The Correlation of Colour and Iron Oxides in Yellow Seal Stones from Northern Laos
Abstract
1. Introduction
2. Materials and Methods
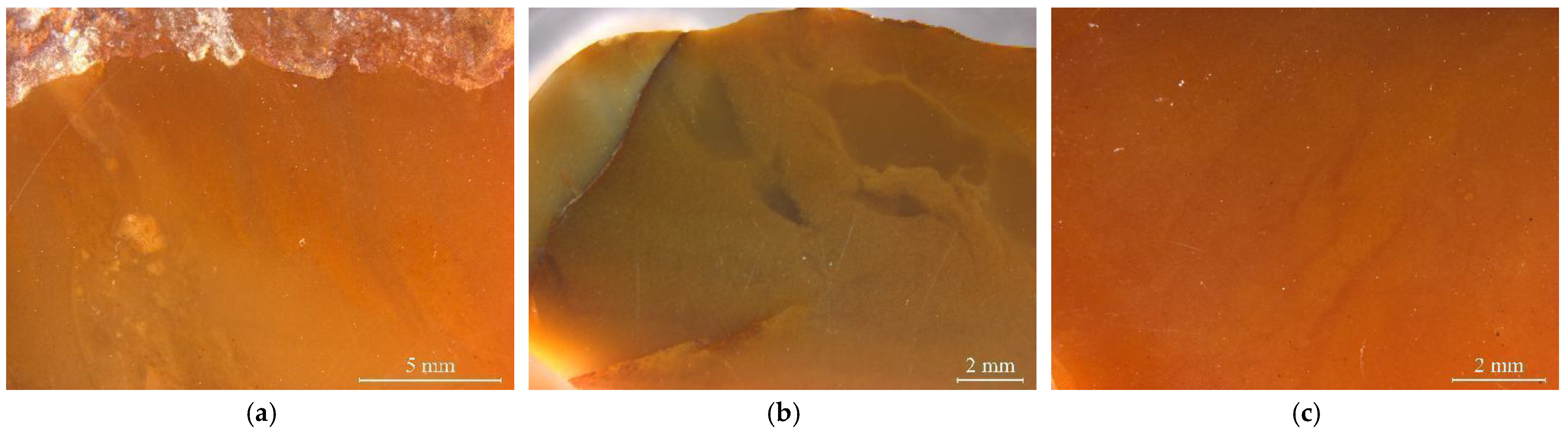
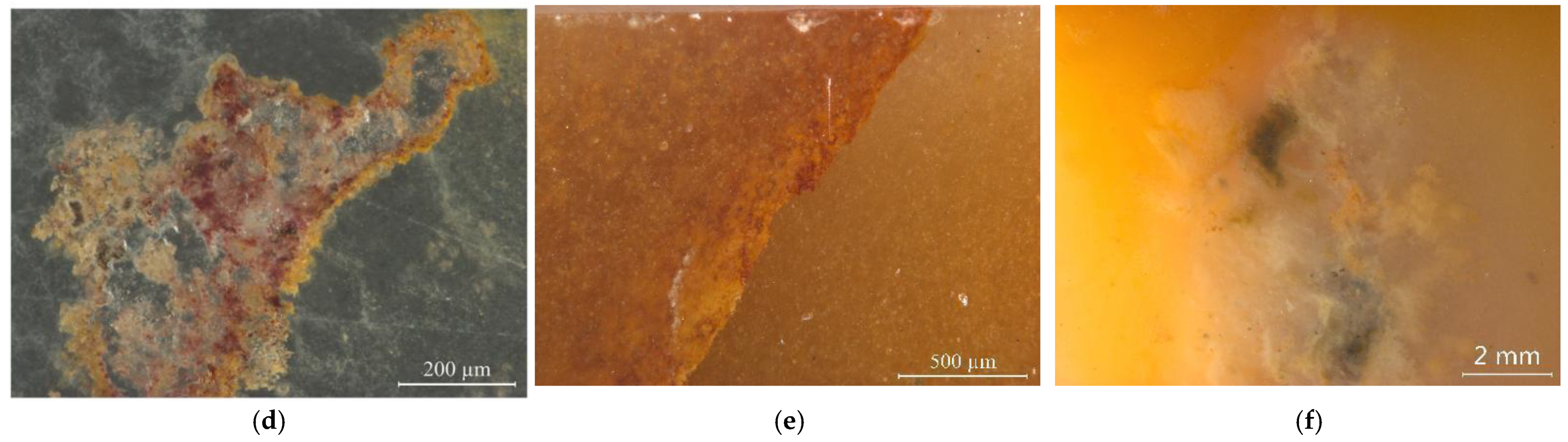
2.1. Samples
2.2. Laser Raman Spectroscopy
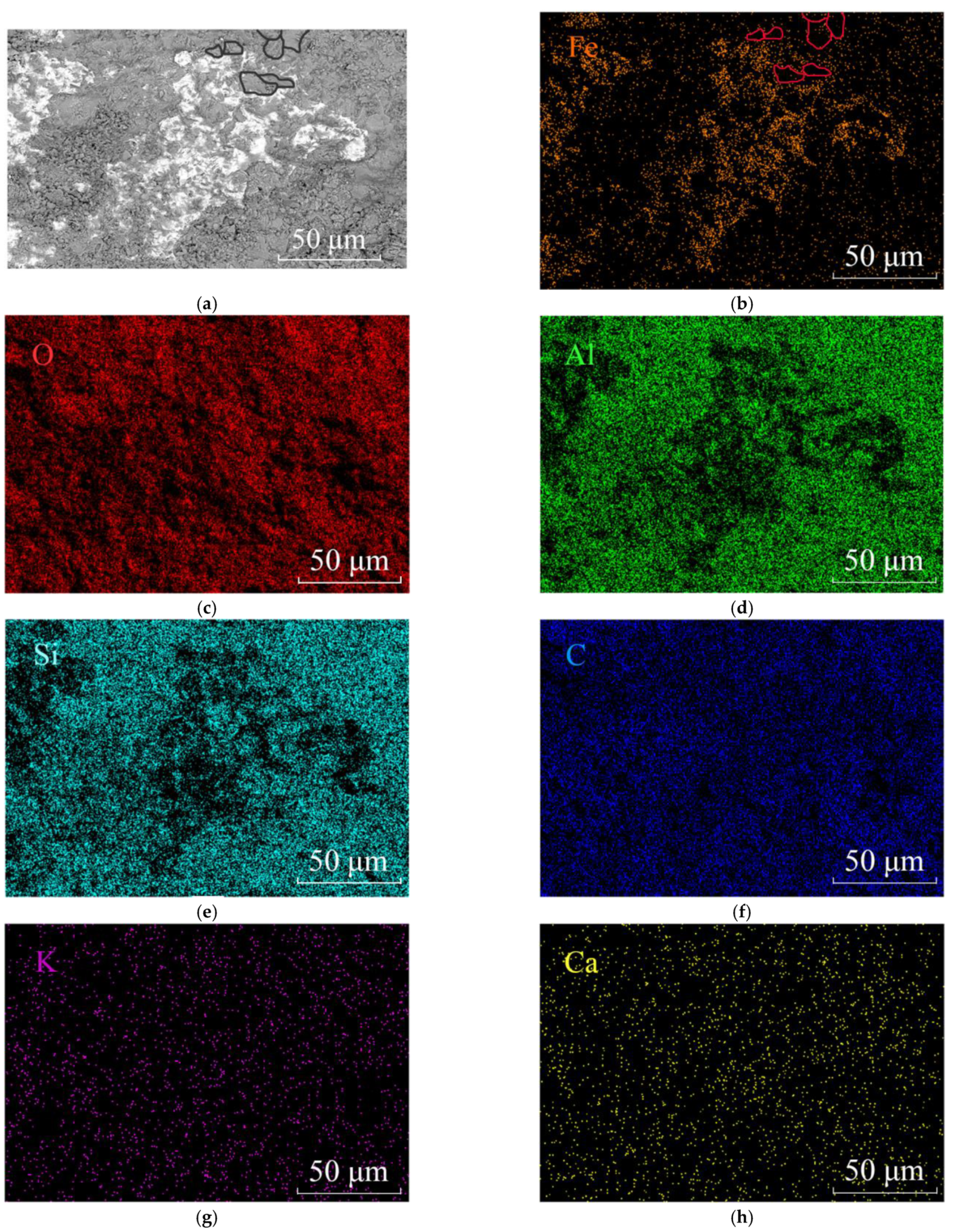
2.3. Scanning Electron Microscope
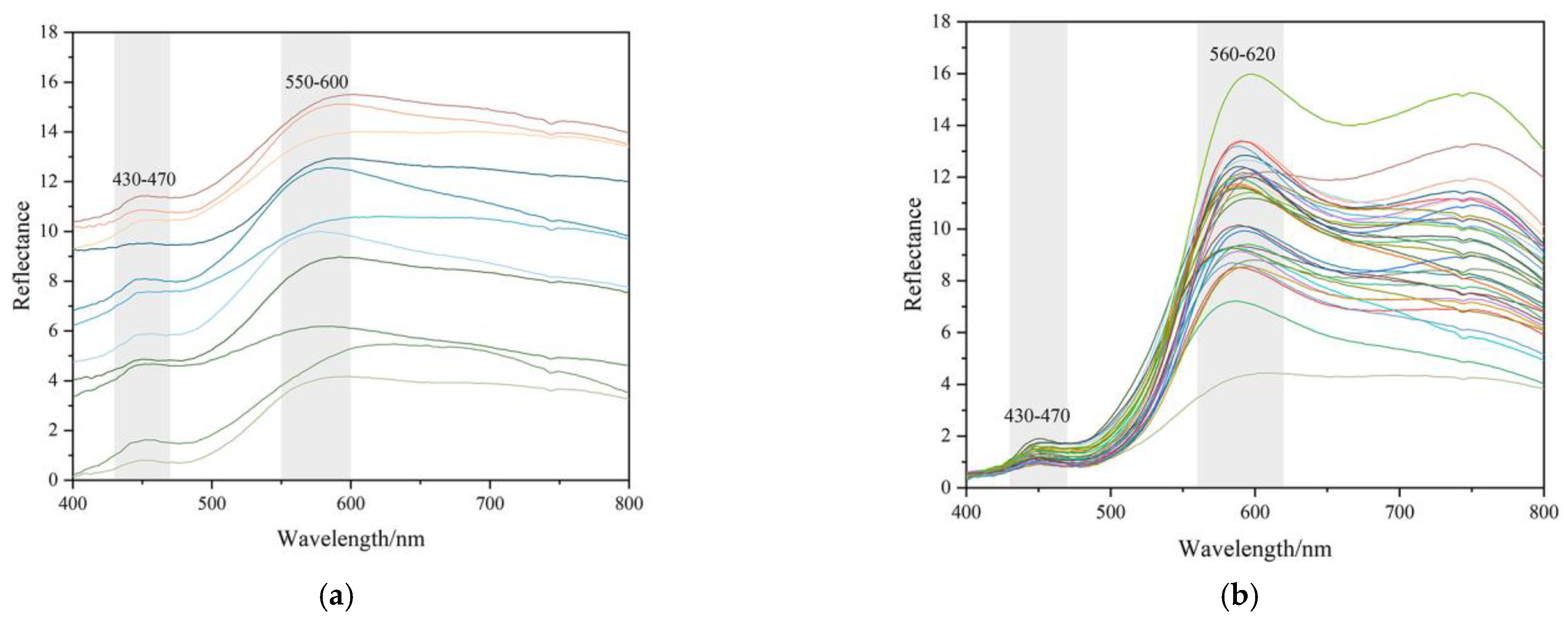
2.4. UV-Vis Reflectance Spectra
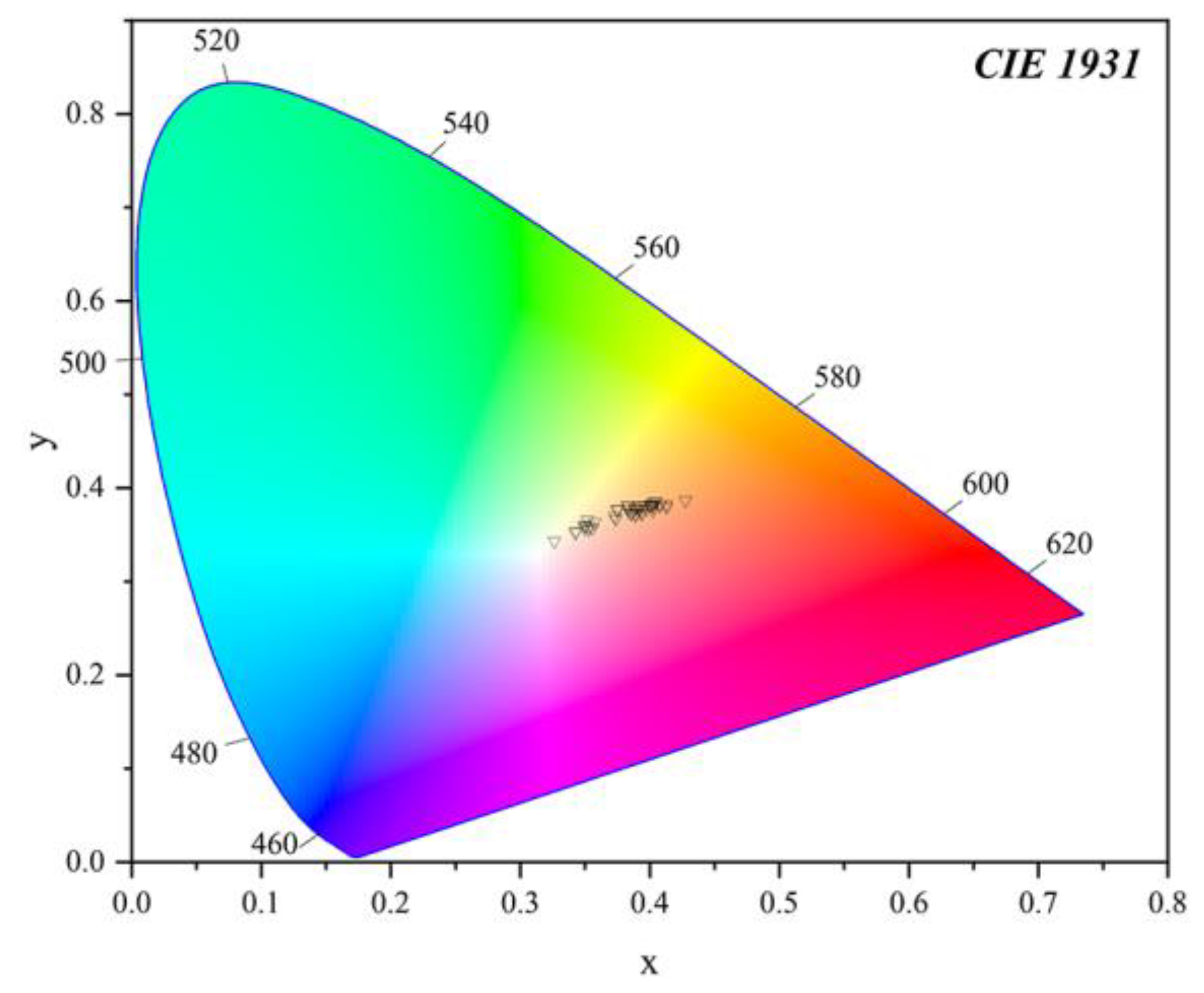
2.5. Colourimetric Analysis
3. Results and Discussion
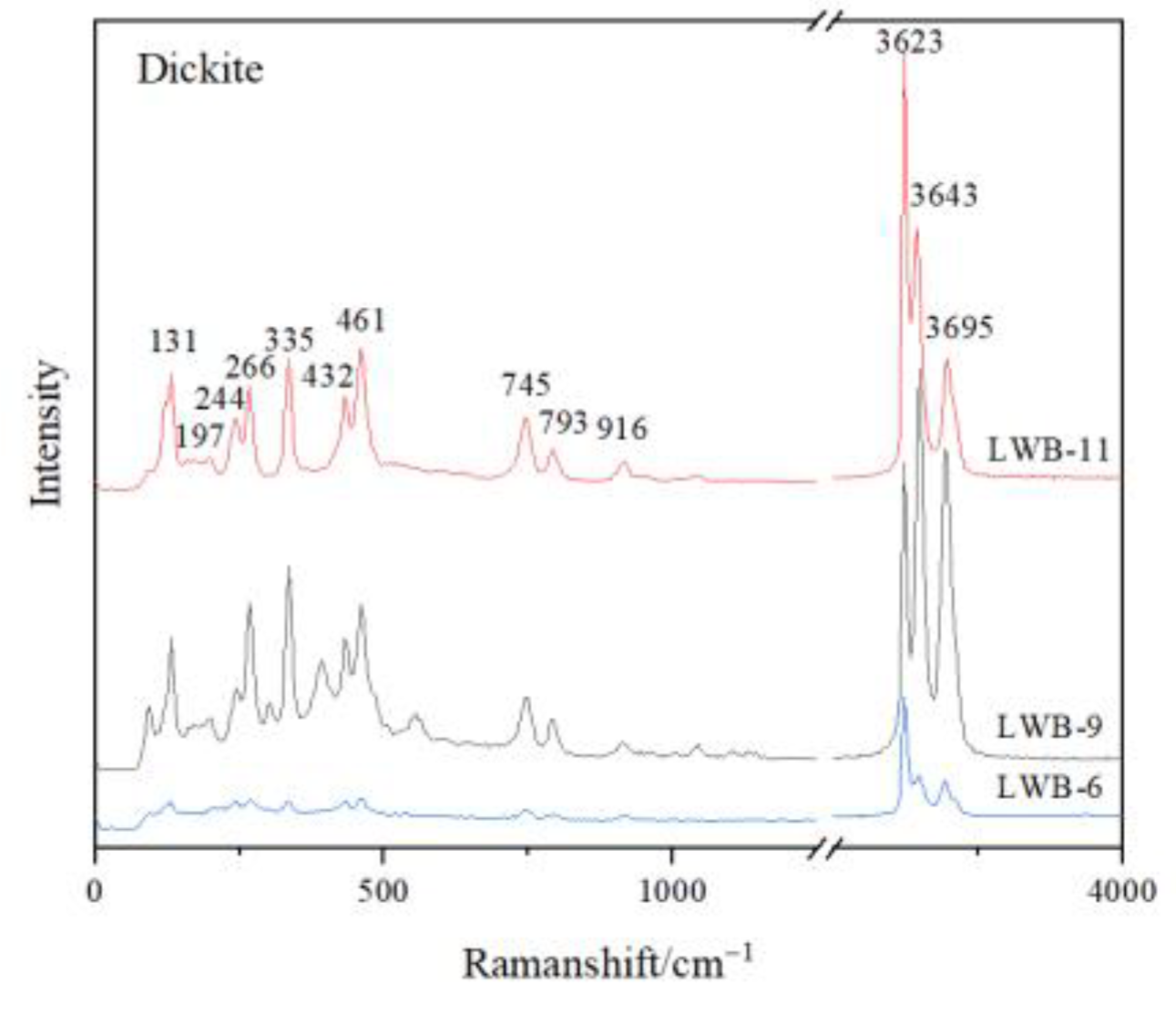
3.1. Laser Raman Spectrum
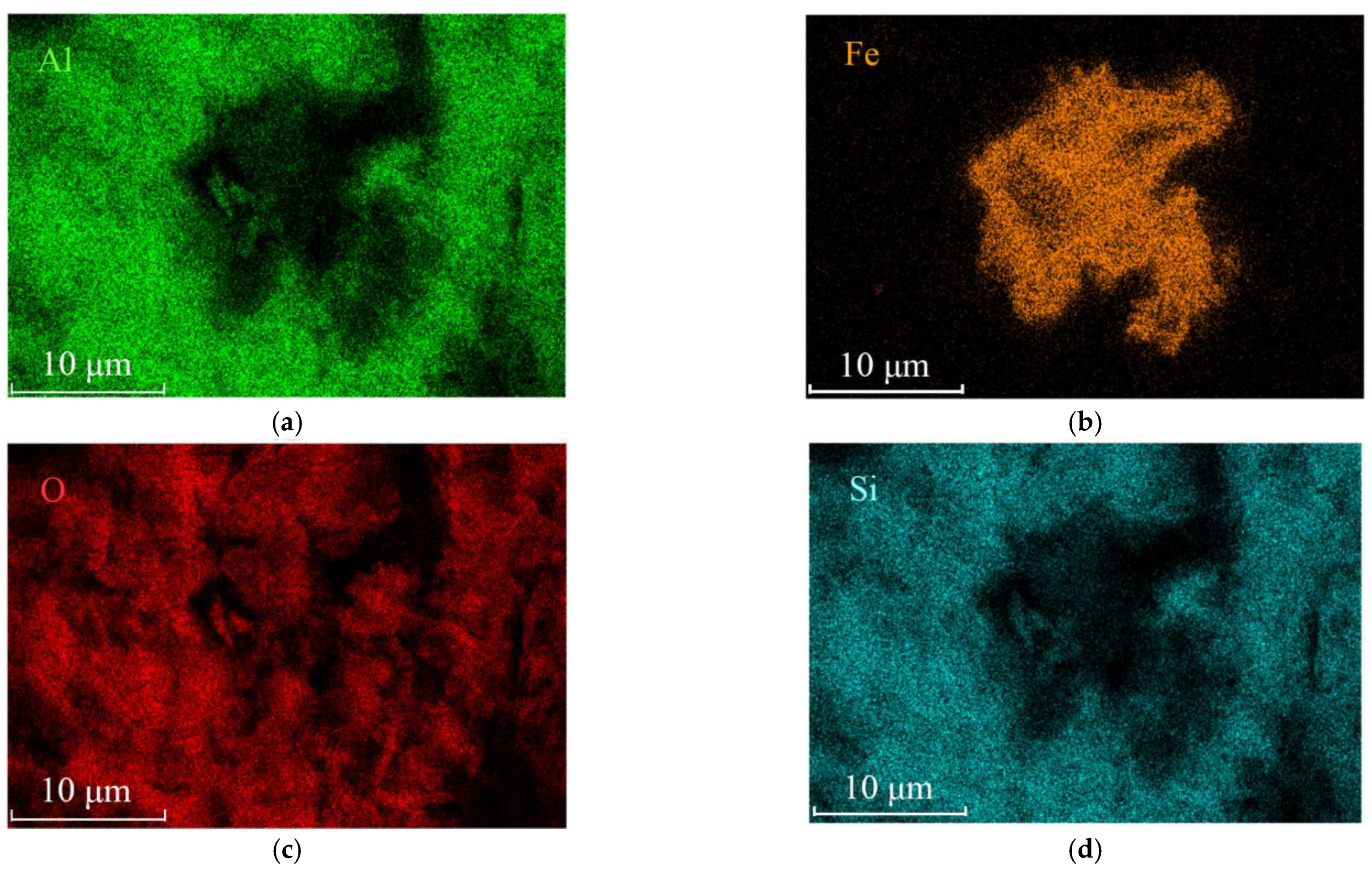
3.2. Scanning Electron Microscope
3.3. Colourimetric Analysis
3.4. UV-Vis Reflectance Spectra
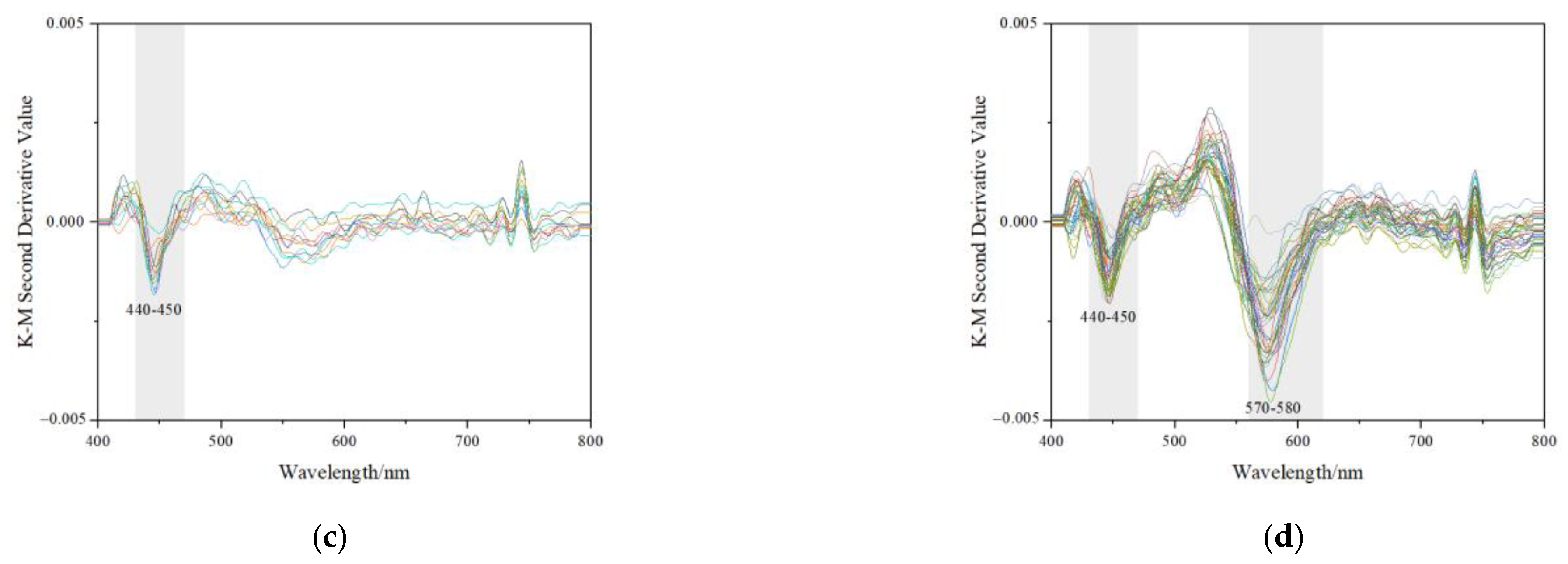
3.5. Characteristics of Second Derivative Reflectance Spectra
3.6. Correlation Analysis between Colour Parameters and Content of Goethite and Hematite
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, Y.; Chen, T. Mineral Composition and Trace Element Characteristics of Laos Northern Yellow Stone. Acta Petrol. Et Mineral. 2019, 38, 399–409. [Google Scholar]
- Liu, Y.; Chen, T.; Han, W.; Wang, C. A Study of Color and Luobowen of Tianhuang Stone. Acta Petrol. Et Mineral. 2013, 32, 549–556. [Google Scholar]
- Çiflikli, M. Hydrothermal Alteration-Related Kaolinite/Dickite Occurrences in Ignimbrites: An Example from Miocene Ignimbrite Units in Avanos, Central Turkey. Arab. J. Geosci. 2020, 13, 1044. [Google Scholar] [CrossRef]
- Han, W.; Ke, J.; Chen, H.; Lu, T.; Yin, K. Diffuse Reflectance Spectroscopy of Red Colored “Laowo Stone. Spectrosc. Spectr. Anal. 2016, 36, 2634–2638. [Google Scholar]
- Suppiah, D.D.; Johan, M.R. Influence of Solution PH on the Formation of Iron Oxide Nanoparticles. Mater. Res. Express 2018, 6, 015008. [Google Scholar] [CrossRef]
- Xie, T.; Lu, S.; Rao, L.; Zhang, L.; Wang, X.; Wang, W.; Wang, Q. Dissolution Factors and Oxidative Potential of Acid Soluble Irons from Chlorite Mineral Particles. Atmos. Environ. 2021, 255, 118436. [Google Scholar] [CrossRef]
- Wu, C.; Long, H.; Cheng, T.; Liu, L.; Qian, P.; Wang, H.; Ren, S.; Zhou, L.; Zheng, X. Quantitative Estimations of Iron Oxide Minerals in the Late Pleistocene Paleosol of the Yangtze River Delta: Implications for the Chemical Weathering, Sedimentary Environment, and Burial Conditions. CATENA 2021, 207, 105662. [Google Scholar] [CrossRef]
- Cornell, R.; Schwertmann, U. The Iron Oxides: Structures, Properties, Reactions, Occurences and Uses, 2nd ed.; Wiley-vch: Weinheim, Germany, 2003. [Google Scholar]
- Zhou, Y.; Liu, Z.; Zhao, Z.; Guo, Y. Quantitative Study on Colour and Spectral Characteristics of Beihong Agate. Minerals 2022, 12, 677. [Google Scholar] [CrossRef]
- Hu, P.; Jiang, Z.; Liu, Q.; Heslop, D.; Roberts, A.P.; Torrent, J.; Barrón, V. Estimating the Concentration of Aluminum-Substituted Hematite and Goethite Using Diffuse Reflectance Spectrometry and Rock Magnetism: Feasibility and Limitations. J. Geophys. Res. Solid Earth 2016, 121, 4180–4194. [Google Scholar] [CrossRef]
- Balsam, W.; Ji, J.; Renock, D.; Deaton, B.C.; Williams, E. Determining Hematite Content from NUV/Vis/NIR Spectra: Limits of Detection. Am. Mineral. 2014, 99, 2280–2291. [Google Scholar] [CrossRef]
- Grygar, T.; Dědeček, J.; Kruiver, P.P.; Dekkers, M.J.; Bezdička, P.; Schneeweiss, O. Iron Oxide Mineralogy in Late Miocene Red Beds from La Gloria, Spain: Rock-Magnetic, Voltammetric and Vis Spectroscopy Analyses. CATENA 2003, 53, 115–132. [Google Scholar] [CrossRef]
- Ding, Z.L.; Sun, J.M.; Liu, T.S.; Zhu, R.X.; Yang, S.L.; Guo, B. Wind-Blown Origin of the Pliocene Red Clay Formation in the Central Loess Plateau, China. Earth Planet. Sci. Lett. 1998, 161, 135–143. [Google Scholar] [CrossRef]
- Li, X.; Hu, X.; Cai, Y.; Han, Z. Quantitative Analysis of Iron Oxide Concentrations within Aptian–Albian Cyclic Oceanic Red Beds in ODP Hole 1049C, North Atlantic. Sediment. Geol. 2011, 235, 91–99. [Google Scholar] [CrossRef]
- Makiel, M.; Skiba, M.; Kisiel, M.; Maj-Szeliga, K.; Błachowski, A.; Szymański, W.; Salata, D. Formation of Iron Oxyhydroxides as a Result of Glauconite Weathering in Soils of Temperate Climate. Geoderma 2022, 416, 115780. [Google Scholar] [CrossRef]
- Schanda, J. CIE Colorimetry. Colorimetry 2007, 3, 25–78. [Google Scholar] [CrossRef]
- Scheinost, A.C. Use and Limitations of Second-Derivative Diffuse Reflectance Spectroscopy in the Visible to Near-Infrared Range to Identify and Quantify Fe Oxide Minerals in Soils. Clays Clay Miner. 1998, 46, 528–536. [Google Scholar] [CrossRef]
- Torrent, J.; Liu, Q.; Bloemendal, J.; Barrón, V. Magnetic Enhancement and Iron Oxides in the Upper Luochuan Loess-Paleosol Sequence, Chinese Loess Plateau. Soil Sci. Soc. Am. J. 2007, 71, 1570–1578. [Google Scholar] [CrossRef]
- Freire-Lista, D.M.; Campos, B.B.; do Rosário Costa, M.; Sanjurjo-Sánchez, J. Main Building Granite of São Tiago de Folhadela Parish Church (North of Portugal). Petrography, Glyptography, Construction Phases and Decay by Pyrite Oxidation. Constr. Build. Mater. 2022, 350, 128904. [Google Scholar] [CrossRef]
- Winkler, E.M. (Ed.) Natural Rust on Stone. In Stone: Properties, Durability in Man’s Environment; Springer: Vienna, Austria, 1975; pp. 164–168. [Google Scholar] [CrossRef]
- Zolotov, M.Y.; Shock, E.L. Formation of Jarosite-Bearing Deposits through Aqueous Oxidation of Pyrite at Meridiani Planum, Mars. Geophys. Res. Lett. 2005, 32, L21203. [Google Scholar] [CrossRef]
- Bedoya-Gonzalez, D.; Hilberg, S.; Redhammer, G.; Rinder, T. A Petrographic Investigation of the Carboniferous Sequence from the Ibbenbüren Mine: Tracing the Origin of the Coal Mine Drainage. Minerals 2021, 11, 483. [Google Scholar] [CrossRef]
- Cairncross, B.; Windisch, W.; Smit, H.; Fraser, A.; Gutzmer, J. The Vergenoeg: Gauteng Province, South Africa Fluorite Mine. Rocks Miner. 2008, 83, 410–421. [Google Scholar] [CrossRef]
- Sawłowicz, Z.; Malinowski, Ł.; Giże, A.; Stanek, J.; Przybyło, J. Mineralogical-Geochemical Study of Corroded Iron-Based Metals from a Salt Mine Environment. Corrosion 2020, 76, 666–677. [Google Scholar] [CrossRef]
- Lupulescu, M.; Chamberlain, S.C.; Walter, M.; Wallace, S. Diagenetic Uvite with Overgrown Dravite Bigelow, St. Lawrence County, New York. Rocks Miner. 2010, 85, 250–259. [Google Scholar] [CrossRef]
- Domingo, C.; Rodríguez-Clemente, R.; Blesa, M. Morphological Properties of α-FeOOH, γ-FeOOH and Fe3O4 Obtained by Oxidation of Aqueous Fe(II) Solutions. J. Colloid Interface Sci. 1994, 165, 244–252. [Google Scholar] [CrossRef]
- Bayoumi, M.B.; Abu Khoziem, H.A. Mineralogical Investigation of the Alteration Aspects in Gabal El Sela Area, South Eastern Desert, Egypt. Arab. J. Geosci. 2021, 14, 1268. [Google Scholar] [CrossRef]
- Gentile, L. Ferrihydrite Nanoparticles Entrapped in Shear-Induced Multilamellar Vesicles. J. Colloid Interface Sci. 2022, 606, 1890–1896. [Google Scholar] [CrossRef]
- Agresti, F.; Zin, V.; Barison, S.; Sani, E.; Meucci, M.; Mercatelli, L.; Nodari, L.; Rossi, S.; Bobbo, S.; Fabrizio, M. NIR Transmittance Tuneability under a Magnetic Field of Colloidal Suspensions of Goethite (α-FeOOH) Nanorods. RSC Adv. 2017, 7, 12429–12436. [Google Scholar] [CrossRef]
- Schwertmann, U.; Murad, E. Effect of PH on the Formation of Goethite and Hematite from Ferrihydrite. Clays Clay Miner. 1983, 31, 277–284. [Google Scholar] [CrossRef]
- Bigham, J.M.; Nordstrom, D.K. Iron and Aluminum Hydroxysulfates from Acid Sulfate Waters. Rev. Mineral. Geochem. 2000, 40, 351–403. [Google Scholar] [CrossRef]
- Bao, H.; Koch, P.L.; Thiemens, M.H. Oxygen Isotopic Composition of Ferric Oxides from Recent Soil, Hydrologic, and Marine Environments. Geochim. Et Cosmochim. Acta 2000, 64, 2221–2231. [Google Scholar] [CrossRef]
- Schwertmann, U.; Stanjek, H.; Becher, H.-H. Long-Term in Vitro Transformation of 2-Line Ferrihydrite to Goethite/Hematite at 4, 10, 15 and 25°C. Clay Miner. 2004, 39, 433–438. [Google Scholar] [CrossRef]
- Luo, M.R.; Li, C. CIE Color Appearance Models and Associated Color Spaces. Colorimetry 2007, 11, 261–294. [Google Scholar] [CrossRef]
- Nagano, T.; Nakashima, S. Study of Colors and Degrees of Weathering of Granitic Rocks by Visible Diffuse Reflectance Spectroscopy. Geochem. J. 1989, 23, 75–83. [Google Scholar] [CrossRef]
- Cuadros, J.; Sánchez-Marañón, M.; Mavris, C.; Fiore, S.; Bishop, J.L.; Melgosa, M. Color Analysis and Detection of Fe Minerals in Multi-Mineral Mixtures from Acid-Alteration Environments. Appl. Clay Sci. 2020, 193, 105677. [Google Scholar] [CrossRef]
- Nagano, T.; Isobe, H.; Nakashima, S.; Ashizaki, M. Characterization of Iron Hydroxides in a Weathered Rock Surface by Visible Microspectroscopy. Appl. Spectrosc. 2002, 56, 651–657. [Google Scholar] [CrossRef]
- Sánchez-Marañón, M.; Molinero-García, A.; Delgado, R.; García del Moral, L.F.; Martín-García, J.M. Spectral Analysis of Fe Oxidation in the Early Stages of Weathering and Soil Formation. CATENA 2023, 222, 106850. [Google Scholar] [CrossRef]
- Zhao, H.; Qiang, X.; Xu, X.; Sun, Y. Iron Oxide Characteristics of the Chinese Loess-Red Clay Sequences and Their Implications for the Evolution of the East Asian Summer Monsoon since the Late Oligocene. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2020, 543, 109604. [Google Scholar] [CrossRef]
- Szeberényi, J.; Kovács, J.; Bradák, B.; Barta, G.; Csonka, D.; Medveďová, A.; Roštínský, P.; Kiss, K.; Varga, G. Experiencing New Perspectives in the Application of Reflectance Spectroscopy in Loess Research. Quat. Int. 2020, 552, 36–49. [Google Scholar] [CrossRef]
- Barron, V.; Torrent, J. Use of the Kubelka—Munk Theory to Study the Influence of Iron Oxides on Soil Colour. J. Soil Sci. 1986, 37, 499–510. [Google Scholar] [CrossRef]
- Torrent, J.; Barrón, V. Diffuse Reflectance Spectroscopy of Iron Oxides. Encycl. Surf. Colloid Sci. 2002, 1, 1438–1446. [Google Scholar]
- Ion, R.-M.; Barbu, M.G.; Gonciar, A.; Vasilievici, G.; Gheboianu, A.I.; Slamnoiu-Teodorescu, S.; David, M.E.; Iancu, L.; Grigorescu, R.M. A Multi-Analytical Investigation of Roman Frescoes from Rapoltu Mare (Romania). Coatings 2022, 12, 530. [Google Scholar] [CrossRef]
- Fontes, M.P.F.; Carvalho, I.A., Jr. Color Attributes and Mineralogical Characteristics, Evaluated by Radiometry, of Highly Weathered Tropical Soils. Soil Sci. Soc. Am. J. 2005, 69, 1162–1172. [Google Scholar] [CrossRef]
- Chao, T.T.; Theobald, P.K. The Significance of Secondary Iron and Manganese Oxides in Geochemical Exploration. Econ. Geol. 1976, 71, 1560–1569. [Google Scholar] [CrossRef]




| Dominant Wavelength (nm) | ||||||||
|---|---|---|---|---|---|---|---|---|
| yellow parts | LWB-5L | 35.97 | 2.52 | 8.21 | 0.33 | 72.90 | 8.59 | 576.59 |
| LWB-10L | 47.99 | 2.48 | 5.27 | 0.19 | 64.81 | 5.83 | 578.90 | |
| LWB-17L | 33.65 | 0.35 | 4.55 | 0.18 | 85.61 | 4.57 | 572.58 | |
| LW-Y-3L | 36.45 | 2.95 | 9.89 | 0.38 | 73.40 | 10.32 | 576.53 | |
| LW-Y-4L | 38.88 | 1.07 | 9.85 | 0.34 | 83.78 | 9.90 | 573.22 | |
| LW-Y-6L | 38.66 | 1.82 | 6.69 | 0.25 | 74.76 | 6.94 | 575.88 | |
| LW-Y-8L | 41.95 | 1.94 | 8.98 | 0.31 | 77.79 | 9.19 | 575.00 | |
| LW-Y-10L | 34.44 | 3.65 | 7.94 | 0.35 | 65.33 | 8.74 | 579.11 | |
| LW-Y-14L | 37.62 | 2.20 | 8.35 | 0.31 | 75.26 | 8.63 | 575.82 | |
| LW-Y-16L | 40.26 | 2.73 | 8.19 | 0.30 | 71.56 | 8.63 | 576.98 | |
| LW-Y-17L | 39.46 | 2.99 | 8.79 | 0.33 | 71.20 | 9.29 | 577.15 | |
| orange-yellow parts | LWB-1L | 39.28 | 8.00 | 14.75 | 0.60 | 61.52 | 16.78 | 581.21 |
| LWB-2L | 42.81 | 8.97 | 18.02 | 0.67 | 63.53 | 20.13 | 580.65 | |
| LWB-2D | 43.66 | 9.79 | 19.44 | 0.71 | 63.26 | 21.77 | 580.89 | |
| LWB-3D | 41.32 | 11.61 | 19.89 | 0.79 | 59.73 | 23.03 | 582.58 | |
| LWB-4D | 43.32 | 10.45 | 20.41 | 0.75 | 62.90 | 22.93 | 581.18 | |
| LWB-4L | 33.99 | 3.98 | 8.04 | 0.36 | 63.68 | 8.97 | 579.69 | |
| LWB-5D | 40.76 | 7.87 | 16.66 | 0.64 | 64.70 | 18.42 | 580.08 | |
| LWB-6D | 45.02 | 5.44 | 17.45 | 0.57 | 72.70 | 18.28 | 577.13 | |
| LWB-7D | 45.10 | 7.49 | 19.03 | 0.64 | 68.52 | 20.45 | 578.75 | |
| LWB-8D | 44.93 | 7.10 | 17.67 | 0.60 | 68.10 | 19.04 | 578.80 | |
| LWB-10D | 43.77 | 7.16 | 17.35 | 0.61 | 67.57 | 18.77 | 578.99 | |
| LWB-12L | 35.54 | 7.73 | 17.24 | 0.71 | 65.85 | 18.89 | 579.88 | |
| LWB-12D | 38.66 | 12.39 | 22.14 | 0.90 | 60.76 | 25.37 | 582.58 | |
| LWB-14D | 41.73 | 8.33 | 19.89 | 0.71 | 67.28 | 21.56 | 579.39 | |
| LWB-15D | 36.36 | 7.77 | 18.23 | 0.72 | 66.92 | 19.81 | 579.54 | |
| LWB-18L | 41.38 | 4.65 | 16.59 | 0.57 | 74.34 | 17.23 | 576.56 | |
| LW-Y-1D | 42.84 | 4.19 | 15.05 | 0.51 | 74.45 | 15.62 | 576.40 | |
| LW-Y-1L | 42.65 | 4.06 | 15.28 | 0.51 | 75.13 | 15.80 | 576.19 | |
| LW-Y-2L | 43.66 | 7.07 | 18.32 | 0.63 | 68.90 | 19.64 | 578.59 | |
| LW-Y-2D | 44.16 | 9.15 | 19.67 | 0.70 | 65.05 | 21.70 | 580.15 | |
| LW-Y-3D | 43.19 | 7.64 | 17.52 | 0.63 | 66.44 | 19.11 | 579.44 | |
| LW-Y-4D | 43.59 | 10.31 | 20.41 | 0.75 | 63.19 | 22.87 | 581.04 | |
| LW-Y-5D | 39.05 | 4.96 | 12.70 | 0.49 | 68.68 | 13.63 | 578.31 | |
| LW-Y-6D | 38.58 | 7.89 | 14.79 | 0.61 | 61.93 | 16.76 | 581.07 | |
| LW-Y-8D | 42.72 | 7.13 | 15.69 | 0.58 | 65.55 | 17.24 | 579.61 | |
| LW-Y-9D | 42.98 | 8.53 | 19.31 | 0.69 | 66.16 | 21.12 | 579.72 | |
| LW-Y-11D | 40.09 | 5.73 | 15.32 | 0.56 | 69.49 | 16.35 | 578.21 | |
| LW-Y-12D | 46.27 | 11.88 | 22.10 | 0.79 | 61.73 | 25.09 | 581.76 | |
| LW-Y-13D | 42.98 | 9.19 | 19.48 | 0.71 | 64.75 | 21.54 | 580.30 | |
| LW-Y-14D | 40.39 | 8.78 | 16.12 | 0.65 | 61.42 | 18.36 | 581.39 | |
| LW-Y-15L | 40.81 | 7.25 | 15.17 | 0.58 | 64.47 | 16.81 | 580.01 | |
| LW-Y-15D | 42.85 | 10.49 | 18.29 | 0.71 | 60.17 | 21.09 | 582.11 | |
| LW-Y-17D | 44.39 | 9.56 | 16.97 | 0.64 | 60.59 | 19.48 | 581.70 | |
| LW-Y-18D | 42.98 | 7.92 | 18.55 | 0.66 | 66.87 | 20.17 | 579.38 | |
| Samples | Y1 | Goethite (g/kg) | Y2 | Hematite (g/kg) | |
|---|---|---|---|---|---|
| yellow parts | LWB-5L | 0.001075941 | 0.22835225 | —— | —— |
| LWB-10L | 0.001984312 | 0.471795657 | —— | —— | |
| LWB-17L | 0.001812021 | 0.425621738 | —— | —— | |
| LW-Y-3L | 0.001569042 | 0.360503127 | —— | —— | |
| LW-Y-4L | 0.001989744 | 0.47325136 | —— | —— | |
| LW-Y-6L | 0.00176468 | 0.412934281 | —— | —— | |
| LW-Y-8L | 0.00272929 | 0.671449621 | —— | —— | |
| LW-Y-10L | 0.000875987 | 0.174764567 | —— | —— | |
| LW-Y-14L | 0.002027255 | 0.483304445 | —— | —— | |
| LW-Y-16L | 0.001603278 | 0.369678603 | —— | —— | |
| LW-Y-17L | 0.002057389 | 0.491380249 | —— | —— | |
| orange-yellow parts | LWB-1L | 0.001059933 | 0.224062162 | 0.0012252 | 0.402530309 |
| LWB-2L | 0.002222709 | 0.535685955 | 0.002443285 | 0.892200609 | |
| LWB-2D | 0.001280728 | 0.28323506 | 0.002732704 | 1.008547074 | |
| LWB-3D | 0.000955706 | 0.196129244 | 0.003031046 | 1.128480675 | |
| LWB-4D | 0.001826245 | 0.429433762 | 0.003257142 | 1.219371117 | |
| LWB-4L | 0.001108467 | 0.237069152 | 0.000866089 | 0.258167593 | |
| LWB-5D | 0.001313221 | 0.29194323 | 0.002473944 | 0.904525304 | |
| LWB-6D | 0.002801402 | 0.690775641 | 0.002235463 | 0.80865613 | |
| LWB-7D | 0.001698799 | 0.395278193 | 0.002306093 | 0.837049482 | |
| LWB-8D | 0.001967412 | 0.467266419 | 0.001983025 | 0.707175859 | |
| LWB-10D | 0.0017378 | 0.405730505 | 0.001743261 | 0.61079074 | |
| LWB-12L | 0.00143351 | 0.324180683 | 0.00359445 | 1.354968756 | |
| LWB-12D | 0.001386035 | 0.311457298 | 0.006150555 | 2.38252327 | |
| LWB-14D | 0.001518542 | 0.346969169 | 0.005319845 | 2.048577627 | |
| LWB-15D | 0.001285326 | 0.284467247 | 0.003875173 | 1.467819471 | |
| LWB-18L | 0.001784134 | 0.41814803 | 0.002998162 | 1.115261068 | |
| LW-Y-1D | 0.002090698 | 0.50030709 | 0.001453646 | 0.494365656 | |
| LW-Y-1L | 0.000582 | 0.095916496 | 0.001921795 | 0.682561744 | |
| LW-Y-2L | 0.002387378 | 0.579817254 | 0.003423759 | 1.286350983 | |
| LW-Y-2D | 0.001425405 | 0.32200866 | 0.004308197 | 1.641895239 | |
| LW-Y-3D | 0.002250389 | 0.543104239 | 0.002312591 | 0.839661424 | |
| LW-Y-4D | 0.00165117 | 0.3825136 | 0.004120847 | 1.566580404 | |
| LW-Y-5D | 0.001549591 | 0.355290394 | 0.00202292 | 0.723213885 | |
| LW-Y-6D | 0.001488122 | 0.338816764 | 0.002209787 | 0.798334202 | |
| LW-Y-8D | 0.001552343 | 0.356027791 | 0.003057663 | 1.139180642 | |
| LW-Y-9D | 0.001626641 | 0.375939707 | 0.002184462 | 0.788153614 | |
| LW-Y-11D | 0.001370853 | 0.307388542 | 0.002466733 | 0.901626586 | |
| LW-Y-12D | 0.001775296 | 0.41577941 | 0.003562259 | 1.342028192 | |
| LW-Y-13D | 0.001365152 | 0.305860637 | 0.004128769 | 1.569765008 | |
| LW-Y-14D | 0.001384571 | 0.311064945 | 0.002815282 | 1.041743287 | |
| LW-Y-15L | 0.001159357 | 0.250707808 | 0.002290062 | 0.830604936 | |
| LW-Y-15D | 0.001392494 | 0.313188411 | 0.003010108 | 1.120063488 | |
| LW-Y-17D | 0.001844488 | 0.434322812 | 0.002955704 | 1.098192886 | |
| LW-Y-18D | 0.001443802 | 0.326939003 | 0.002497205 | 0.913876211 | |
| Colour Parameters | Correlations with Goethite | Correlations with Hematite | Multiple Linear Correlation | ||||
|---|---|---|---|---|---|---|---|
| Results | Pearson’s r | Significance | Pearson’s r | Significance | R | R2 | |
| L* (Lightness) | 0.475 | 0.005 | 0.038 | 0.829 | 0.477 | 0.228 | |
| a* (redness) | −0.080 | 0.653 | 0.611 | <0.001 | 0.614 | 0.377 | |
| b* (yellowness) | 0.194 | 0.271 | 0.706 | <0.001 | 0.737 | 0.543 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, J.; Chen, T.; Zheng, J.; Wu, J.; Xu, Y. The Correlation of Colour and Iron Oxides in Yellow Seal Stones from Northern Laos. Minerals 2023, 13, 291. https://doi.org/10.3390/min13020291
Tian J, Chen T, Zheng J, Wu J, Xu Y. The Correlation of Colour and Iron Oxides in Yellow Seal Stones from Northern Laos. Minerals. 2023; 13(2):291. https://doi.org/10.3390/min13020291
Chicago/Turabian StyleTian, Jinglin, Tao Chen, Jinyu Zheng, Jiaxin Wu, and Yating Xu. 2023. "The Correlation of Colour and Iron Oxides in Yellow Seal Stones from Northern Laos" Minerals 13, no. 2: 291. https://doi.org/10.3390/min13020291
APA StyleTian, J., Chen, T., Zheng, J., Wu, J., & Xu, Y. (2023). The Correlation of Colour and Iron Oxides in Yellow Seal Stones from Northern Laos. Minerals, 13(2), 291. https://doi.org/10.3390/min13020291








