Effect of CO2 Concentration on the Microbial Activity of Orenia metallireducens (Strain Z6) in Surface Inert Materials
Abstract
1. Introduction
2. Methods and Material
2.1. Chemicals and Mineral Preparation and Characterization
2.2. Development of Batch Cultures
2.3. Sample Collection, Pretreatment and Analyses
3. Results and Discussion
3.1. Effects of CO2 Concentration on Geochemistry and Microbial Metabolism
3.2. Varied Microbial Activity Between the Cultures Grown with Glass Beads and Quartz Sand
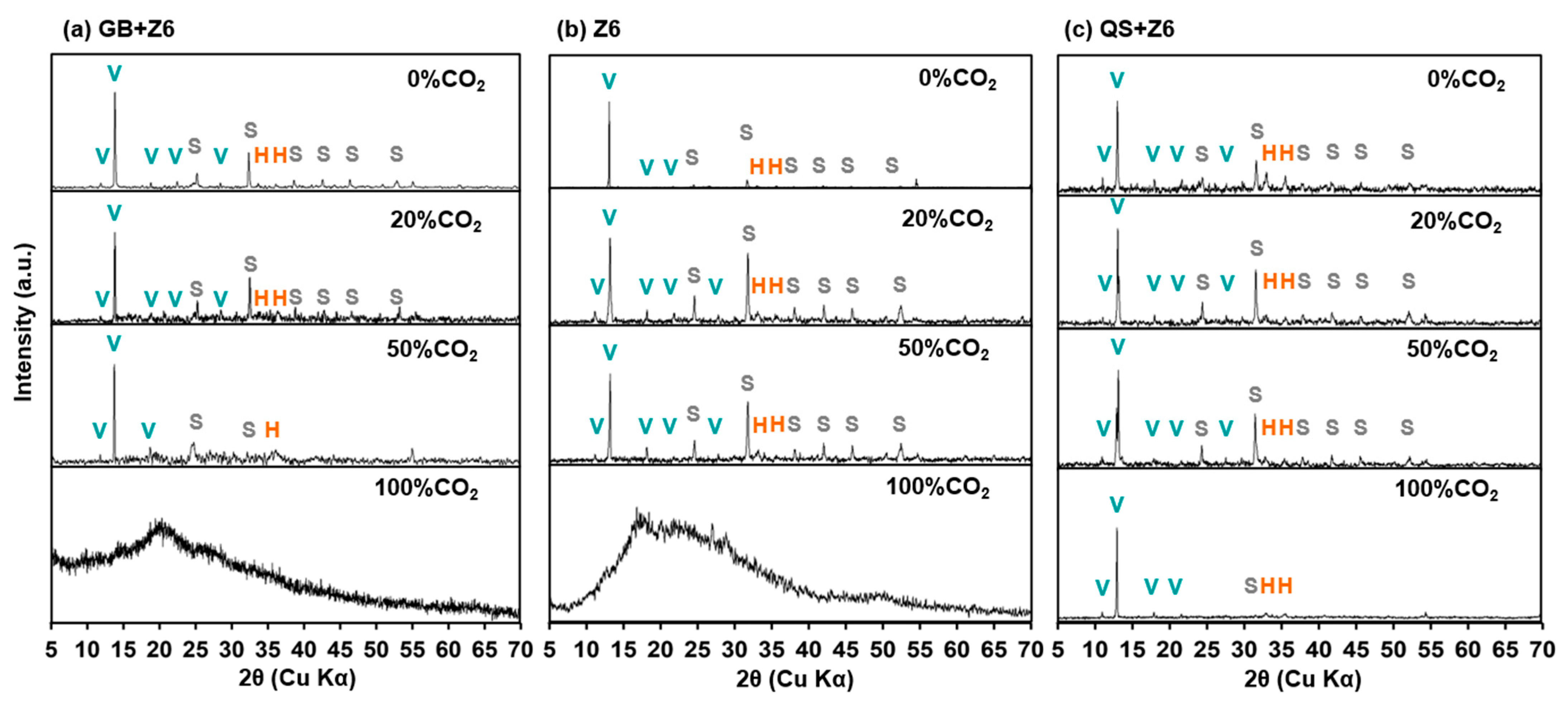
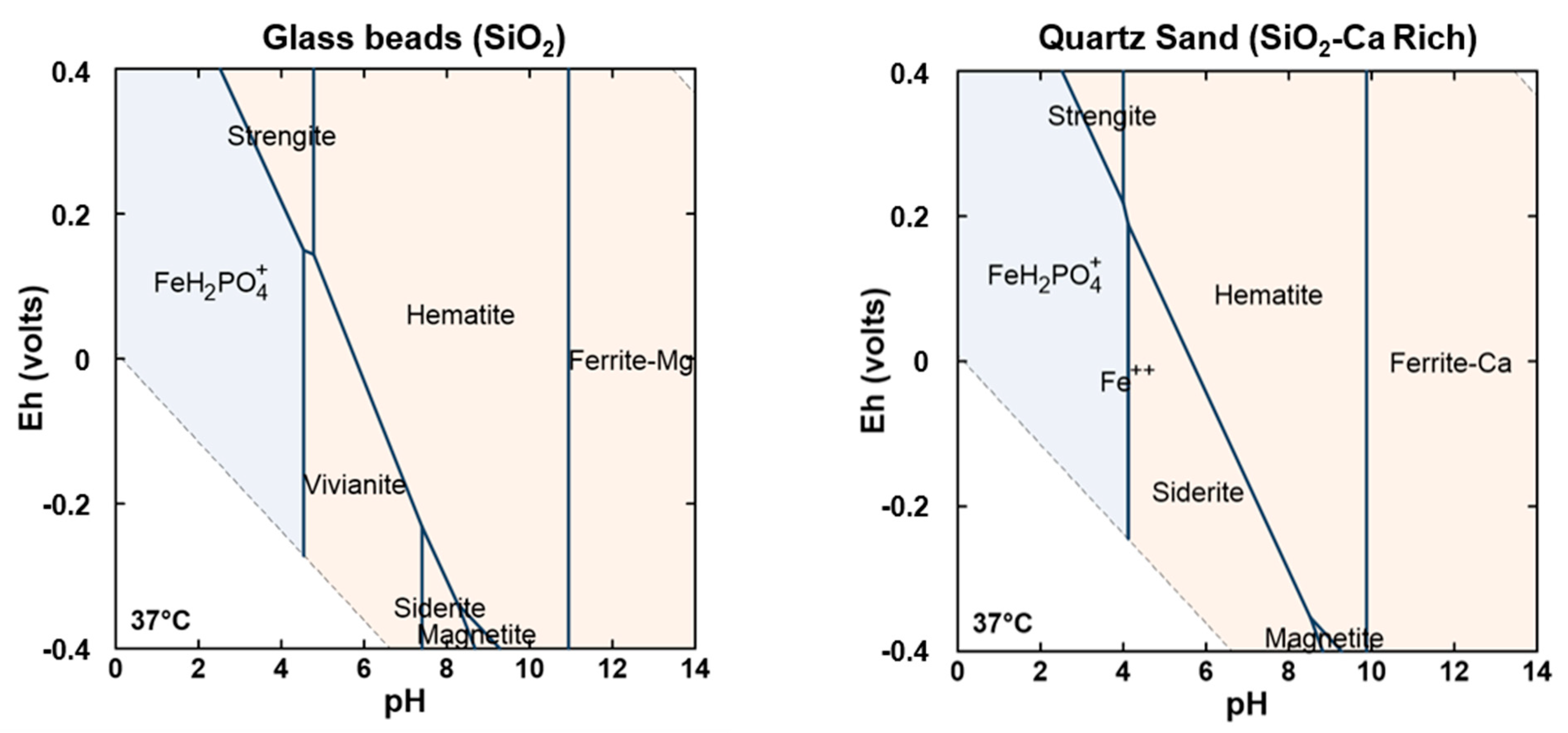
3.3. Secondary Mineral Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Salvatore, M.; Ferrara, A.; Rossi, S.; Biancalani, R.; Federici, S.; Jacobs, H.; Flammini, A. Agriculture, Forestry and Other Land Use Emissions by Sources and Removals by Sinks 1990−2011 Analysis; FAO: Rome, Italy, 2014. [Google Scholar]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.F.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef]
- Aminu, M.D.; Nabavi, S.A.; Rochelle, C.A.; Manović, V. A review of developments in carbon dioxide storage. Appl. Energy 2017, 208, 1389–1419. [Google Scholar] [CrossRef]
- Abdel-Mohsen, O.; Mohamed, M.E.G.; Suhaib, M.H.; Evan, K. Paleologos Chapter 2—Carbon capture and utilization. In Sustainable Utilization of Carbon Dioxide in Waste Management; Abdel-Mohsen, O., Mohamed, M.E.G., Suhaib, M.H., Evan, K.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 67–113. [Google Scholar]
- Trias, R.; Menez, B.; le Campion, P.; Zivanovic, Y.; Lecourt, L.; Lecoeuvre, A.; Schmitt-Kopplin, P.; Uhl, J.; Gislason, S.R.; Alfreethsson, H.A.; et al. High reactivity of deep biota under anthropogenic CO2 injection into basalt. Nat. Commun. 2017, 8, 1063. [Google Scholar] [CrossRef] [PubMed]
- Snæbjörnsdóttir, S.Ó.; Sigfússon, B.; Marieni, C.; Goldberg, D.; Gíslason, S.R.; Oelkers, E.H. Carbon dioxide storage through mineral carbonation. Nat. Rev. Earth Environ. 2020, 1, 90–102. [Google Scholar] [CrossRef]
- Raza, A.; Glatz, G.; Gholami, R.; Mahmoud, M.; Alafnan, S. Carbon mineralization and geological storage of CO2 in basalt: Mechanisms and technical challenges. Earth-Sci. Rev. 2022, 229, 104036. [Google Scholar] [CrossRef]
- Dong, Y.; Sanford, R.A.; Chang, Y.J.; McInerney, M.J.; Fouke, B.W. Hematite Reduction Buffers Acid Generation and Enhances Nutrient Uptake by a Fermentative Iron Reducing Bacterium, Orenia metallireducens Strain Z6. Environ. Sci. Technol. 2017, 51, 232–242. [Google Scholar] [CrossRef]
- Li, S.; Feng, Q.; Liu, J.; He, Y.; Shi, L.; Boyanov, M.I.; O’Loughlin, E.J.; Kemner, K.M.; Sanford, R.A.; Shao, H.; et al. Carbonate minerals and dissimilatory iron-reducing organisms trigger synergistic abiotic and biotic chain Reactions under elevated CO2 concentration. Environ. Sci. Technol. 2022, 56, 16428–16440. [Google Scholar] [CrossRef]
- Dong, Y.; Sanford, R.A.; Boyanov, M.I.; Flynn, T.M.; O’Loughlin, E.J.; Kemner, K.M.; George, S.; Fouke, K.E.; Li, S.; Huang, D.; et al. Controls on iron reduction and biomineralization over broad environmental conditions as suggested by the Firmicutes Orenia metallireducens strain Z6. Environ. Sci. Technol. 2020, 54, 10128–10140. [Google Scholar] [CrossRef]
- Shah, S.A.; Shao, Y.; Zhang, Y.; Zhao, H.; Zhao, L. Texture and trace element geochemistry of auartz: A review. Minerals 2022, 12, 1042. [Google Scholar] [CrossRef]
- Pan, X.; Li, S.; Li, Y.; Guo, P.; Zhao, X.; Cai, Y. Resource, characteristic, purification and application of quartz: A review. Miner. Eng. 2022, 183, 107600. [Google Scholar] [CrossRef]
- Li, S.; Liu, X.; Li, Z.; Liu, H.; Hu, D. Combination of direct boiling and glass beads increases the purity and accuracy of bacterial DNA extraction. Biotechnol. J. 2023, 18, e2300135. [Google Scholar] [CrossRef]
- Bowles, J.F.W. The Iron Oxides: Structure, Properties Reactions Occurrence and Uses. Mineral. Mag. 1997, 61, 740–741. [Google Scholar] [CrossRef]
- Dong, Y.; Kumar, C.G.; Chia, N.; Kim, P.-J.; Miller, P.A.; Price, N.D.; Cann, I.K.O.; Flynn, T.M.; Sanford, R.A.; Krapac, I.G.; et al. Halomonas sulfidaeris-dominated microbial community inhabits a 1.8 km-deep subsurface Cambrian Sandstone reservoir. Environ. Microbiol. 2014, 16, 1695–1708. [Google Scholar] [CrossRef] [PubMed]
- Lathuillière, M.J.; Pinto, O.B., Jr.; Johnson, M.S.; Jassal, R.S.; Dalmagro, H.J.; Leite, N.K.; Speratti, A.B.; Krampe, D.; Couto, E.G. Soil CO2 concentrations and efflux dynamics of a tree island in the pantanal wetland. J. Geophys. Res. Biogeosci. 2017, 122, 2154–2169. [Google Scholar] [CrossRef]
- Wilkin, R.T.; Digiulio, D.C. Geochemical impacts to groundwater from geologic carbon sequestration: Controls on pH and inorganic carbon concentrations from reaction path and kinetic modeling. Environ. Sci. Technol. 2010, 44, 4821–4827. [Google Scholar] [CrossRef]
- Viollier, E.; Inglett, P.W.; Hunter, K.S.; Roychoudhury, A.N.; Cappellen, P.V. The ferrozine method revisited: Fe(II)/Fe(III) determination in natural waters. Appl. Geochem. 2000, 15, 785–790. [Google Scholar] [CrossRef]
- Rittmann, B.E.; McCarty, P.L. Environmental Biotechnology: Principles and Applications; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- O’Loughlin, E.J.; Boyanov, M.I.; Flynn, T.M.; Gorski, C.A.; Hofmann, S.M.; McCormick, M.L.; Scherer, M.M.; Kemner, K.M. Effects of bound phosphate on the bioreduction of lepidocrocite (gamma-FeOOH) and maghemite (gamma-Fe2O3) and formation of secondary minerals. Environ. Sci. Technol. 2013, 47, 9157–9166. [Google Scholar] [CrossRef]
- Veerasingam, S.; Veerasingam, S.; Venkatachalapathy, R. Estimation of carbonate concentration and characterization of marine sediments by Fourier Transform Infrared Spectroscopy. Infrared Phys. Technol. 2014, 66, 136–140. [Google Scholar] [CrossRef]
- Gilliam, T.; Wiles, C. Stabilization and Solidification of Hazardous, Radioactive, and Mixed Wastes: 2nd Volume; ASTM International: West Conshohocken, PA, USA, 1992. [Google Scholar] [CrossRef]
- Gulliver, D.M.; Lowry, G.V.; Gregory, K.B. CO2 concentration and pH alters subsurface microbial ecology at reservoir temperature and pressure. RSC Adv. 2014, 4, 17443–17453. [Google Scholar] [CrossRef]
- Wilkins, M.J.; Hoyt, D.W.; Marshall, M.J.; Alderson, P.A.; Plymale, A.E.; Markillie, L.M.; Tucker, A.E.; Walter, E.D.; Linggi, B.E.; Dohnalkova, A.C.; et al. CO2 exposure at pressure impacts metabolism and stress responses in the model sulfate-reducing bacterium Desulfovibrio vulgaris strain Hildenborough. Front. Microbiol. 2014, 5, 507. [Google Scholar] [CrossRef]
- Wu, B.; Shao, H.B.; Wang, Z.P.; Hu, Y.D.; Tang, Y.J.J.; Jun, Y.S. Viability and metal reduction of Shewanella oneidensis MR-1 under CO2 stress: Implications for ecological effects of CO2 leakage from geologic CO2 sequestration. Environ. Sci. Technol. 2010, 44, 9213–9218. [Google Scholar] [CrossRef] [PubMed]
- Fredrickson, J.K.; Zachara, J.M.; Kennedy, D.W.; Dong, H.; Onstott, T.C.; Hinman, N.W.; Li, S.M. Biogenic iron mineralization accompanying the dissimilatory reduction of hydrous ferric oxide by a groundwater bacterium. Geochim. Cosmochim. Acta 1998, 62, 3239–3257. [Google Scholar] [CrossRef]
- List, C.; Hosseini, Z.; Meibom, K.L.; Hatzimanikatis, V.; Bernier-Latmani, R. Impact of iron reduction on the metabolism of Clostridium acetobutylicum. Environ. Microbiol. 2019, 21, 3548–3563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Davis, T.A.; Matthews, M.A.; Drews, M.J.; LaBerge, M.; An, Y.H.H. Sterilization using high-pressure carbon dioxide. J. Supercrit. Fluids 2006, 38, 354–372. [Google Scholar] [CrossRef]
- Mitchell, A.C.; Phillips, A.J.; Hamilton, M.A.; Gerlach, R.; Hollis, W.K.; Kaszuba, J.P.; Cunningham, A.B. Resilience of planktonic and biofilm cultures to supercritical CO2. J. Supercrit. Fluids 2008, 47, 318–325. [Google Scholar] [CrossRef]
- Sun, Q.; Lian, B. The different roles of Aspergillus nidulans carbonic anhydrases in wollastonite weathering accompanied by carbonation. Geochim. Cosmochim. Acta 2019, 244, 437–450. [Google Scholar] [CrossRef]
- Kirk, M.F.; Santillan, E.F.U.; Sanford, R.A.; Altman, S.J. CO2-induced shift in microbial activity affects carbon trapping and water quality in anoxic bioreactors. Geochim. Cosmochim. Acta 2013, 122, 198–208. [Google Scholar] [CrossRef]
- Howe, K.J.; Ishida, K.P.; Clark, M.M. Use of ATR/FTIR spectrometry to study fouling of microfiltration membranes by natural waters. Desalination 2002, 147, 251–255. [Google Scholar] [CrossRef]
- Matrajt, G.; Borg, J.; Raynal, P.I.; Djouadi, Z.; D’hendecourt, L.; Flynn, G.J.; Deboffle, D. FTIR and Raman analyses of the Tagish Lake meteorite: Relationship with the aliphatic hydrocarbons observed in the Diffuse Interstellar Medium. Astron. Astrophys. 2004, 416, 983–990. [Google Scholar] [CrossRef]
- Langford, H.; Hodson, A.J.; Banwart, S.A. Using FTIR spectroscopy to characterise the soil mineralogy and geochemistry of cryoconite from Aldegondabreen glacier, Svalbard. Appl. Geochem. 2011, 26, S206–S209. [Google Scholar] [CrossRef]
- Reig, F.B.; Adelantado, J.V.G.; Moreno, M.C.M.M. FTIR quantitative analysis of calcium carbonate (calcite) and silica (quartz) mixtures using the constant ratio method. Application to geological samples. Talanta 2002, 58, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Moros, J.; Cassella, R.J.; Barciela-Alonso, M.C.; Moreda-Piñeiro, A.; Herbello-Hermelo, P.; Bermejo-Barrera, P.; Garrigues, S.; Guardia, M.D.L. Estuarine sediment quality assessment by Fourier-transform infrared spectroscopy. Vib. Spectrosc. 2010, 53, 204–213. [Google Scholar] [CrossRef]
- Yi, M. Synthesis of Hydroxyapatite microspheres by hydro-thermal method under the control of sodium citrate. J. Inorg. Mater. 2014, 29, 284–288. [Google Scholar]
- Zeng, X.; Yamane, H.; Takahashi, M.; Masuda, T. Structure and properties of ionomers based on enthylene-co-methacrylic acid copolymer: Effects of thermal history. Mater. Sci. Res. Int. 1999, 5, 33–37. [Google Scholar]
- Roh, Y.; Moon, H.-S. Iron reduction by a psychrotolerant Fe (III)-reducing bacterium isolated from ocean sediment. Geosci. J. 2001, 5, 183–190. [Google Scholar] [CrossRef]
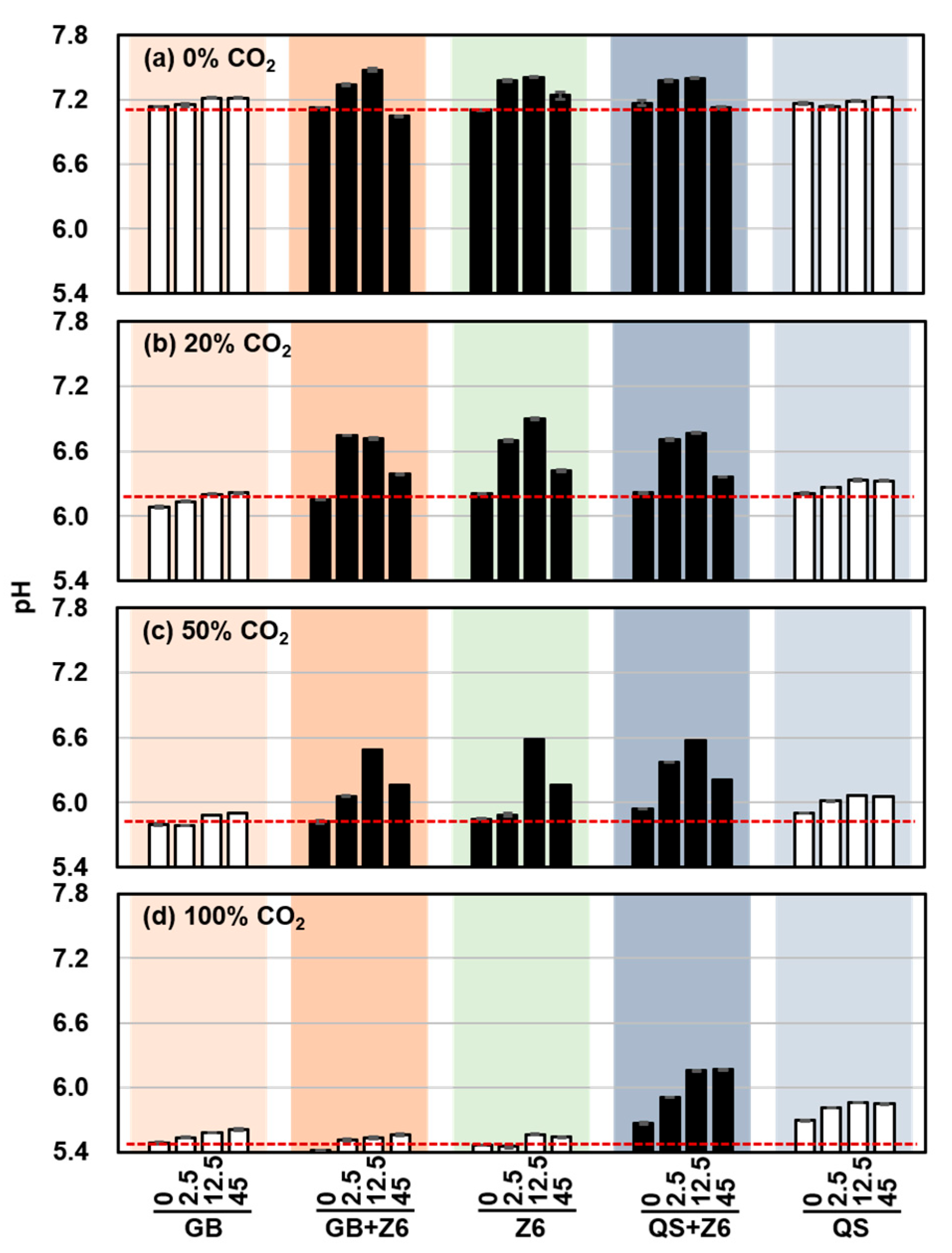
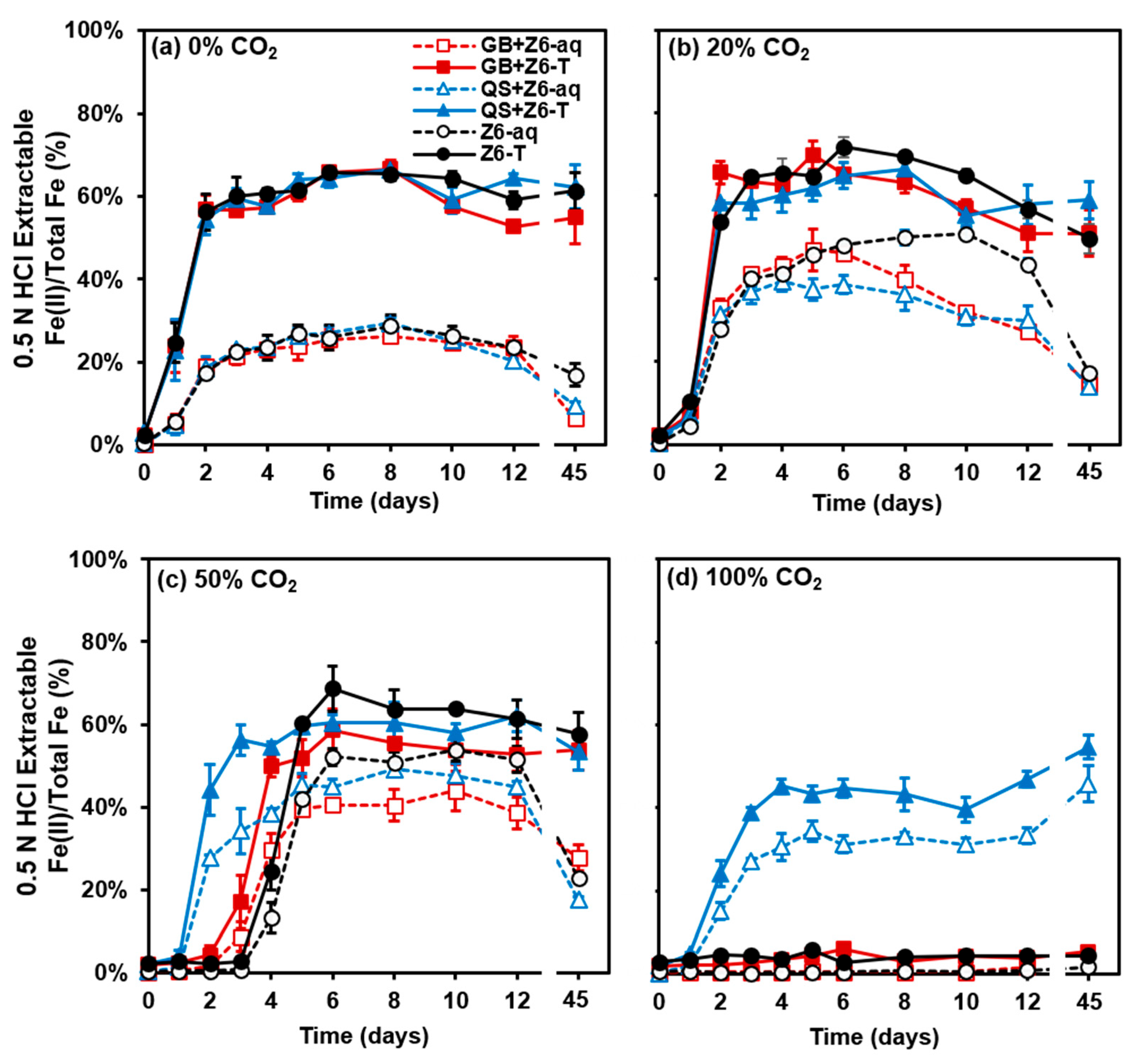
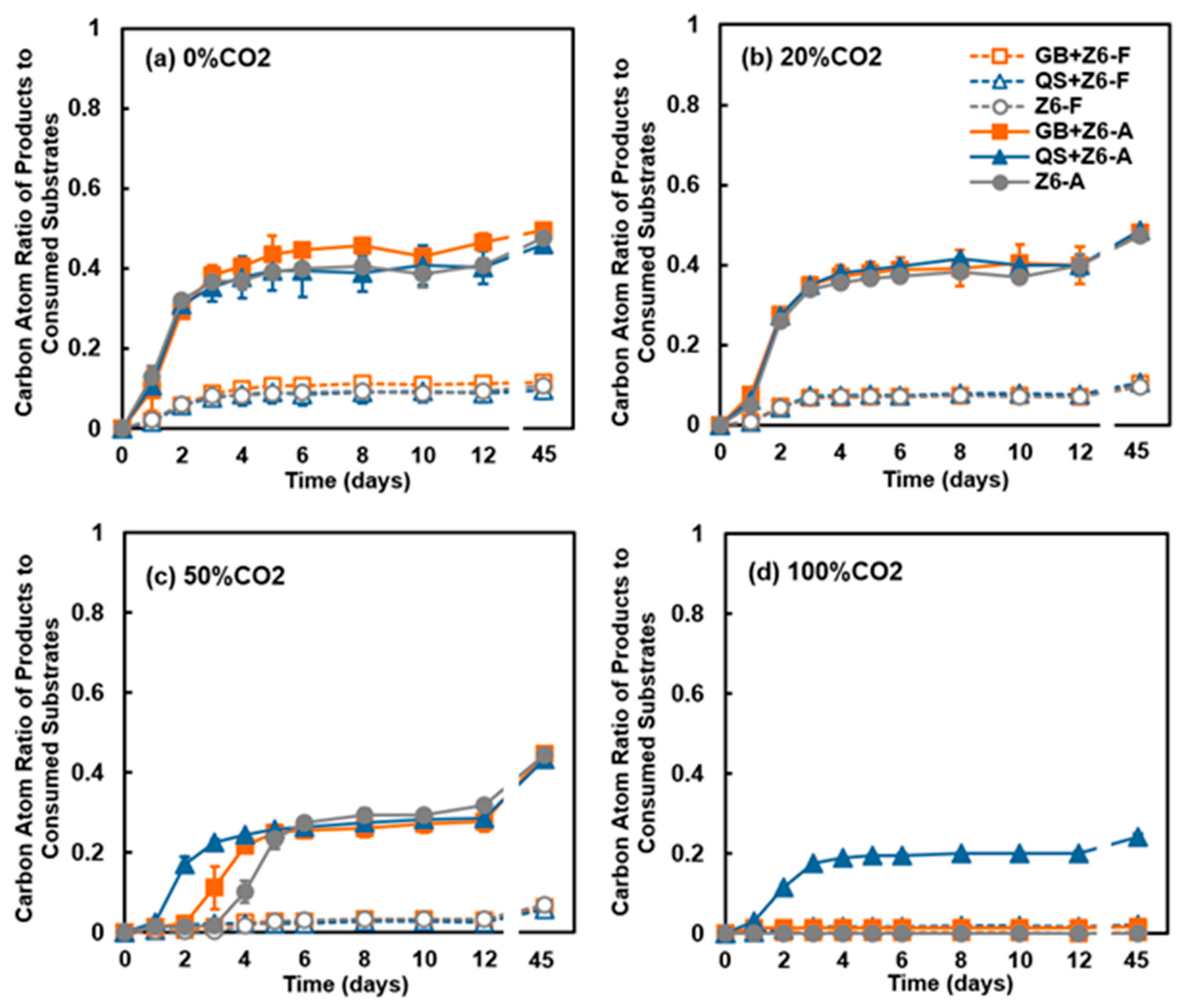

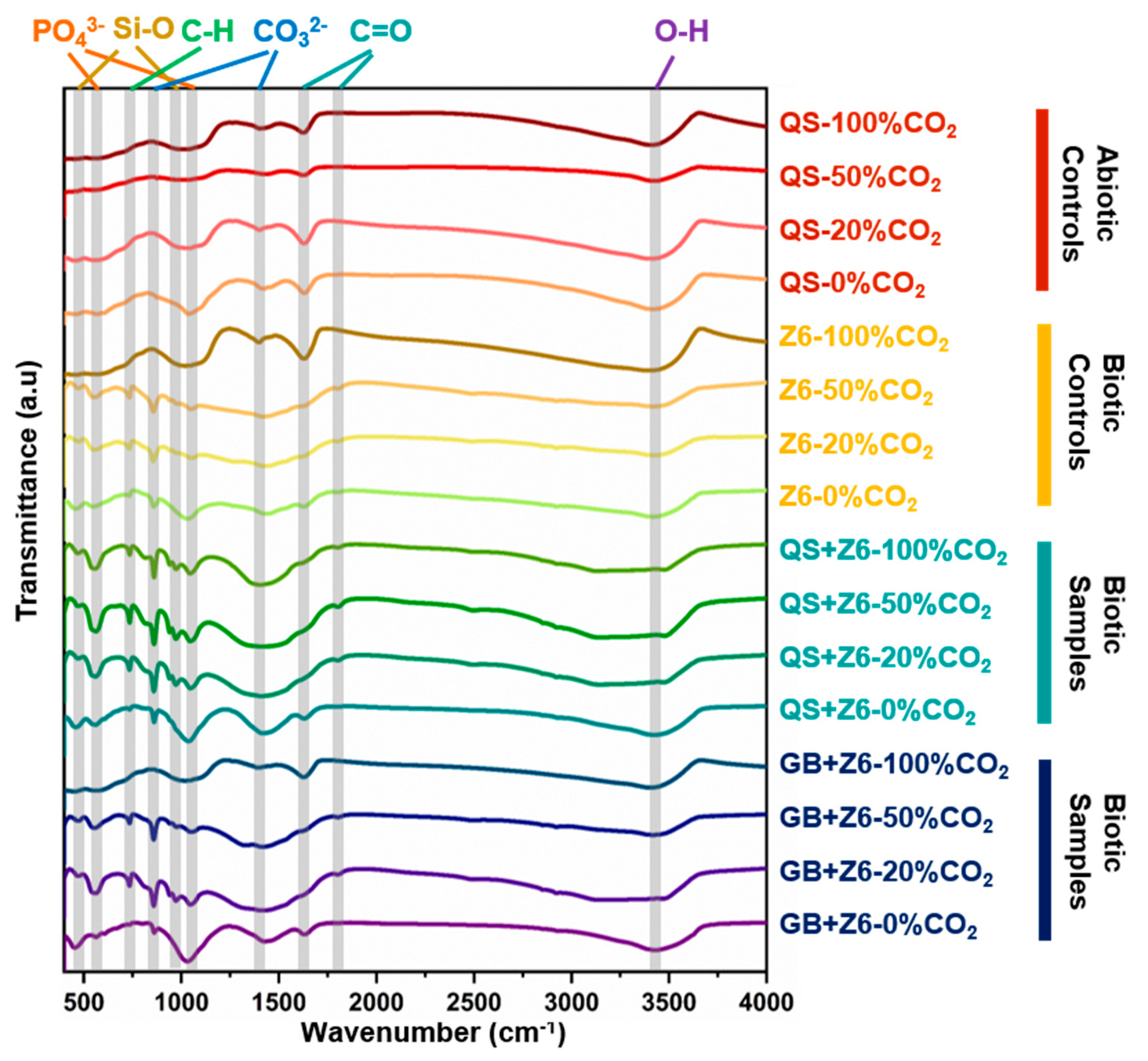
| Minerals | BET a (m2/g) | APS b (µm) | ANC c (kg/t) | Ca (mg/kg) | Fe (mg/kg) |
|---|---|---|---|---|---|
| Glass Beads | 0.1903 ± 0.004 | 1457.95 | 0.57 | 80,019.3 | 1369.34 |
| Quartz Sand | 1.083 ± 0.085 | 1231.07 | 518.97 | 330,960.13 | 201.04 |
| ID | Conditions b | Gas | Mineral (mmol/L) | Cell Inoculation c | kint (mM/day) | Maxium Fe(II)aq:Fe(II)TOT (Time, day) d | Final Fe(II)aq:Fe(II)TOT | Secondary Minerals e |
|---|---|---|---|---|---|---|---|---|
| XRD | ||||||||
| 1 | Z6 (0% CO2) | N2 | - | Y | 5.39 | 0.44 (8) | 0.27 | sid, viv, hem |
| 2 | GB (0% CO2) | N2 | 130 | N | - | - | - | - |
| 3 | GB + Z6 (0% CO2) | N2 | 130 | Y | 5.44 | 0.39 (8) | 0.12 | sid, viv, hem |
| 4 | QS (0% CO2) | N2 | 130 | N | - | - | - | - |
| 5 | QS + Z6 (0% CO2) | N2 | 130 | Y | 5.18 | 0.44 (8) | 0.15 | sid, viv, hem |
| 6 | Z6 (20% CO2) | N2:CO2 (80:20, v:v) | - | Y | 5.14 | 0.78 (10) | 0.35 | sid, viv, hem |
| 7 | GB (20% CO2) | N2:CO2 (80:20, v:v) | 130 | N | - | - | - | - |
| 8 | GB + Z6 (20% CO2) | N2:CO2 (80:20, v:v) | 130 | Y | 6.34 | 0.71 (6) | 0.29 | sid, viv, hem |
| 9 | QS (20% CO2) | N2:CO2 (80:20, v:v) | 130 | N | - | - | - | - |
| 10 | QS + Z6 (20% CO2) | N2:CO2 (80:20, v:v) | 130 | Y | 5.63 | 0.59 (6) | 0.24 | sid, viv, hem |
| 11 | Z6 (50% CO2) | N2:CO2 (50:50, v:v) | - | Y | 0.00 | 0.84 (10) | 0.39 | sid, viv, hem |
| 12 | GB (50% CO2) | N2:CO2 (50:50, v:v) | 130 | N | - | - | - | - |
| 13 | GB + Z6 (50% CO2) | N2:CO2 (50:50, v:v) | 130 | Y | 0.23 | 0.82 (10) | 0.52 | sid, viv, hem |
| 14 | QS (50% CO2) | N2:CO2 (50:50, v:v) | 130 | N | - | - | - | - |
| 15 | QS + Z6 (50% CO2) | N2:CO2 (50:50, v:v) | 130 | Y | 4.21 | 0.82 (10) | 0.33 | sid, viv, hem |
| 16 | Z6 (100% CO2) | CO2 | - | N | 0.16 | - | - | - |
| 17 | GB (100% CO2) | CO2 | 130 | N | - | - | - | - |
| 18 | GB + Z6 (100% CO2) | CO2 | 130 | N | 0.03 | - | - | - |
| 19 | QS (100% CO2) | CO2 | 130 | N | - | - | - | - |
| 20 | QS + Z6 (100% CO2) | CO2 | 130 | Y | 2.23 | 0.79 (10) | 0.84 | sid, viv, hem |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Song, W.; Liu, J.; Boyanov, M.I.; O’Loughlin, E.J.; Kemner, K.M.; Sanford, R.A.; Shao, H.; Feng, Q.; He, Y.; et al. Effect of CO2 Concentration on the Microbial Activity of Orenia metallireducens (Strain Z6) in Surface Inert Materials. Minerals 2025, 15, 112. https://doi.org/10.3390/min15020112
Li S, Song W, Liu J, Boyanov MI, O’Loughlin EJ, Kemner KM, Sanford RA, Shao H, Feng Q, He Y, et al. Effect of CO2 Concentration on the Microbial Activity of Orenia metallireducens (Strain Z6) in Surface Inert Materials. Minerals. 2025; 15(2):112. https://doi.org/10.3390/min15020112
Chicago/Turabian StyleLi, Shuyi, Wentao Song, Juan Liu, Maxim I. Boyanov, Edward J. O’Loughlin, Kenneth M. Kemner, Robert A. Sanford, Hongbo Shao, Qi Feng, Yu He, and et al. 2025. "Effect of CO2 Concentration on the Microbial Activity of Orenia metallireducens (Strain Z6) in Surface Inert Materials" Minerals 15, no. 2: 112. https://doi.org/10.3390/min15020112
APA StyleLi, S., Song, W., Liu, J., Boyanov, M. I., O’Loughlin, E. J., Kemner, K. M., Sanford, R. A., Shao, H., Feng, Q., He, Y., Dong, Y., & Shi, L. (2025). Effect of CO2 Concentration on the Microbial Activity of Orenia metallireducens (Strain Z6) in Surface Inert Materials. Minerals, 15(2), 112. https://doi.org/10.3390/min15020112








