Effective Treatment of Acid Mine Drainage with Microbial Fuel Cells: An Emphasis on Typical Energy Substrates
Abstract
1. Introduction
2. Materials and Methods
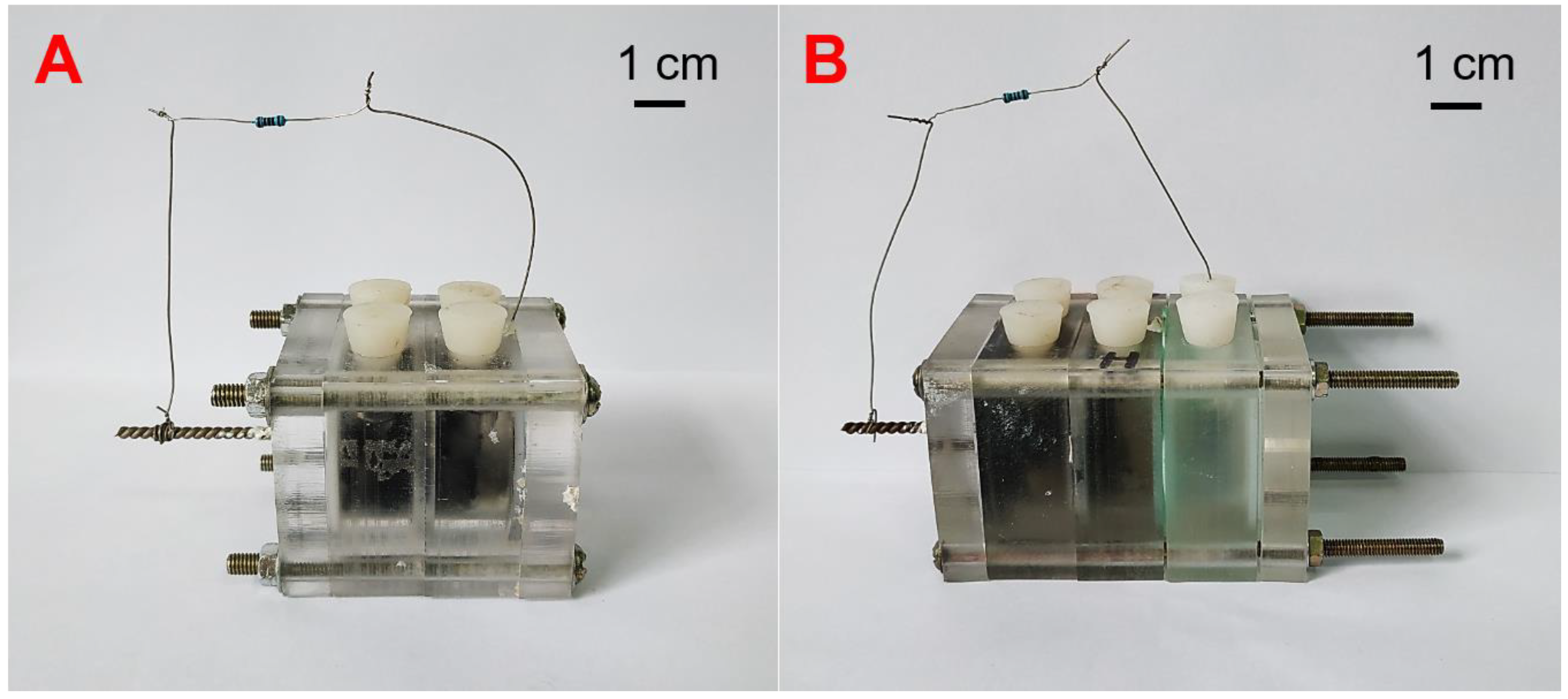
2.1. The Configuration of Microbial Fuel Cell (MFC) Reactors
2.2. Startup and Operation of MFC
2.3. Analysis and Calculations
2.4. Genomic DNA Extraction and MiSeq Sequencing of Bioelectroactive Biofilms
3. Results and Discussion
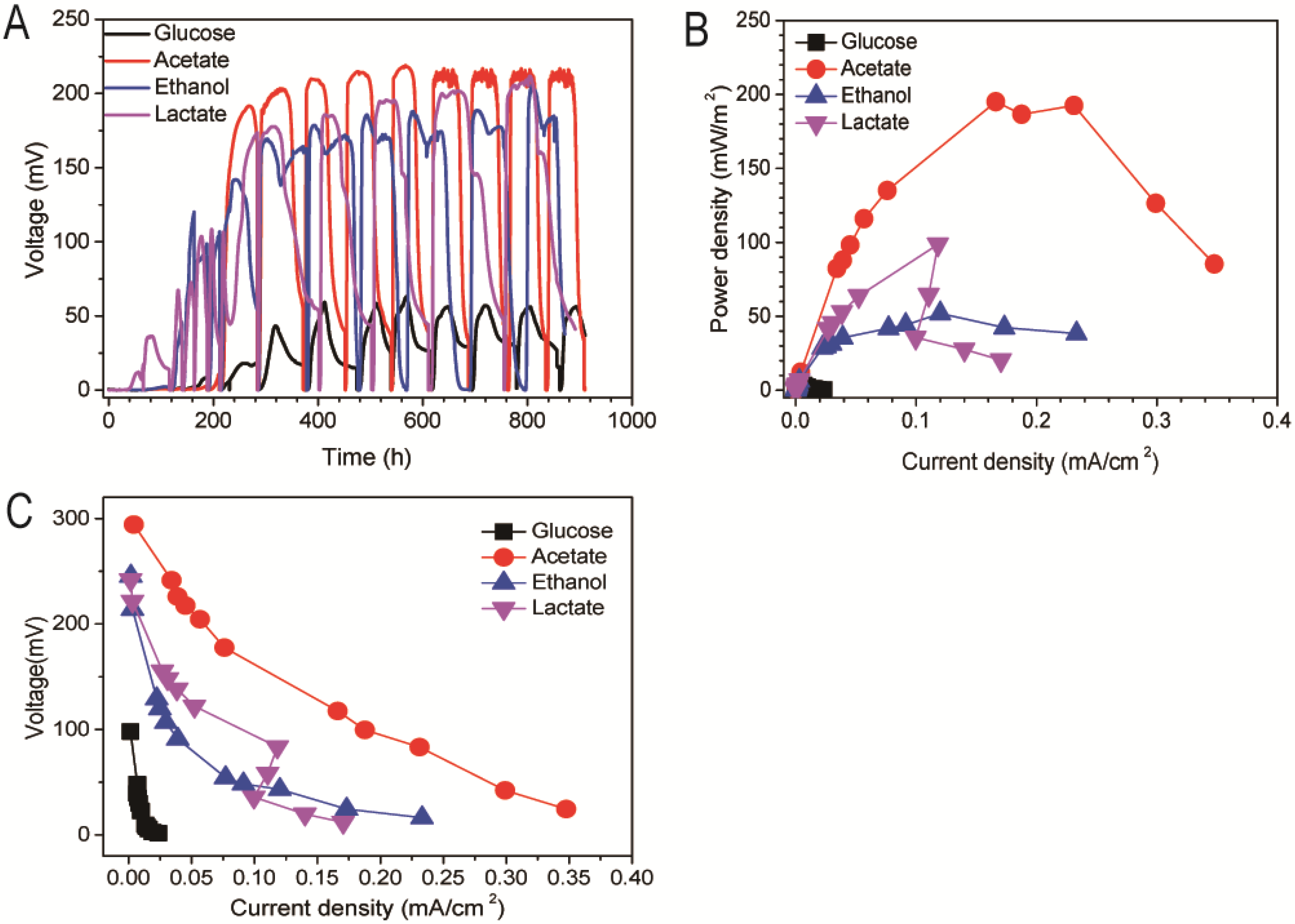
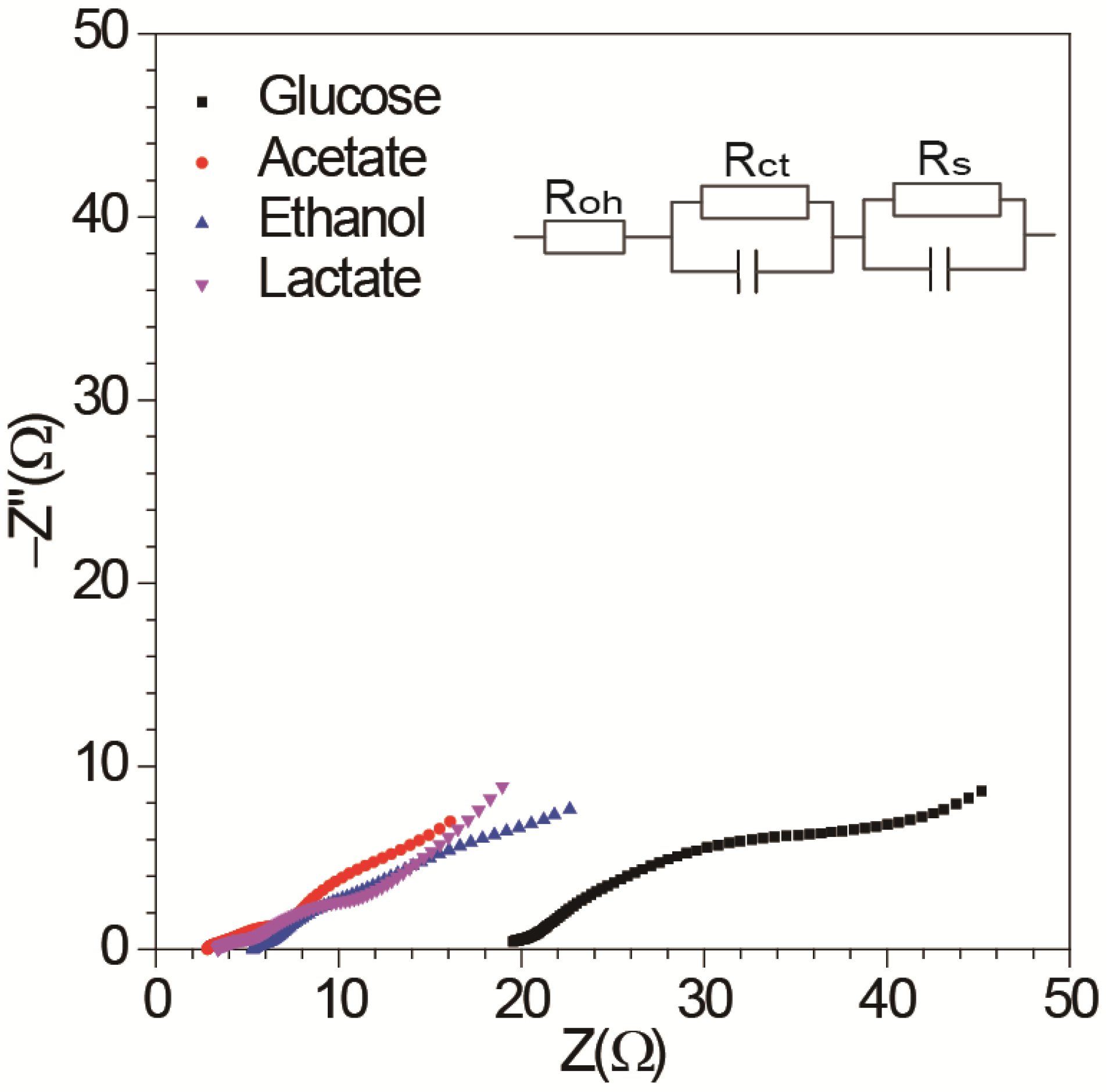
3.1. Effect of Different Energy Substrates on Single Chamber MFC Performance
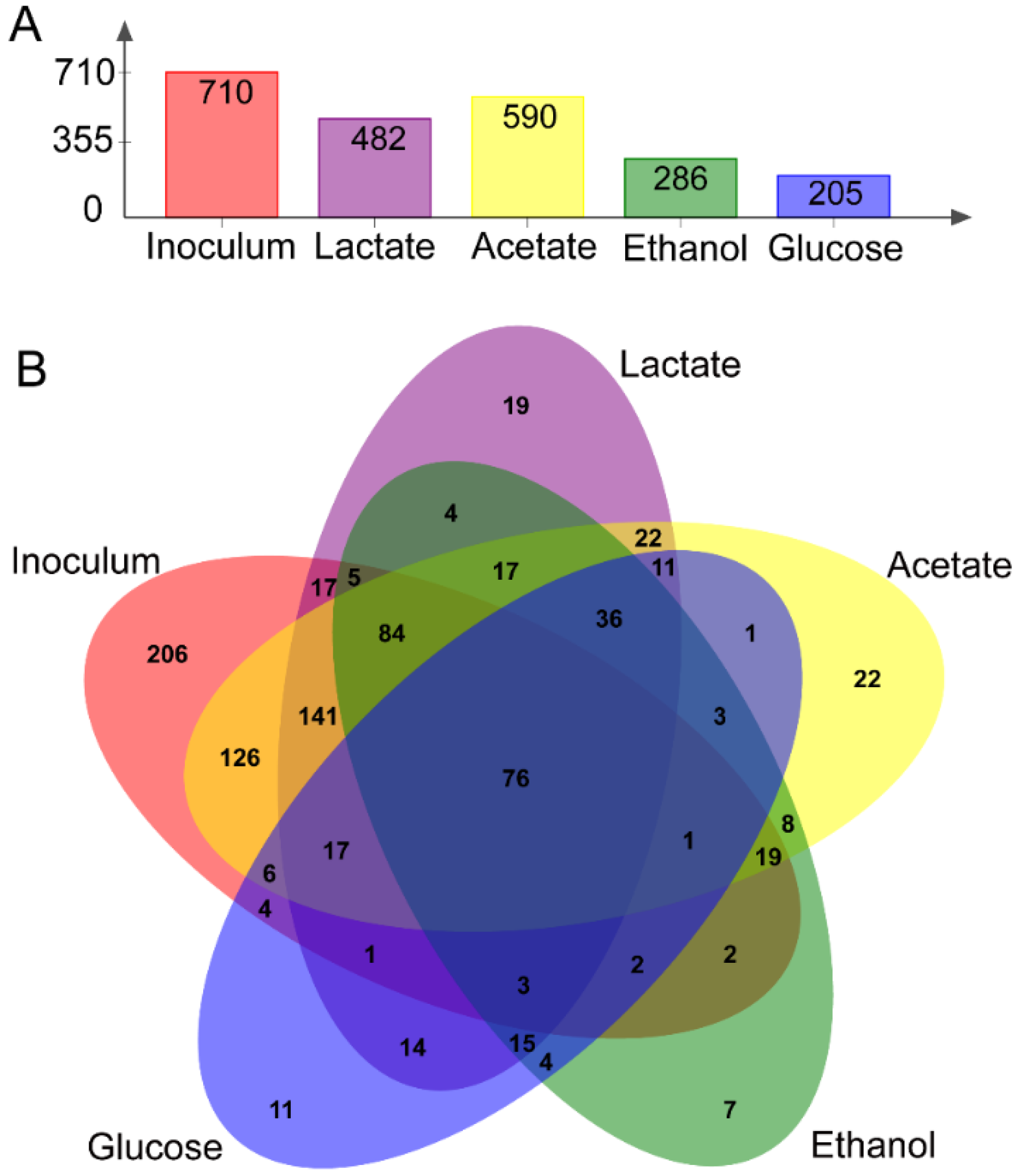
3.2. Microbial Community of Anodic Bioelectroactive Biofilms
3.3. Contribution of Electroactive Biofilms on Anolyte’s Chemical Oxygen Demand Removal and Catholyte’s Copper Recovery
3.4. Morphologies of Electrode and XRD Analysis
3.5. Comparison of this Study with Previous Studies
| Energy Substrates | Research Focuses | Reference | |
|---|---|---|---|
| Microbial Community of Anodic Biofilm | Wastewater (Containing Cu2+) | ||
| acetate, lactate, ethanol, glucose | Yes, high throughput sequencing technique. | Yes | This study |
| acetate, butyrate, propionate, glucose | Yes, using the culture-dependent technique. | No | [12] |
| acetate, ethanol, glucose | No | No | [40] |
| acetate, butyrate, glucose | Yes, using denaturing gradient gel electrophoresis. | No | [37] |
| glucose, methanol, propyl alcohol | Yes, high throughput sequencing technique. | No | [13] |
| acetate, glucose, starch, dextran, butyrate | No | No | [41] |
| glucose, lactose, cheese | No | No | [42] |
| glucose, acetate, xylose | No | No | [43] |
| acetate | No | Yes | [44] |
| acetate | No | Yes | [45] |
| glucose | No | Yes | [46] |
| acetate | No | Yes | [47] |
| acetate | No | Yes | [48] |
| acetate | No | Yes | [17] |
| acetate | No | Yes | [49] |
| acetate | No | Yes | [50] |
| acetate | No | Yes | [51] |
| acetate | Yes, high throughput sequencing technique; anodic biofilm was directly exposed to Cu2+. | Yes | [14] |
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ai, C.; Liang, Y.; Miao, B.; Chen, M.; Zeng, W.; Qiu, G. Identification and analysis of a novel gene cluster involves in Fe2+ oxidation in Acidithiobacillus ferrooxidans ATCC 23270, a typical biomining acidophile. Curr. Microbiol. 2018, 75, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Park, I.; Tabelin, C.B.; Jeon, S.; Li, X.; Seno, K.; Ito, M.; Hiroyoshi, N. A review of recent strategies for acid mine drainage prevention and mine tailings recycling. Chemosphere 2019, 219, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, T.; Herrera, P.S.; Uchiyama, H.; Miyamae, H.; Iyatomi, N.; Hashimoto, K.; Tabelin, C.B. The two-step neutralization ferrite-formation process for sustainable acid mine drainage treatment: Removal of copper, zinc and arsenic, and the influence of coexisting ions on ferritization. Sci. Total Environ. 2020, 715, 136877. [Google Scholar] [CrossRef] [PubMed]
- Simate, G.S.; Ndlovu, S. Acid mine drainage: Challenges and opportunities. J. Environ. Chem. Eng. 2014, 2, 1785–1803. [Google Scholar] [CrossRef]
- Tolonen, E.T.; Sarpola, A.; Hu, T.; Ramo, J.; Lassi, U. Acid mine drainage treatment using by-products from quicklime manufacturing as neutralization chemicals. Chemosphere 2014, 117, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Logan Bruce, E.; Rabaey, K. Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Science 2012, 337, 686–690. [Google Scholar] [CrossRef]
- Ai, C.; Hou, S.; Yan, Z.; Zheng, X.; Amanze, C.; Chai, L.; Qiu, G.; Zeng, W. Recovery of metals from acid mine drainage by bioelectrochemical system inoculated with a novel exoelectrogen, Pseudomonas sp. E8. Microorganisms 2020, 8, 41. [Google Scholar] [CrossRef]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef]
- Wang, H.; Ren, Z.J. A comprehensive review of microbial electrochemical systems as a platform technology. Biotechnol. Adv. 2013, 31, 1796–1807. [Google Scholar] [CrossRef]
- Logan, B.E.; Regan, J.M. Electricity-producing bacterial communities in microbial fuel cells. Trends Microbiol. 2006, 14, 512–518. [Google Scholar] [CrossRef]
- Kiely, P.D.; Regan, J.M.; Logan, B.E. The electric picnic: Synergistic requirements for exoelectrogenic microbial communities. Curr. Opin. Biotech. 2011, 22, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Chae, K.J.; Choi, M.J.; Lee, J.W.; Kim, K.Y.; Kim, I.S. Effect of different substrates on the performance, bacterial diversity, and bacterial viability in microbial fuel cells. Bioresour. Technol. 2009, 100, 3518–3525. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.H.; Qiu, C.H.; Fang, C.F.; Ge, Q.L.; Hui, Y.X.; Han, B.; Pang, S. Characterization of bacterial communities in anode microbial fuel cells fed with glucose, propyl alcohol and methanol. Appl. Biochem. Micro. 2017, 53, 250–257. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, X.; Jin, M.; Li, Y.; Li, S.; Kong, F.; Nan, J.; Wang, A. Copper removal and microbial community analysis in single-chamber microbial fuel cell. Bioresour. Technol. 2018, 253, 372–377. [Google Scholar] [CrossRef]
- Shehab, N.; Li, D.; Amy, G.L.; Logan, B.E.; Saikaly, P.E. Characterization of bacterial and archaeal communities in air-cathode microbial fuel cells, open circuit and sealed-off reactors. Appl. Microbiol. Biot. 2013, 97, 9885–9895. [Google Scholar] [CrossRef]
- Ai, C.; Yan, Z.; Chai, H.; Gu, T.; Wang, J.; Chai, L.; Qiu, G.; Zeng, W. Increased chalcopyrite bioleaching capabilities of extremely thermoacidophilic Metallosphaera sedula inocula by mixotrophic propagation. J. Ind. Microbiol. Biotechnol. 2019, 46, 1113–1127. [Google Scholar] [CrossRef]
- Cheng, S.A.; Wang, B.S.; Wang, Y.H. Increasing efficiencies of microbial fuel cells for collaborative treatment of copper and organic wastewater by designing reactor and selecting operating parameters. Bioresour. Technol. 2013, 147, 332–337. [Google Scholar] [CrossRef]
- Logan B., E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef]
- Herrera, L.; Ruiz, P.; Aguillon, J.C.; Fehrmann, A. A new spectrophotometric method for the determination of ferrous iron in the presence of ferric iron. J. Chem. Technol. Biotechnol. 1989, 44, 171–181. [Google Scholar] [CrossRef]
- Wetlesen, C.U. Rapid spectrophotometric determination of copper in iron, steel and ferrous alloys. Anal. Chim. Acta 1957, 16, 268–270. [Google Scholar] [CrossRef]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microb. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Schloss, P.D.; Gevers, D.; Westcott, S.L. Reducing the effects of PCR amplification and sequencing artifacts on 16S rRNA-based studies. PLoS ONE 2011, 6, e27310. [Google Scholar] [CrossRef] [PubMed]
- Christwardana, M.; Frattini, D.; Accardo, G.; Yoon, S.P.; Kwon, Y. Early-stage performance evaluation of flowing microbial fuel cells using chemically treated carbon felt and yeast biocatalyst. Appl. Energy 2018, 222, 369–382. [Google Scholar] [CrossRef]
- He, Z.; Mansfeld, F. Exploring the use of electrochemical impedance spectroscopy (EIS) in microbial fuel cell studies. Energy Environ. Sci. 2009, 2, 215–219. [Google Scholar] [CrossRef]
- Paitier, A.; Godain, A.; Lyon, D.; Haddour, N.; Vogel, T.M.; Monier, J.M. Microbial fuel cell anodic microbial population dynamics during MFC start-up. Biosens. Bioelectron. 2017, 92, 357–363. [Google Scholar] [CrossRef]
- Cai, W.; Zhang, Z.; Ren, G.; Shen, Q.; Hou, Y.; Ma, A.; Deng, Y.; Wang, A.; Liu, W. Quorum sensing alters the microbial community of electrode-respiring bacteria and hydrogen scavengers toward improving hydrogen yield in microbial electrolysis cells. Appl. Energy 2016, 183, 1133–1141. [Google Scholar] [CrossRef]
- Christwardana, M.; Frattini, D.; Duarte, K.D.Z.; Accardo, G.; Kwon, Y. Carbon felt molecular modification and biofilm augmentation via quorum sensing approach in yeast-based microbial fuel cells. Appl. Energy 2019, 238, 239–248. [Google Scholar] [CrossRef]
- Ai, C.; McCarthy, S.; Eckrich, V.; Rudrappa, D.; Qiu, G.; Blum, P. Increased acid resistance of the archaeon, Metallosphaera sedula by adaptive laboratory evolution. J. Ind. Microbiol. Biotechnol. 2016, 43, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Zhou, D.; Liu, Y.; Yu, R.; Qiu, G.; Zeng, W. Effects of pH value on the expression of key iron/sulfur oxidation genes during bioleaching of chalcopyrite on thermophilic condition. Ann. Microbiol. 2019, 69, 627–635. [Google Scholar] [CrossRef]
- Ai, C.; Yan, Z.; Zhou, H.; Hou, S.; Chai, L.; Qiu, G.; Zeng, W. Metagenomic Insights into the Effects of seasonal temperature variation on the activities of activated sludge. Microorganisms 2019, 7, 713. [Google Scholar] [CrossRef] [PubMed]
- Stöckl, M.; Teubner, N.C.; Holtmann, D.; Mangold, K.M.; Sand, W. Extracellular polymeric substances from Geobacter sulfurreducens biofilms in microbial fuel cells. Acs Appl. Mater. Interfaces 2019, 11, 8961–8968. [Google Scholar] [CrossRef]
- Reguera, G.; McCarthy, K.D.; Mehta, T.; Nicoll, J.S.; Tuominen, M.T.; Lovley, D.R. Extracellular electron transfer via microbial nanowires. Nature 2005, 435, 1098–1101. [Google Scholar] [CrossRef]
- Pant, D.; Van Bogaert, G.; Diels, L.; Vanbroekhoven, K. A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour. Technol. 2010, 101, 1533–1543. [Google Scholar] [CrossRef]
- Zhang, Y.; Min, B.; Huang, L.; Angelidaki, I. Electricity generation and microbial community response to substrate changes in microbial fuel cell. Bioresour. Technol. 2011, 102, 1166–1173. [Google Scholar] [CrossRef]
- Nancharaiah, Y.V.; Venkata Mohan, S.; Lens, P.N. Metals removal and recovery in bioelectrochemical systems: A review. Bioresour. Technol. 2015, 195, 102–114. [Google Scholar] [CrossRef]
- Mathuriya, A.S.; Yakhmi, J.V. Microbial fuel cells to recover heavy metals. Environ. Chem. Lett. 2014, 12, 483–494. [Google Scholar] [CrossRef]
- Sharma, Y.; Li, B. The variation of power generation with organic substrates in single-chamber microbial fuel cells (SCMFCs). Bioresour. Technol. 2010, 101, 1844–1850. [Google Scholar] [CrossRef]
- Min, B.; Logan, B.E. Continuous electricity generation from domestic wastewater and organic substrates in a flat plate microbial fuel cell. Environ. Sci. Technol. 2004, 38, 5809–5814. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulou, G.; Stamatelatou, K.; Bebelis, S.; Lyberatos, G. Electricity generation from synthetic substrates and cheese whey using a two chamber microbial fuel cell. Biochem. Eng. J. 2010, 50, 10–15. [Google Scholar] [CrossRef]
- Thygesen, A.; Poulsen, F.W.; Min, B.; Angelidaki, I.; Thomsen, A.B. The effect of different substrates and humic acid on power generation. Bioresour. Technol. 2009, 100, 1186–1191. [Google Scholar] [CrossRef]
- Heijne, A.T.; Liu, F.; van der Weijden, R.; Weijma, J.; Buisman, C.J.N.; Hamelers, H.V.M. Copper recovery combined with electricity production in a microbial fuel cell. Environ. Sci. Technol. 2010, 44, 4376–4381. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.C.; Zhang, L.J.; Gao, Z.Y.; Wu, W.M. Copper reduction in a pilot-scale membrane-free bioelectrochemical reactor. Bioresour. Technol. 2011, 102, 10334–10339. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.C.; Liang, M.; Li, W.; Zhang, L.J.; Ni, J.R.; Wu, W.M. Removal of copper from aqueous solution by electrodeposition in cathode chamber of microbial fuel cell. J. Hazard. Mater. 2011, 189, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Huang, L.; Quan, X.; Li Puma, G. Electricity generation and bivalent copper reduction as a function of operation time and cathode electrode material in microbial fuel cells. J. Power Sources 2016, 307, 705–714. [Google Scholar] [CrossRef]
- Zhang, L.; Tao, H.; Wei, X.; Lei, T.; Li, J.; Wang, A.; Wu, W. Bioelectrochemical recovery of ammonia–copper(II) complexes from wastewater using a dual chamber microbial fuel cell. Chemosphere 2012, 89, 1177–1182. [Google Scholar] [CrossRef]
- Rodenas Motos, P.; Ter Heijne, A.; van der Weijden, R.; Saakes, M.; Buisman, C.J.; Sleutels, T.H. High rate copper and energy recovery in microbial fuel cells. Front. Microbiol. 2015, 6, 527. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, L.; Wu, D.; Huang, L.; Zhou, P.; Quan, X.; Chen, G. Dependency of simultaneous Cr(VI), Cu(II) and Cd(II) reduction on the cathodes of microbial electrolysis cells self-driven by microbial fuel cells. J. Power Sources 2015, 273, 1103–1113. [Google Scholar] [CrossRef]
- Luo, H.; Liu, G.; Zhang, R.; Bai, Y.; Fu, S.; Hou, Y. Heavy metal recovery combined with H2 production from artificial acid mine drainage using the microbial electrolysis cell. J. Hazard. Mater. 2014, 270, 153–159. [Google Scholar] [CrossRef] [PubMed]

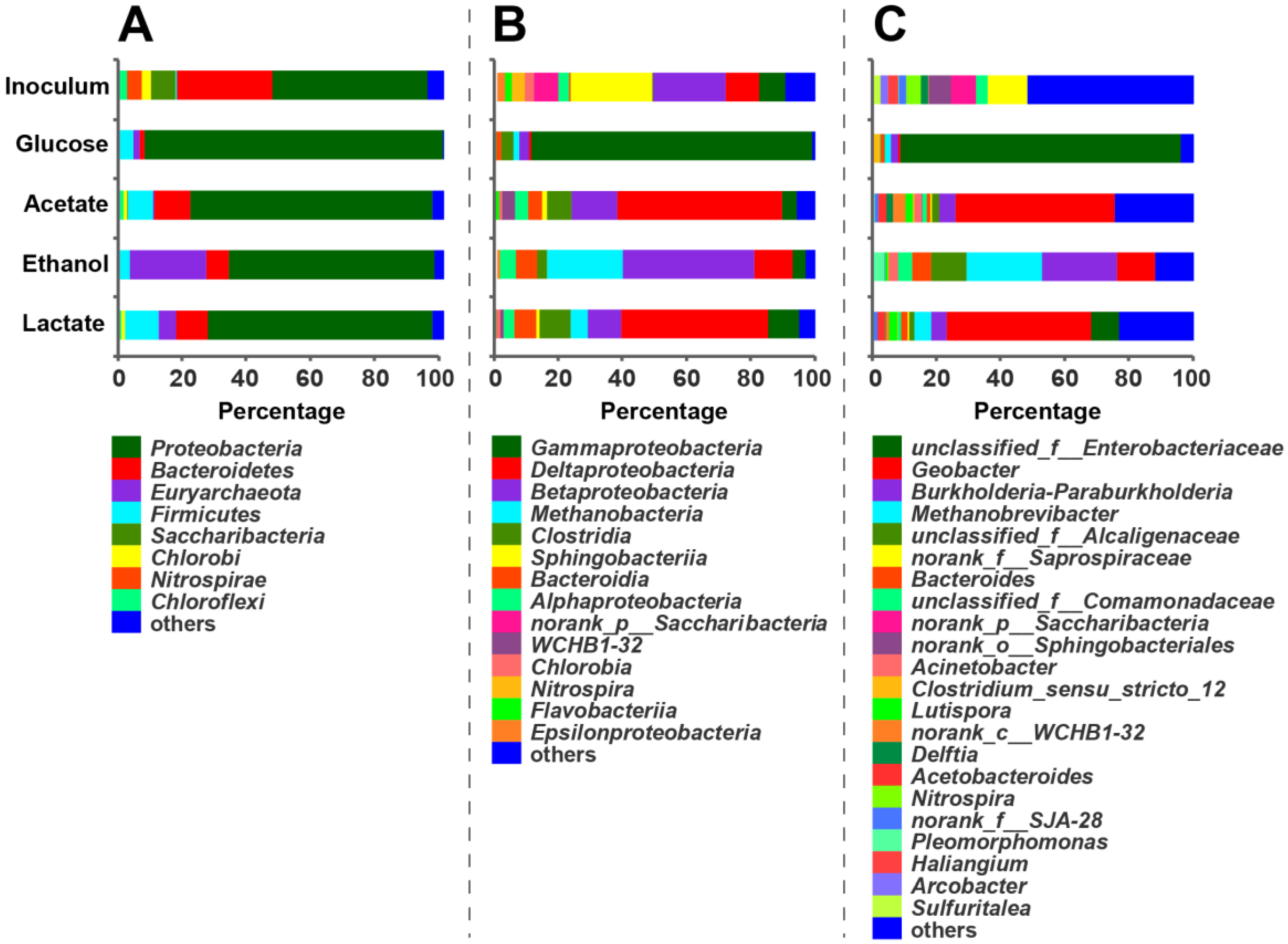


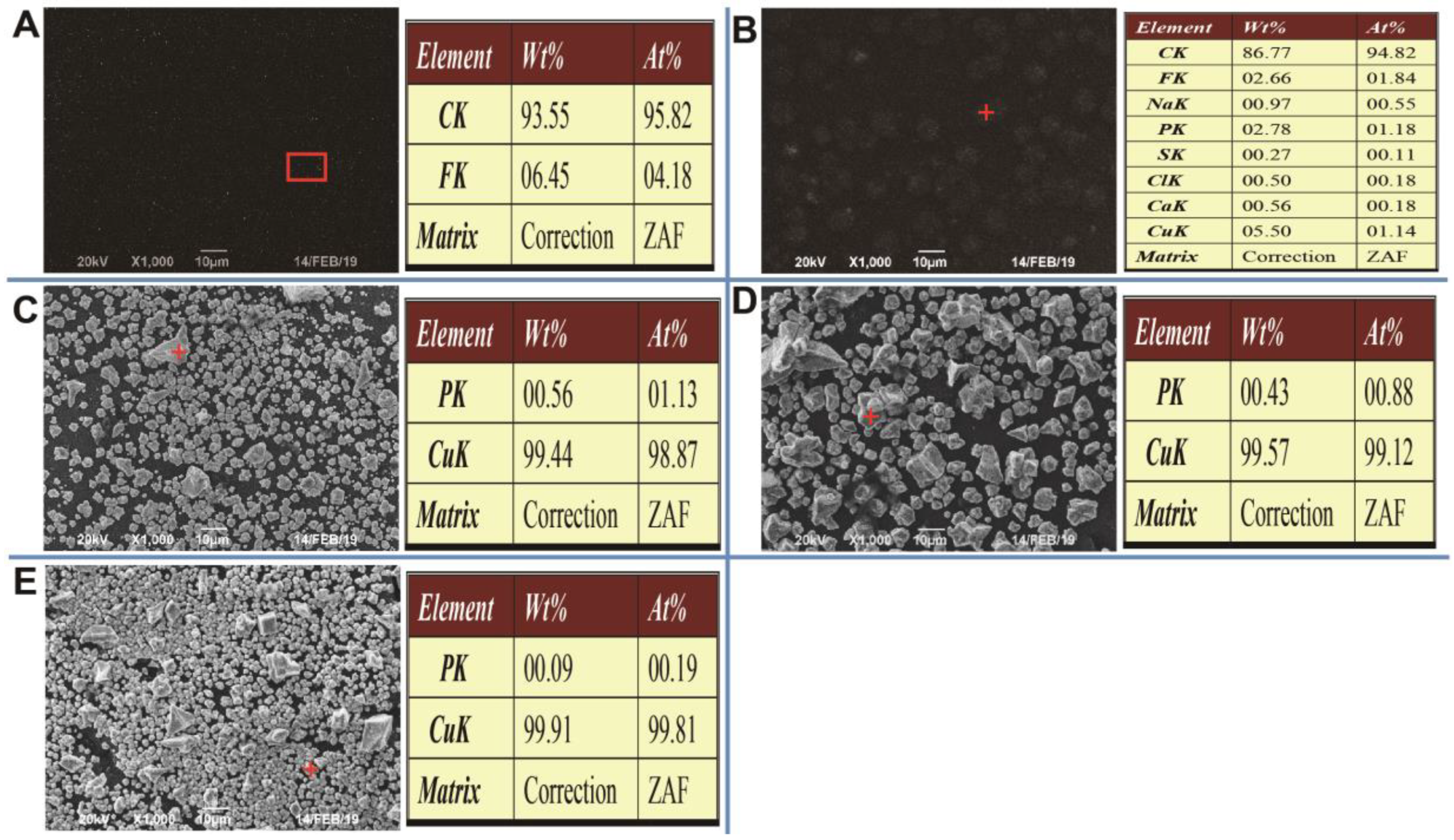
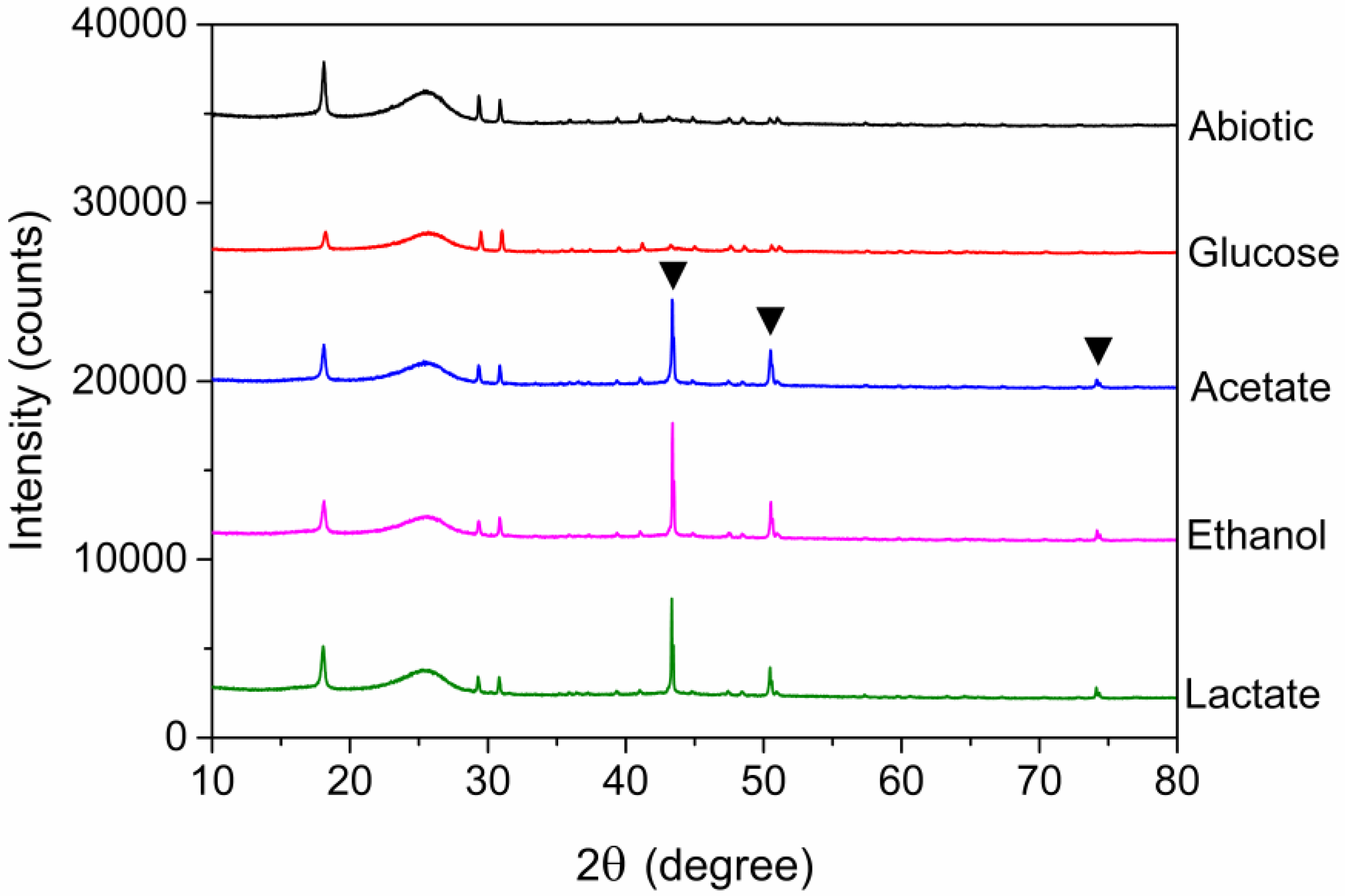
| Sample | Reads | Sobs | Shannon | Simpson | Ace | Chao | Coverage |
|---|---|---|---|---|---|---|---|
| Inoculum | 40818 | 710 | 5.2069 | 0.0138 | 732.31 | 735.02 | 0.9988 |
| Glucose | 97701 | 205 | 0.7931 | 0.7633 | 363.79 | 347.38 | 0.9993 |
| Acetate | 45985 | 590 | 2.9848 | 0.2534 | 670.25 | 672.18 | 0.9975 |
| Ethanol | 43875 | 286 | 2.7412 | 0.1233 | 428.09 | 371.00 | 0.9981 |
| Lactate | 32430 | 482 | 2.9694 | 0.2130 | 589.26 | 586.79 | 0.9963 |
| Archaeal Genus | Percentage in Anode Biofilm (%) | ||||
|---|---|---|---|---|---|
| Inoculum | Glucose | Acetate | Ethanol | Lactate | |
| Methanobacterium | 0 | 0.01 | 0 | 0.04 | 0.15 |
| Methanobrevibacter | 0 | 1.82 | 0 | 23.70 | 5.23 |
| Methanosaeta | 0 | 0 | 0.02 | 0 | 0.05 |
| Methanosarcina | 0 | 0 | 0.19 | 0 | 0 |
| Methanomassiliicoccus | 0 | 0 | 0.25 | 0 | 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ai, C.; Yan, Z.; Hou, S.; Zheng, X.; Zeng, Z.; Amanze, C.; Dai, Z.; Chai, L.; Qiu, G.; Zeng, W. Effective Treatment of Acid Mine Drainage with Microbial Fuel Cells: An Emphasis on Typical Energy Substrates. Minerals 2020, 10, 443. https://doi.org/10.3390/min10050443
Ai C, Yan Z, Hou S, Zheng X, Zeng Z, Amanze C, Dai Z, Chai L, Qiu G, Zeng W. Effective Treatment of Acid Mine Drainage with Microbial Fuel Cells: An Emphasis on Typical Energy Substrates. Minerals. 2020; 10(5):443. https://doi.org/10.3390/min10050443
Chicago/Turabian StyleAi, Chenbing, Zhang Yan, Shanshan Hou, Xiaoya Zheng, Zichao Zeng, Charles Amanze, Zhimin Dai, Liyuan Chai, Guanzhou Qiu, and Weimin Zeng. 2020. "Effective Treatment of Acid Mine Drainage with Microbial Fuel Cells: An Emphasis on Typical Energy Substrates" Minerals 10, no. 5: 443. https://doi.org/10.3390/min10050443
APA StyleAi, C., Yan, Z., Hou, S., Zheng, X., Zeng, Z., Amanze, C., Dai, Z., Chai, L., Qiu, G., & Zeng, W. (2020). Effective Treatment of Acid Mine Drainage with Microbial Fuel Cells: An Emphasis on Typical Energy Substrates. Minerals, 10(5), 443. https://doi.org/10.3390/min10050443




