar] [CrossRef]
- Maragkos, I.; Giannopoulou, I.P.; Panias, D. Synthesis of ferronickel slag-based geopolymers. Miner. Eng. 2009, 22, 196–203. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Perdikatsis, V. Effect of synthesis parameters on the compressive strength of low-calcium ferronickel slag inorganic polymers. J. Hazard. Mater. 2009, 161, 760–768. [Google Scholar] [CrossRef]
- Reig, L.; Soriano, L.; Borrachero, M.V.; Monzo, J.; Paya, J. Influence of calcium aluminate cement (CAC) on alkaline activation of red clay brick waste (RCBW). Cem. Concr. Compos. 2016, 65, 177–185. [Google Scholar] [CrossRef]
- Robayo, R.; Mulford, A.; Munera, J.; Mejia de Gutiérrez, R. Alternative cements based on alkali-activated red clay brick waste. Constr. Build. Mater. 2016, 128, 163–169. [Google Scholar] [CrossRef]
- Rovnanik, P.; Reznik, B.; Rovnanikovà, P. Blended alkali-activated fly ash/brick powder materials. Proc. Eng. 2016, 151, 108–113. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies, 3rd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2001. [Google Scholar]
- Yu, P.; Kirkpatrick, R.J.; Poe, B.; Mcmillan, P.F.; Cong, X. Structure of Calcium Silicate Hydrate (C–S–H): Near-, Mid-, and Far-Infrared Spectroscopy. J. Am. Ceram. Soc. 1999, 18, 742–748. [Google Scholar] [CrossRef]
- Gervais, F.; Blin, A.; Massiot, D.; Coutures, J.P.; Chopinet, M.H.; Naudin, F. Infrared reflectivity spectroscopy of silicate glasses. J. Non Cryst. Solids 1987, 89, 384–401. [Google Scholar] [CrossRef]
- Muthuvel, I.; Gowthami, K.; Thirunarayanan, G.; Suppuraj, P.; Krishnakumar, B.; do Nascimento Sobral, J.F.; Swaminathan, M. Graphene oxide–Fe2V4O13 hybrid material as highly efficient hetero-Fenton catalyst for degradation of methyl orange. Int. J. Ind. Chem. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Sedira, N.; Castro-Gomes, J.; Magrinho, M. Red clay brick and tungsten mining waste-based alkali-activated binder: Microstructural and mechanical properties. Constr. Build. Mater. 2018, 190, 1034–1048. [Google Scholar] [CrossRef]
- Jena, S.; Panigrahi, R. Performance assessment of geopolymer concrete with partial replacement of ferrochrome slag as coarse aggregate. Constr. Build. Mater. 2019, 220, 525–537. [Google Scholar] [CrossRef]
- Dimas, D.; Giannopoulou, I.; Panias, D. Polymerization in sodium silicate solutions: A fundamental process in geopolymerization technology. J. Mater. Sci. 2009, 44, 3719–3730. [Google Scholar] [CrossRef]
- Vergés, M.A.; Costo, R.; Roca, A.G.; Marco, J.F.; Goya, G.F.; Serna, C.J.; Morales, M.P. Uniform and water stable magnetite nanoparticles with diameters around the monodomain-multidomain limit. J. Phys. D Appl. Phys. 2008, 41, 134003–134022. [Google Scholar] [CrossRef]
- Ye, X.; Lin, D.; Jiao, Z.; Zhang, L. The thermal stability of nanocrystalline maghemite. J. Phys. D Appl. Phys. 1998, 31, 2739–2744. [Google Scholar] [CrossRef]
- Swaddle, T.W.; Oltmann, P. Kinetics of the magnetite-maghemite-hematite transformation, with special reference to hydrothermal systems. Can. J. Chem. 1980, 58, 1763–1772. [Google Scholar] [CrossRef]
- Mehta, A.; Siddique, R. Sulfuric acid resistance of fly ash based geopolymer concrete. Constr. Build. Mater. 2017, 146, 136–143. [Google Scholar] [CrossRef]
- Lahoti, M.; Tan, K.H.; Yang, E.-H. A critical review of geopolymer properties for structural fire-resistance applications. Constr. Build. Mater. 2019, 221, 514–526. [Google Scholar] [CrossRef]

| Chemical Composition (wt %) | Bricks | Slag |
|---|---|---|
| SiO2 | 59.1 | 32.7 |
| CaO | 17.8 | 3.7 |
| Al2O3 | 10.2 | 8.3 |
| MgO | 1.9 | 2.8 |
| K2O | 1.9 | - |
| Fe2O3 | 7.4 | 43.8 |
| Cr2O3 | - | 3.1 |
| TiO2 | 1.0 | - |
| MnO | 0.1 | 0.4 |
| SO3 | - | 0.5 |
| LOI | 0.2 | - |
| Total | 99.6 | 95.3 |
| Particle Size Distribution (%) | ||
| d90 (μm) | 94.3 | 45.6 |
| d50 (μm) | 16.7 | 8.9 |
| d10 (μm) | 0.5 | 0.4 |
| IP Code 1 | Solids (S) | Liquids (L) | S/L Ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NaOH (M) | Brick | Slag | NaOH | H2O | Na2SiO3 | H2O/Na2O Molar Ratio | SiO2/Na2O Molar Ratio | ||
| B6 | 6 | 70.9 | - | 2.6 | 10.4 | 16.1 | 22.0 | 1.4 | 2.4 |
| B8 | 8 | 71.4 | - | 3.2 | 9.5 | 15.9 | 18.1 | 1.2 | 2.5 |
| B10 | 10 | 69.0 | - | 4.2 | 9.6 | 17.2 | 15.5 | 1.0 | 2.2 |
| S | 8 | - | 81.1 | 2.1 | 6.3 | 10.5 | 18.1 | 1.2 | 4.3 |
| B75S25 | 8 | 56.3 | 18.8 | 2.8 | 8.3 | 13.8 | 18.1 | 1.2 | 3.0 |
| B50S50 | 8 | 38.2 | 38.2 | 2.7 | 7.8 | 13.1 | 18.1 | 1.2 | 3.2 |
| Code | Compressive Strength 1 (MPa) | Molar Ratios | ||
|---|---|---|---|---|
| SiO2/Al2O3 | (SiO2 + Al2O3)/Na2O | H2O/Na2O | ||
| S | 49.0 | 7.4 | 14.0 | 18.3 |
| B6 2 | 12.3 | 10.8 | 15.8 | 21.8 |
| Β8 | 43.4 | 10.9 | 14.0 | 18.2 |
| B10 | 31.5 | 11.0 | 11.0 | 15.5 |
| B75S25 | 45.2 | 10.1 | 15.0 | 18.2 |
| B50S50 | 48.9 | 9.3 | 14.0 | 17.9 |
| Code | Compressive Strength (MPa) | Water Absorption (%) | Porosity (%) | Density (kg/m3) |
|---|---|---|---|---|
| B8 | 43.4 | 22.2 | 26.5 | 2020 |
| S | 49.0 | 4.2 | 10.8 | 2580 |
| B50S50 | 48.9 | 11.3 | 16.7 | 2100 |
| Raw Materials 1 | Na2O/SiO2 Molar Ratios in Activating Solution | Synthesis Conditions | Compressive Strength (MPa) | Reference | |
|---|---|---|---|---|---|
| Temperature (°C) | Ageing Time (Days) | ||||
| Brick waste, OPC | 0.13 | 25 ± 3 | 28 | 102.6 | [3] |
| Brick waste | 0.63 | 20 | 7 | 41.9 | [7] |
| Brick waste, CAC | 0.63 | 65 | 7 | 92.0 | [38] |
| Brick waste | 0.12 | 70 | 28 | 66.6 | [39] |
| Brick waste, fly ash | 1.00 | 21 | 28 | 47.0 | [40] |
| Brick waste, GBFS | 0.67 | 25 | 28 | 120.0 | [21] |
| Brick waste, metallurgical slag | 0.86 | 90 | 7 | 48.9 | This study |
| Temperature (°C) | Compressive Strength (MPa) | Weight Loss (%) | Shrinkage (%) |
|---|---|---|---|
| 90 | 48.9 | - | - |
| 400 | 39.2 | 12.1 | 4.9 |
| 600 | 36.9 | 12.7 | 6.0 |
| 800 | 32.5 | 13.6 | 7.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soultana, A.; Valouma, A.; Bartzas, G.; Komnitsas, K. Properties of Inorganic Polymers Produced from Brick Waste and Metallurgical Slag. Minerals 2019, 9, 551. https://doi.org/10.3390/min9090551
Soultana A, Valouma A, Bartzas G, Komnitsas K. Properties of Inorganic Polymers Produced from Brick Waste and Metallurgical Slag. Minerals. 2019; 9(9):551. https://doi.org/10.3390/min9090551
Chicago/Turabian StyleSoultana, Athanasia, Aikaterini Valouma, Georgios Bartzas, and Konstantinos Komnitsas. 2019. "Properties of Inorganic Polymers Produced from Brick Waste and Metallurgical Slag" Minerals 9, no. 9: 551. https://doi.org/10.3390/min9090551
APA StyleSoultana, A., Valouma, A., Bartzas, G., & Komnitsas, K. (2019). Properties of Inorganic Polymers Produced from Brick Waste and Metallurgical Slag. Minerals, 9(9), 551. https://doi.org/10.3390/min9090551