Abstract
Crawling is an almost universal stage of locomotor development in infants; however, it is difficult to quantify using typical motion analysis techniques. The crawling stage therefore has underutilized potential to assess development and detect deviations or abnormalities. This study measured longitudinal weightbearing asymmetries in typically developing (TD) crawling children and compared this population to children with limb loss or limb differences (LLD) using a pressure-sensing mat. The LLD group bore significantly more weight using their arms vs. their legs than the TD group (p < 0.001), but even in cases of unilateral limb loss, bilateral weightbearing symmetry was similar to TD, controlling for body mass and age (p = 0.570). As children in the TD group developed and gained body mass, their weight shifted significantly to their left side (η2 = 0.050) and away from their arms and toward their legs (η2 = 0.255). The results provide insight into the biomechanical development of TD infant crawling, and the ways in which an atypically developing population manages weightbearing during crawling. The establishment of symmetry data will be useful, as crawling can serve as an opportunity for earlier detection of neuromotor conditions such as cerebral palsy. Furthermore, insight into the crawling patterns of children with limb loss and limb difference can inform prosthetic prescription and the need to consider a missing weight shift toward the legs as children develop.
1. Introduction
Despite the observation that 95.7% of human infants crawl as part of their neuromotor development toward upright bipedal locomotion [1], there are very few quantitative studies available to understand the biomechanics of infant crawling. Crawling can relay information about an infant’s development and indicate certain health-related conditions [2]. Development through various modes of human infant crawling has been a focus within theoretical frameworks, such as Dynamic Systems Theory, that seek to understand how and why infants learn to move. New motor skills emerge from a child’s ability to interact with and organize multiple complex subsystems embedded within the individual and open to complex conditions in the environment and the demands of the task [3,4]. As infants learn to move, these interactions become motor patterns for crawling and walking.
The transitions from crawling to unsupported upright locomotion and from early steps to more stable walking represent important windows to understanding the development of multiple systems in typically and atypically developing children [5]. By extension, if quantified data from typically developing infants are established, crawling and early walking are opportunities to identify previously undiagnosed atypical development. Several studies have sought to characterize these seemingly chaotic periods of motor growth. Ivanenko et al. observed that toddlers utilize a systematically different pattern of vertical trunk oscillations compared to adults, and that toddlers lack efficient transfer of energy to and from kinetic and gravitational potential energy, per an inverted pendulum model of gait [6]. Adolph observed motor strategies in healthy, typically developing infants whose body dimensions were manipulated by wearing either unweighted or heavy weighted vests as they negotiated adjustable steep and shallow sloping surfaces [7,8]. Some infants who moved freely on slopes in unweighted vests altered or refused movement when wearing weighted vests on the same slope. The infants in heavy vests had less exploration, stepping, and swaying at the top of the slope. Adolph suggested that growth creates natural changes in body dimensions that make the infant’s body more stable; for example, the center of mass is lowered as the body grows. Adolph observed that because infants learn to crawl and walk while their body dimensions are changing, locomotion requires continual adaptation to cope with changing body size, environment, ground surfaces, and activity [9].
While these and other studies have provided valuable insight into the development of crawling in infants, they are limited in terms of quantitative biomechanical data. Relatively few studies have used traditional marker-based motion analysis with infants (examples include [10,11,12]). Similarly, few studies (e.g., [13]) have collected kinetic data. The focus on observation over instrumentation is understandable given the challenges of data collection in mobile infants; however, it has limited our ability to understand factors associated with development that are less easily observed, such as dynamic weightbearing symmetry [14,15].
Studies of symmetry in walking development date back decades. In 1969, Scrutton analyzed the footprints of children aged 1 to 4 years old whose feet were coated in talcum powder to determine spatial parameters such as step length and foot progression angle [16]. The study confirmed expected bilateral symmetry in normalized step lengths, with slightly higher rates of asymmetry in the youngest children in the population. In 1993, Cioni et al. used observational analysis of videos of full-term and pre-term infants at the onset of independent walking [17]. The authors established “asymmetry” as one of their observational criteria, defined based on transverse plane rotation during progression. The measure was observed in both populations in early walking but had resolved into a fully symmetrical pattern in both groups when observed at 4 months following the onset of walking. Weightbearing symmetry is an intuitive measure of neuromotor organization and musculoskeletal structure; however, it has not been assessed in infant crawling due to the challenges associated with measurement in this population. Typically, motion analysis laboratories use force platforms for kinetic measurement and require contact with one (and only one) limb on each force platform to provide the necessary ground reaction force vector [18]. This approach is impractical for infant crawling.
This study utilized a distributed array of ground contact pressure transducers to assess the weightbearing contribution of each of the four limbs during crawling in a typically developing population and a population of children with limb loss or limb difference. The results contribute to the understanding of weightbearing symmetry and determine if known skeletal differences actually result in expected weightbearing asymmetries.
2. Materials and Methods
Participants were grouped into two populations: typically developing (TD) infants and infants with limb loss or limb difference (LLD). The study was approved by the institutional review boards of Kennesaw State University and Children’s Healthcare of Atlanta, and parental permission was obtained prior to screening and data collection.
Children in the TD group were screened using the third edition of the Ages & Stages Questionnaire--ASQ-3 [19]. This is a widely used, validated parent-report measure of child development with well-established age-based norms. The instrument assesses development in the areas of communication, gross motor, fine motor, problem solving, and personal-social. Cutoff scores determine typical development in each area. We accepted children with scores above the cutoff in both gross motor and fine motor areas. Children in the TD group were seen every two weeks from onset of crawling to transition to walking as the primary means of locomotion (>50%).
Children in the LLD group (Table 1) were referred through either the Orthopedics or the Orthotics and Prosthetics departments at Children’s Healthcare of Atlanta. Children were included who were utilizing unassisted crawling as primary means of locomotion and had congenital or acquired unilateral or bilateral limb loss in at least one limb at or proximal to the ankle or wrist joint. To include a range of potential asymmetries, we did not restrict the sample to lower limb, unilateral, etc., although the majority of participants were unilateral lower limb involved (Table 1). Some children with limb loss received a prosthetic limb during the study but were analyzed without a prosthesis. The ASQ-3 was not administered for this group because their gross motor development was known to be atypical. Children in the LLD group were assessed only once, not every two weeks, because this sample was drawn from a much wider geographic region and return visits were often impractical.

Table 1.
Participant information for the LLD group. Age refers to non-corrected age at study date in months. Affected limbs are coded as Right (R) or Left (L) and Leg (L) or Arm (A).
Crawling was assessed using a 4.9 m × 0.6 m Zeno pressure transducer mat (ProtoKinetics, Havertown, PA, USA) in a setup similar to that in ref. [20]. The mat incorporates a distributed array of 1 cm2 force sensors that output 16 levels of pressure sampling at 120 Hz. Sensor gains were adjusted to a range of pressures appropriate for infant body weights. PKMAS4 software version 1.00C1i14, which was originally designed for animal-based studies of quadrupedal gait, was used for data collection and reduction. A distributed pressure array mat was also used by Yozu et al.; their mat sampled at 80 Hz and covered 0.88 m × 0.96 m [13]. Two cameras recorded sagittal and frontal plane video as a reference for data processing. In each session, the infant’s clothing was removed, leaving only a diaper, to maintain consistent surface contact and friction and joint mobility. Each child was encouraged to crawl back and forth along the length of the mat by positioning caregivers, siblings, or toys along or at the opposite end of the mat. Objects were kept far enough in front of the infant to avoid any reaching with the non-weightbearing arm during crawling. Crawling passes were considered useful when they contained at least five steady-state crawling cycles for any reference limb. If the infant’s temperament permitted, we sought to obtain three to five passes for each session.
Points of contact were manually labeled as right or left arm or leg. For the legs, any potential discrete contacts with the same knee and foot were combined into a single leg contact (Figure 1). The time-series pressure level under each point of contact was integrated across the entire step using PKMAS4 software. Two symmetry measures for this integrated pressure were analyzed, arms vs. legs or anterior vs. posterior (IPR A-P) and left side combined arm and leg vs. right side combined arm and leg (IPR L-R). The first, IPR A-P, measures the role of arms vs. legs in both supporting body weight and providing additional propulsive accelerations and braking decelerations. A perfectly balanced child with their body center of mass located at the midway point between the four support centers of pressure should maintain an average IPR A-P of 1.0.
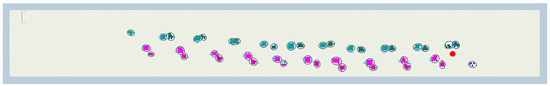
Figure 1.
Screen image from PKMAS4 software indicating contact points during a single crawling trial from left to right. Completed contact points are indicated with solid colors and ellipses. Green tones are left limbs; pink tones are right limbs. Darker colors are hand contacts, and lighter colors are knee contacts, including the foot if it made contact. On the right side of the figure, active cells show the current pressure level, and the red dot represents the body’s center of pressure. The second measure, IPR L-R, measures any asymmetry in weightbearing between the left arm and leg and the right arm and leg. Perfect balance between sides would produce an average APR L-R of 1.0. each measure was averaged across each steady-state step cycle during a given trial.
For the TD group, the latter measure was always reported as left divided by right. For the LLD group, an additional measure was created based on the involved side, dividing the contralateral arm and leg IPs by the ipsilateral arm and leg IPs (IPR C-I). A hypothesis that a child with limb loss or limb difference involving one leg would “favor” and bear more weight on the opposite intact leg would therefore be equivalent to hypothesizing that IPR C-I is greater than one.
IPR A-P, IPR L-R, and IPR C-I symmetries were analyzed using a multiple linear regression model with panel-corrected standard error estimators to account for correlation across time. Factors included development status (TD vs. LLD), age at time of data collection, and body mass at time of data collection. The latter two factors were considered across all data points and then again using only data from the TD group to account for typical progression toward walking. Alpha was set at 0.05. In addition to test statistics and p-values being reported, η2 effect sizes were also calculated for each independent variable in each model as a way of quantifying the importance of independent variables in explaining the variability in the respective outcomes without sample size influencing the interpretation. Effect size classifications were made according to the traditional cut points given by Cohen [21].
3. Results
The analysis included 39 children: 27 children in the TD group (with multiple visits to the laboratory every two weeks) and 12 children in the LLD group (with a single visit). Data corruption prevented the analysis of LL04 and 07. A total of 714 crawling trials were assessed. The LLD group was significantly older (p = 0.026) than the TD group due to gross motor delays associated with limb loss or difference; however, body mass and length were not significantly different.
3.1. Differences in Weightbearing Symmetry Between Groups
IPR A-P was, on average, greater than one in both groups, with mean ± standard deviation of 1.40 ± 0.44 in the LLD group and 1.16 ± 0.46 in the TD group (Figure 2). After controlling for age and body mass, there was a significant difference (p < 0.001) in IPR A-P between groups, with a medium effect size (Table 2).
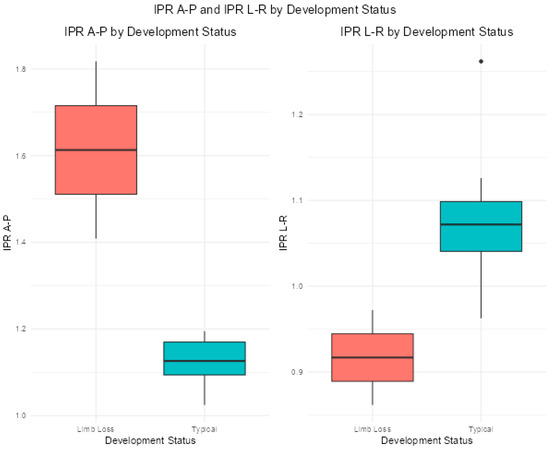
Figure 2.
Boxplots comparing means for IPR A-P and IPR L-R for each group.

Table 2.
Regression results for comparisons of weightbearing symmetry between groups.
IPR L-R was, on average, slightly less than one (0.96 ± 0.25) in the LLD group and greater than one (1.12 ± 0.38) in the LLD group. The IPR L-R regression model required a transformation in order for the assumptions of inference (normality and constant variance) to be reasonably met. Per the model, the square root of the L-R value for TD was 0.08 greater than for children in the LLD group, on average, controlling for age and mass, with a significant difference (p < 0.001) (Table 2). However, when IPR L-R was modified for the TD group to reflect contralateral limb divided by ipsilateral limb (IPR C-I) and compared to IPR L-R in the TD group, the difference was no longer significant (p = 0.570).
3.2. Differences in Weightbearing Symmetry with Development in TD Children
This analysis focused on the TD group only. These children were seen every two weeks from onset of crawling to transition to walking, so the assessment determined how the two measures of weightbearing symmetry changed as a child developed a more mature crawling pattern. Independent assessments were performed for the role of time (recorded as the child’s age in weeks at the time of each data collection session) and growth (recorded as each child’s body mass at the time of each data collection session).
Considering IPR A-P, for each additional week older, the model predicted an expected increase of 0.005, on average, controlling for body mass (p = 0.029). And for each additional kg increase in body mass, the model predicted an expected average decrease of 0.131, controlling for age (p < 0.001) (Table 3, Figure 3).

Table 3.
Regression results for developmental changes in weightbearing symmetry with age (weeks).
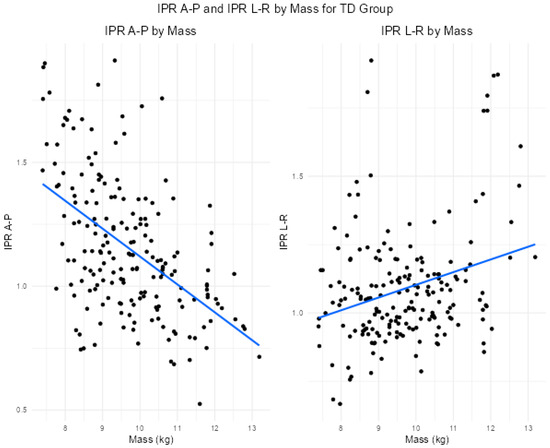
Figure 3.
Scatterplot for IPR A-P and IPR M-L versus body mass in kg in the typically developing cohort. Blue lines indicate line of best fit.
For IPR L-R, age was not significant (p = 0.275). For each additional kg increase in body mass, the model predicted an expected average increase in the square root of IPR L-R of 0.02, controlling for age (p < 0.001) (Table 4, Figure 3).

Table 4.
Regression results for developmental changes in weightbearing symmetry with body mass (kg).
4. Discussion
The purpose of this study was to improve the understanding of weightbearing symmetry during crawling in typically and atypically developing infants. The limb loss/limb difference population was chosen to represent atypical development, since they present defined anatomical asymmetries, as opposed to less well-defined neuromotor asymmetries that might be present in a neurologically involved population, such as children with cerebral palsy.
The study offers an unprecedented comparison of kinetic measures collected during infant crawling. There are substantial normative databases for walking in both adults and young children, and assumptions regarding symmetry are easily tested. In crawling, however, typical gait lab kinetics (embedded force platforms or instrumented treadmills) are impractical, so large datasets do not exist. So, while we might have assumed that typically developing children might be kinetically symmetrical during crawling, we have never before been able to test this assumption. The ensemble averages obtained here, across 708 unique trials from 27 children who varied naturally in age, size, and stage of development, showed that crawling was generally symmetrical but slightly favored weightbearing of the arms over the legs (1.14 ratio) and, for some reason, slightly favored the left side over the right side (1.10 ratio).
4.1. Anterior–Posterior Symmetry
All but one child in the LLD population had lower limb involvement, which was expected to produce differences in IPR A-P. The clinical implications have perhaps been anecdotally discussed, but to our knowledge have never considered in the literature. When part of a limb is absent, the total mass of the limb is reduced. Lower limb loss therefore shifts the body center of mass superiorly (or, in the case of quadrupedal crawling, anteriorly). This shift would suggest that the arms would bear more weight than the legs. However, the legs, designed for propulsion in eventual bipedal gait, have stronger muscles and therefore might be expected to generate greater downward acceleration, shifting the ratio toward the legs. In our LLD population, certain muscles were absent or dysfunctional, which again shifted expectations. Our results indicated that, even when controlling for age and body mass, children with (generally lower) limb loss or limb difference bore more weight with their arms than typically developing children. This observation may have implications for transitioning to walking with a prosthesis, as an intuitive theory would suggest that weightbearing should shift toward the legs.
4.2. Bilateral Symmetry
The results for bilateral symmetry were unexpected. Although the majority of participants in the LLD group had right leg involvement, the distribution was even enough that the L-R ratio would likely regress to the mean. For this reason, the LLD data were adjusted to reflect a contralateral–ipsilateral ratio and then compared to the arbitrary left–right ratio applied for the TD group. However, in the regression model, the L-R ratio showed significant differences, while the C-I ratio did not. The effect size for the L-R ratio was small, so a reasonable conclusion is that children with lower limb loss or difference tend to achieve a bilaterally symmetrical crawling gait similar to that of their typically developing peers. It should be noted that most of the LLD population with lower limb involvement were affected distal to the knee joint, which plays an important role in hands-and-knees crawling. As clinicians consider early prosthetic knee joint prescription for more proximally involved crawlers, the role of the prosthesis in crawling biomechanics should be considered [11].
4.3. Typical Development of Symmetry in Infant Crawling
The study also provides a glimpse into how symmetry develops as typically developing children start crawling and then refine their locomotor pattern as they grow. Biomechanically, one might expect bilateral symmetry to be generally maintained and perhaps more consistent with development. Regarding A-P asymmetry, a shift toward weightbearing in the legs could be expected as preparation for upright walking.
Data from 27 typically developing children showed that, indeed, bilateral symmetry was consistent. Based on the standard deviation, it did not appear that bilateral symmetry was achieved more consistently as the children grew. Binning data into three mass levels (low, medium, and high) showed IPR L-R standard deviations of 0.26, 0.20, and 0.29, respectively.
The hypothesis regarding a shift toward lower limb weightbearing with growth had the largest effect size in our analysis. Mass is a better indicator of growth-related change than age, likely because children begin crawling at different ages, and we were using uncorrected gestational ages. As the body mass of typically developing children in our sample increased, they bore more weight using their legs and less weight using their arms.
4.4. Limitations
The study had limitations. The LLD population was small and should be viewed as a convenience sample, given the small number of children diagnosed with congenital or early-acquired LLD and the challenges of recruiting children at the crawling stage who presented at a large children’s hospital with limb loss or limb difference. In addition, the LLD group was seen only once, while the TD group was seen longitudinally every two weeks, due to the geographic breadth over which the LLD group was recruited. This limited analysis of symmetrical changes with motor development and physical growth to the TD group only.
4.5. Clinical Implications
The results provide insight into the biomechanical development of infant crawling and the ways in which an atypically developing population manages weightbearing during crawling. The establishment of symmetry data will be useful, as crawling can serve as an opportunity for earlier detection of neuromotor conditions such as cerebral palsy. Furthermore, insight into the crawling patterns of children with limb loss and limb difference can help allied healthcare teams better understand the role of a prosthesis in a younger population and the need to consider a missing weight shift toward the legs as children develop.
5. Conclusions
In conclusion, this study found that children with limb loss or limb differences bore significantly more weight using their arms vs. their legs than their typically developing peers, but even in the cases of unilateral limb loss, their bilateral weightbearing symmetry was similar to that of typically developing children, controlling for body mass and age. Over time, as children in the TD group developed, their weight shifted significantly to their left side (small effect size) and away from their arms and toward their legs (large effect size).
Author Contributions
Conceptualization, M.D.G., C.C. and J.C.; methodology, M.D.G. and A.B.; formal analysis, A.B.; investigation, M.D.G., J.C., M.K., E.S., L.B. and L.N.; writing—original draft preparation, M.D.G. and C.C.; writing—review and editing, M.D.G., J.C., C.C., M.K., E.S., L.B., L.N. and A.B.; visualization, M.D.G.; supervision, M.D.G.; project administration, M.D.G., M.K. and J.C.; funding acquisition, M.D.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Gerber Foundation, grant number 9352.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the ethics committees of Children’s Healthcare of Atlanta (00001472) on 19 September 2023 and Kennesaw State University (IRB FY23-24) on 10 October 2022. Parental permission was obtained prior to screening and data collection.
Data Availability Statement
The original data presented in the study are openly available in Digital Commons at https://digitalcommons.kennesaw.edu/datasets/7/, DOI 10.32727/27.2025.1. (accessed on 25 July 2025).
Acknowledgments
The authors acknowledge the contributions of the team of Kennesaw State University MSPO students and First Year and Sophomore Scholars.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| IPR | Integrated Pressure Ratio |
| A-P | Anterior–Posterior |
| L-R | Left–Right |
| C-I | Contralateral–Ipsilateral |
| TD | Typically Developing |
| LLD | Limb Loss/Limb Difference |
References
- WHO Multicentre Growth Reference Study Group; de Onis, M. Who motor development study: Windows of achievement for six gross motor development milestones. Acta Paediatr. 2006, 450, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Yonghi, L.; Matsumura, U.; Tsurusaki, T. Diversity and regularity in infant crawling with typical development. J. Phys. Ther. Sci. 2020, 32, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Forssberg, H. Neural control of human motor development. Curr. Opin. Neurobiol. 1999, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Goldfield, E.C. Transition from rocking to crawling-postural constraints on infant movement. Dev. Psychol. 1989, 25, 913–919. [Google Scholar] [CrossRef]
- Kretch, K.S.; Dusing, S.C.; Harbourne, R.T.; Hsu, L.-Y.; Sargent, B.A.; Willett, S.L. Early mobility and crawling: Beliefs and practices of pediatric physical therapists in the united states. Pediatr. Phys. Ther. 2024, 36, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Ivanenko, Y.P.; Dominici, N.; Lacquaniti, F. Development of independent walking in toddlers. Exerc. Sport Sci. Rev. 2007, 35, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Adolph, K.E.; Avolio, A.M. Walking infants adapt to changing body dimensions. J. Exp. Psychol. Hum. Percept. Perform. 2000, 26, 1148–1166. [Google Scholar] [CrossRef] [PubMed]
- Adolph, K.E. Babies’ steps make giant strides toward a science of development. Infant Behav. Dev. 2002, 25, 86–90. [Google Scholar] [CrossRef]
- Adolph, K.E.; Vereijken, B.; Denny, M.A. Denny. Learning to crawl. Child. Dev. 1998, 69, 1299–1312. [Google Scholar] [CrossRef] [PubMed]
- Righetti, L.; Nylen, A.; Rosander, K.; Ijspeert, A.J. Kinematic and gait similarities between crawling human infants and other quadruped mammals. Front. Neurol. 2015, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Geil, M.D.; Coulter-O’Berry, C. Temporal and spatial parameters of crawling in children with limb loss: Implications on prosthetic knee prescription. J. Prosthet. Orthot. 2010, 22, 21–25. [Google Scholar] [CrossRef]
- Geil, M.D.; Coulter-O’bErry, C.; Schmitz, M.; Heriza, C.P. Crawling kinematics in an early knee protocol for pediatric prosthetic prescription. J. Prosthet. Orthot. 2013, 25, 22–29. [Google Scholar] [CrossRef]
- Yozu, A.; Haga, N.; Tojima, M.; Zhang, Y.; Sumitani, M.; Otake, Y. Vertical peak ground force in human infant crawling. Gait Posture 2013, 37, 293–295. [Google Scholar] [CrossRef] [PubMed]
- Deffeyes, J.E.; Harbourne, R.T.; DeJong, S.L.; Kyvelidou, A.; Stuberg, W.A.; Stergiou, N. Use of information entropy measures of sitting postural sway to quantify developmental delay in infants. J. Neuroeng. Rehabil. 2009, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Liu, Y.; Yi, L.; Fang, J.; Yang, Y.; Wei, K. Abnormal gait patterns in autism spectrum disorder and their correlations with social impairments. Autism Res. 2020, 13, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Scrutton, D.R. Footprint sequences of normal children under five years old. Dev. Med. Child. Neurol. 1969, 11, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Cioni, G.; Duchini, F.; Milianti, B.; Paolicelli, P.; Sicola, E.; Boldrini, A.; Ferrari, A. Differences and variations in the patterns of early independent walking. Early Hum. Dev. 1993, 35, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Winter, D.A. Biomechanics and Motor Control of Human Movement, 3rd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2005. [Google Scholar]
- Squires, J.; Bricker, D. Ages & Stages Questionnaires®, Third Edition (asq®-3): A Parent-Completed Child Monitoring System. 3; Paul H. Brookes Publishing Co., Inc.: Baltimore, MD, USA, 2009. [Google Scholar]
- Geil, M.D. Impact of a three-week full leg cast immobilization on infant crawling kinetics and spatiotemporal parameters. PLoS ONE 2025, 20, e0318106. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).