Pilot Study: Effects of High-Intensity Training on Gait Symmetry and Locomotor Performance in Neurodivergent Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. High-Intensity Interval Training (HIIT) Protocol
2.3. Kinematic and Spatiotemporal Testing
2.4. Energetics
2.5. Statistical Analyses
3. Results
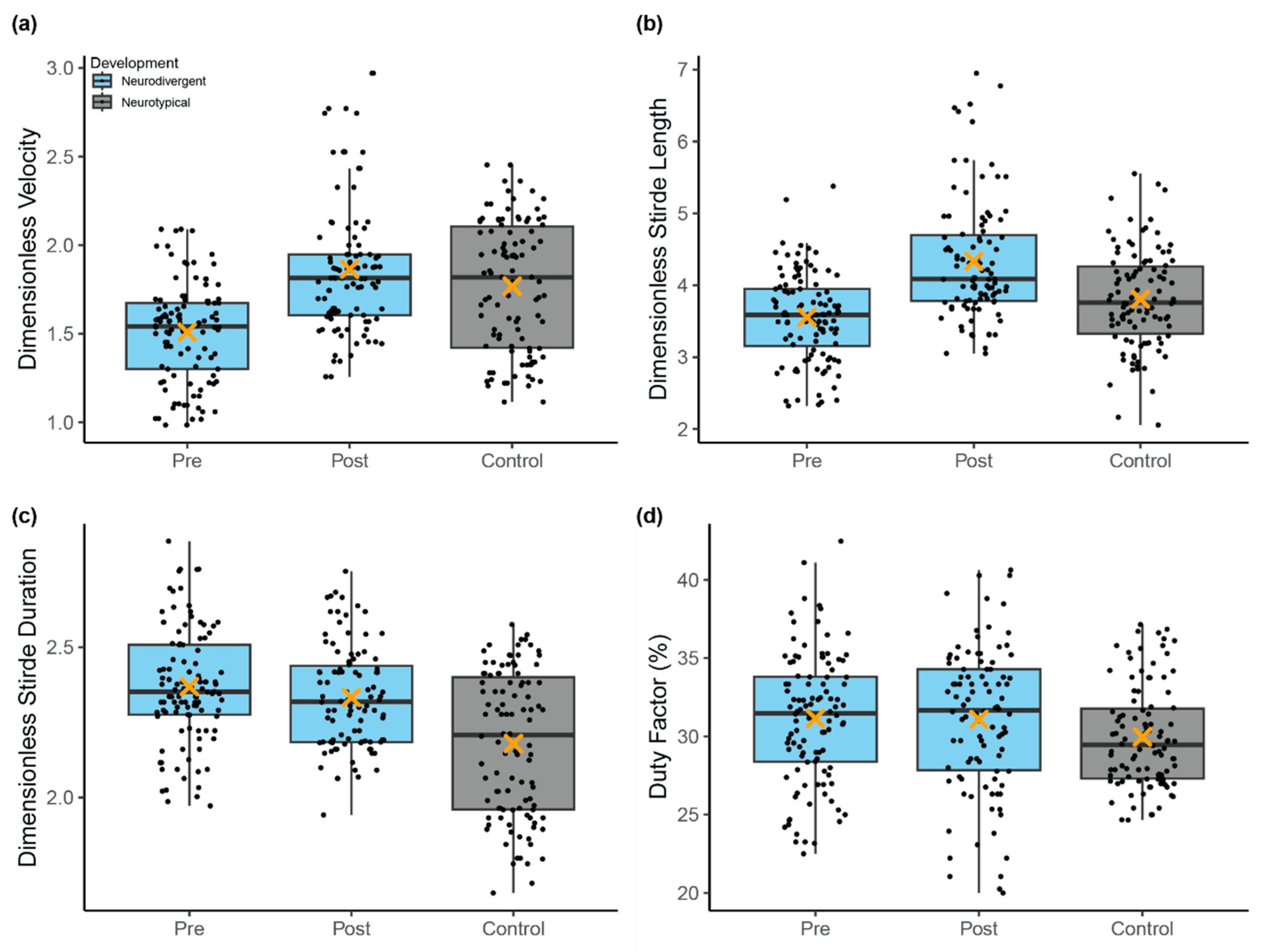
3.1. Spatiotemporal Gait Characteristics
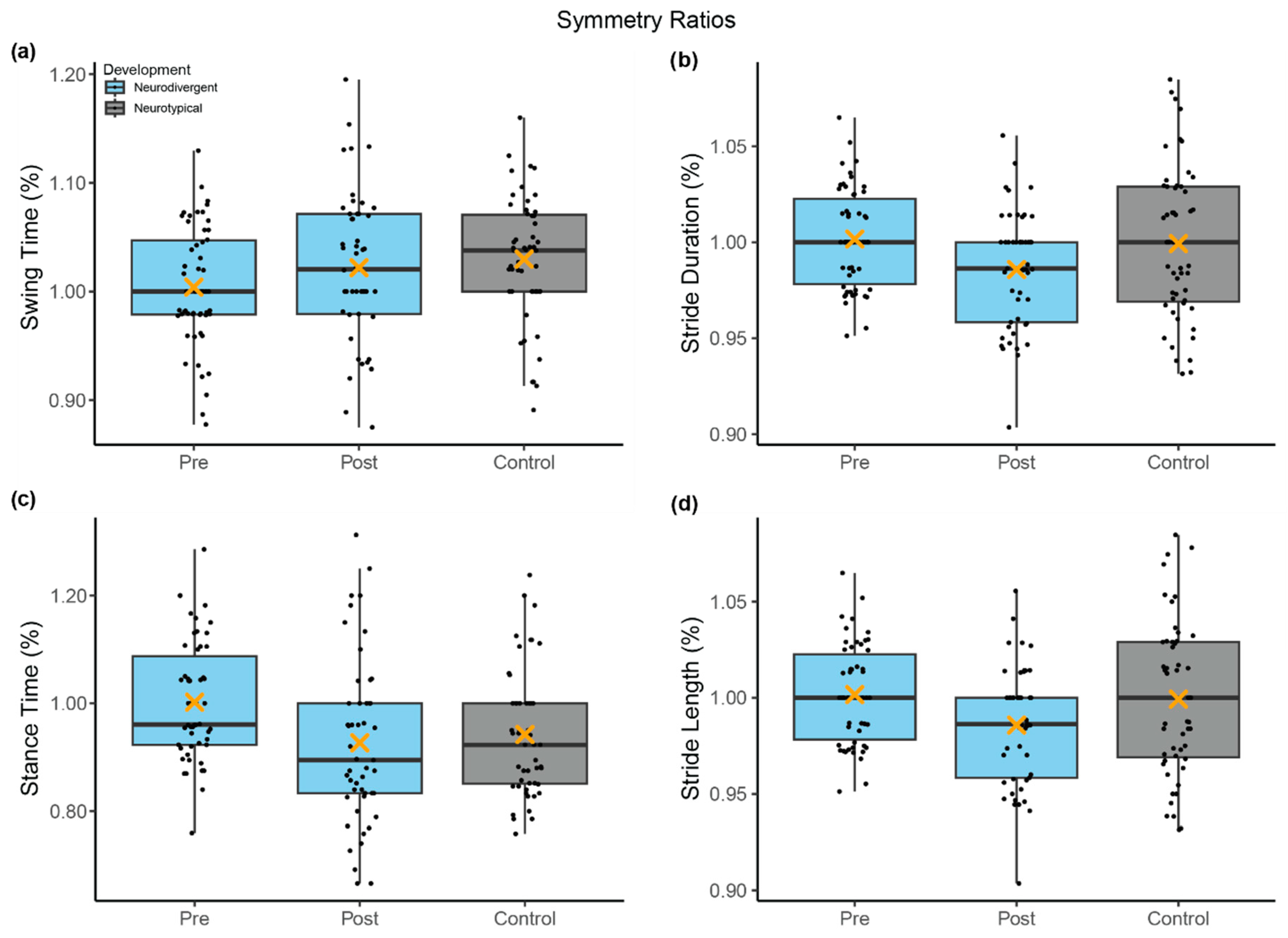
3.2. Gait Symmetry
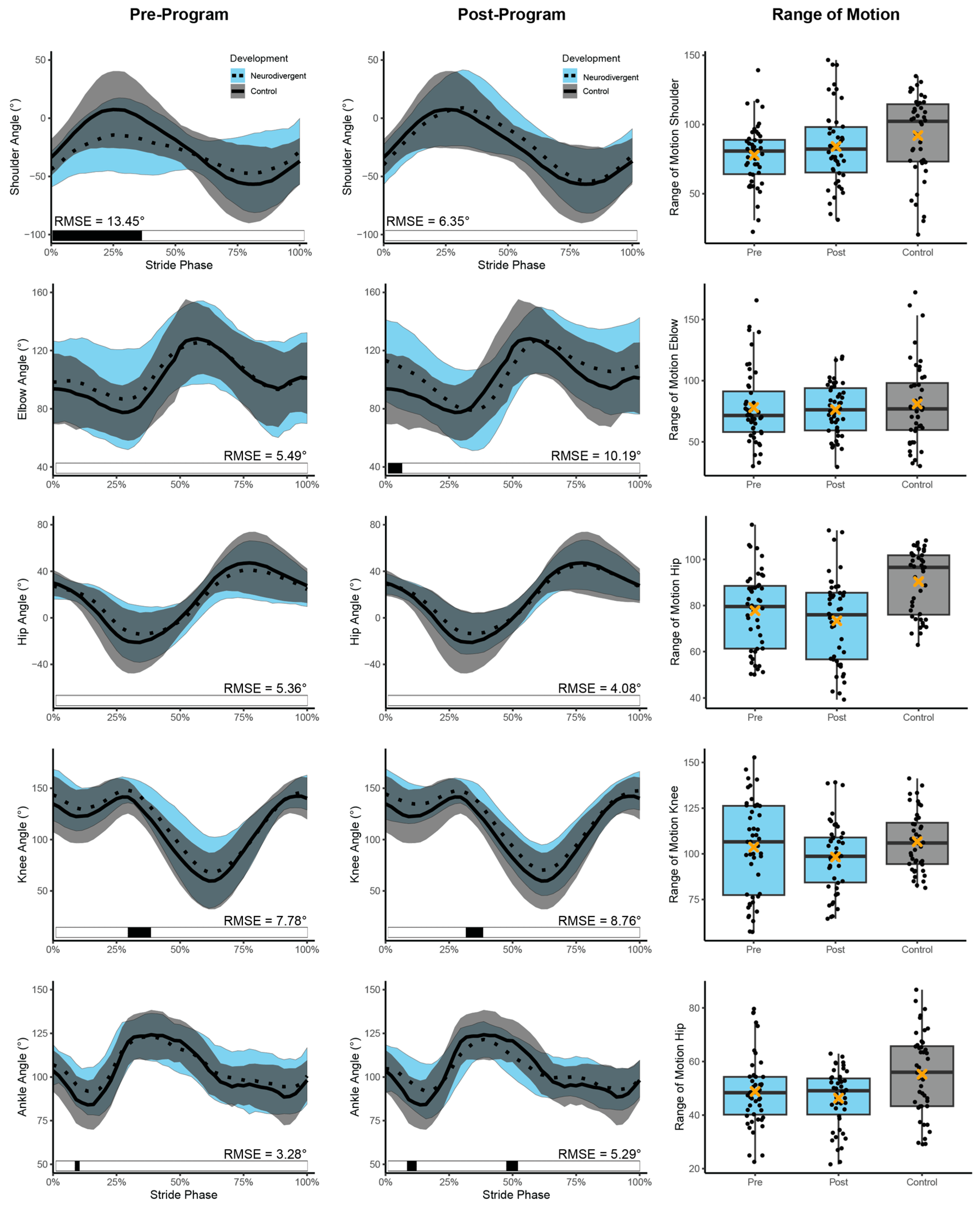
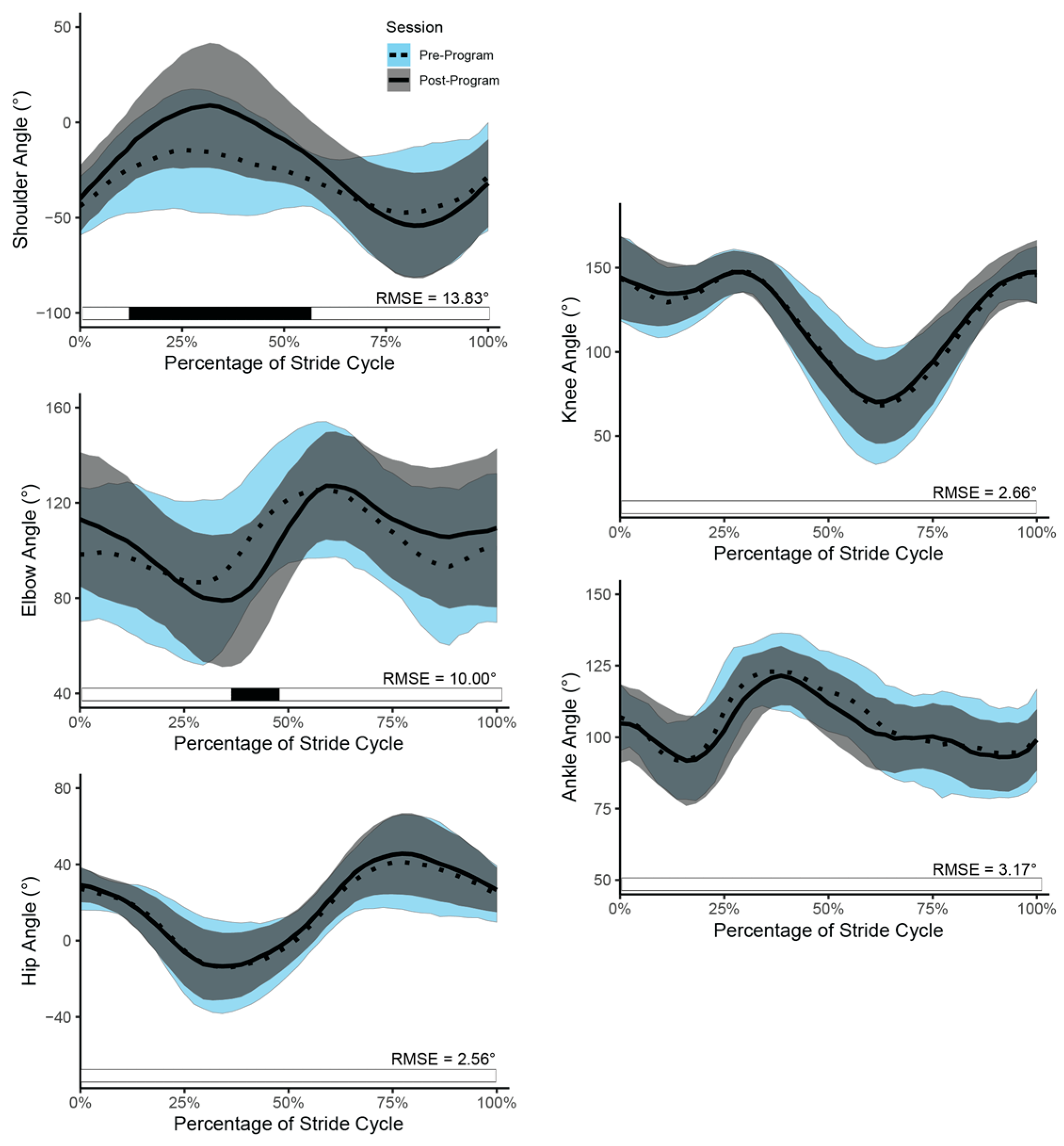
3.3. Running Kinematics
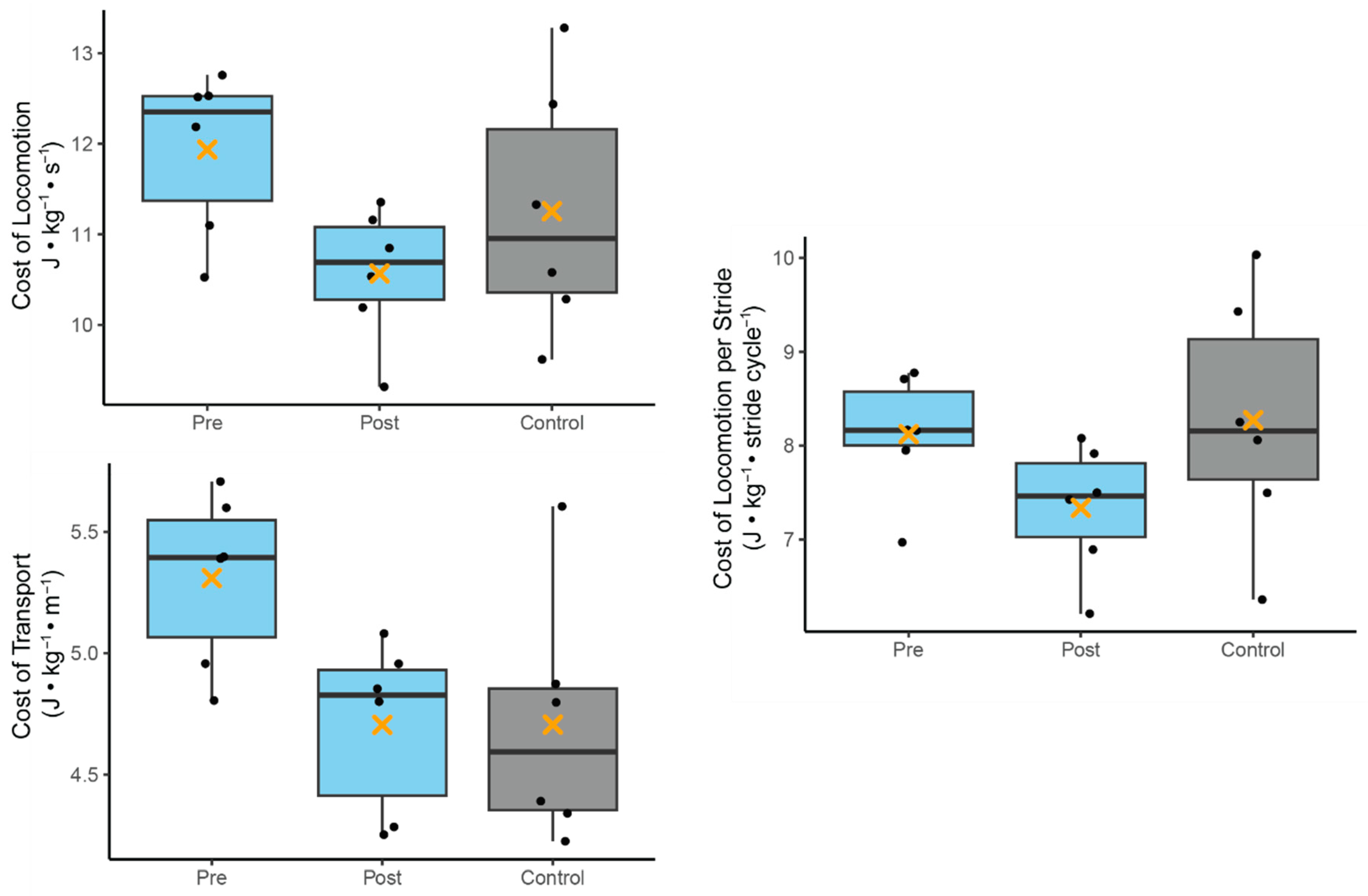
3.4. Running Energetics
4. Discussion
4.1. Limitations
4.2. Clinical Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASD | Autism Spectrum Disorder |
| HIIT | High Intensity Interval Training |
| ISF | Inclusive Sports and Fitness |
| ND | Neurodivergent |
| NT | Neurotypical |
| RMR | Resting Metabolic Rate |
| MR | Metabolic Rate |
| COL | Cost of Locomotion |
| COT | Cost of Transport |
References
- American Psychiatric Association (Ed.) Diagnostic and Statistical Manual of Mental Disorders: DSM-5-TRTM, 5th ed.; Text Revision; American Psychiatric Association Publishing: Washington, DC, USA, 2022; ISBN 978-0-89042-576-3. [Google Scholar]
- Kangarani-Farahani, M.; Malik, M.A.; Zwicker, J.G. Motor Impairments in Children with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. J. Autism. Dev. Disord. 2023, 54, 1997. [Google Scholar] [CrossRef] [PubMed]
- Abdel Karim, A.E.; Mohammed, A.H. Effectiveness of Sensory Integration Program in Motor Skills in Children with Autism. Egypt. J. Med. Hum. Genet. 2015, 16, 375–380. [Google Scholar] [CrossRef]
- Bennett, H.J.; Haegele, J.A. Running Biomechanics of Adolescents with Autism Spectrum Disorder. J. Biomech. Eng. 2021, 143, 111005. [Google Scholar] [CrossRef]
- Dufek, J.S.; Eggleston, J.D.; Harry, J.R.; Hickman, R.A. A Comparative Evaluation of Gait between Children with Autism and Typically Developing Matched Controls. Med. Sci. 2017, 5, 1. [Google Scholar] [CrossRef]
- Fournier, K.A.; Hass, C.J.; Naik, S.K.; Lodha, N.; Cauraugh, J.H. Motor Coordination in Autism Spectrum Disorders: A Synthesis and Meta-Analysis. J. Autism. Dev. Disord. 2010, 40, 1227–1240. [Google Scholar] [CrossRef]
- Kindregan, D.; Gallagher, L.; Gormley, J. Gait Deviations in Children with Autism Spectrum Disorders: A Review. Autism Res. Treat. 2015, 2015, e741480. [Google Scholar] [CrossRef]
- Bhat, A.N. Motor Impairment Increases in Children with Autism Spectrum Disorder as a Function of Social Communication, Cognitive and Functional Impairment, Repetitive Behavior Severity, and Comorbid Diagnoses: A SPARK Study Report. Autism Res. 2021, 14, 202–219. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, N.J.; Tonge, B.J.; Iansek, R.; McGinley, J.; Brereton, A.V.; Enticott, P.G.; Bradshaw, J.L. Gait Function in Newly Diagnosed Children with Autism: Cerebellar and Basal Ganglia Related Motor Disorder. Dev. Med. Child Neurol. 2006, 48, 819–824. [Google Scholar] [CrossRef]
- Vernazza-Martin, S.; Martin, N.; Vernazza, A.; Lepellec-Muller, A.; Rufo, M.; Massion, J.; Assaiante, C. Goal Directed Locomotion and Balance Control in Autistic Children. J. Autism Dev. Disord. 2005, 35, 91–102. [Google Scholar] [CrossRef]
- Weiss, M.; Moran, M.; Parker, M.E.; Foley, J. Gait Analysis of Teenagers and Young Adults Diagnosed with Autism and Severe Verbal Communication Disorders. Front. Integr. Neurosci. 2013, 7, 33. [Google Scholar] [CrossRef]
- Bennett, H.J.; Jones, T.; Valenzuela, K.A.; Haegele, J.A. Coordination Variability during Running in Adolescents with Autism Spectrum Disorder. Autism 2022, 26, 1201–1215. [Google Scholar] [CrossRef] [PubMed]
- Bennett, H.J.; Ringleb, S.I.; Bobzien, J.; Haegele, J.A. Are Gait Biomechanics Related to Physical Activity Engagement? An Examination of Adolescents with Autism Spectrum Disorder. Med. Sci. Sports Exerc. 2022, 54, 447. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, M.; Longworth, M.; Chester, V.L. Gait Patterns in Children with Autism. Clin. Biomech. 2011, 26, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Manicolo, O.; Brotzmann, M.; Hagmann-von Arx, P.; Grob, A.; Weber, P. Gait in Children with Infantile/Atypical Autism: Age-Dependent Decrease in Gait Variability and Associations with Motor Skills. Eur. J. Paediatr. Neurol. 2019, 23, 117–125. [Google Scholar] [CrossRef]
- Rinehart, N.J.; Tonge, B.J.; Bradshaw, J.L.; Iansek, R.; Enticott, P.G.; McGinley, J. Gait Function in High-Functioning Autism and Asperger’s Disorder. Eur. Child Adolesc. Psychiatry 2006, 15, 256–264. [Google Scholar] [CrossRef]
- Sebastião, E.; Bollaert, R.E.; Hubbard, E.A.; Motl, R.W. Gait Variability and Energy Cost of Oveground Walking in Persons with Multiple Sclerosis: A Cross-Sectional Study. Am. J. Phys. Med. Rehabil. 2018, 97, 646–650. [Google Scholar] [CrossRef]
- Brown, A.M.; Zifchock, R.A.; Hillstrom, H.J. The Effects of Limb Dominance and Fatigue on Running Biomechanics. Gait Posture 2014, 39, 915–919. [Google Scholar] [CrossRef]
- Gao, Z.; Fekete, G.; Baker, J.S.; Liang, M.; Xuan, R.; Gu, Y. Effects of Running Fatigue on Lower Extremity Symmetry among Amateur Runners: From a Biomechanical Perspective. Front. Physiol. 2022, 13, 899818. [Google Scholar] [CrossRef]
- Radzak, K.N.; Putnam, A.M.; Tamura, K.; Hetzler, R.K.; Stickley, C.D. Asymmetry between Lower Limbs during Rested and Fatigued State Running Gait in Healthy Individuals. Gait Posture 2017, 51, 268–274. [Google Scholar] [CrossRef]
- Bricout, V.-A.; Pace, M.; Dumortier, L.; Baillieul, F.; Favre-Juvin, A.; Guinot, M. Reduced Cardiorespiratory Capacity in Children with Autism Spectrum Disorders. J. Clin. Med. 2018, 7, 361. [Google Scholar] [CrossRef]
- Coffey, C.; Sheehan, D.; Faigenbaum, A.D.; Healy, S.; Lloyd, R.S.; Kinsella, S. Comparison of Fitness Levels between Elementary School Children with Autism Spectrum Disorder and Age-Matched Neurotypically Developing Children. Autism Res. 2021, 14, 2038–2046. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.-Y. Motor Proficiency and Physical Fitness in Adolescent Males with and without Autism Spectrum Disorders. Autism 2014, 18, 156–165. [Google Scholar] [CrossRef]
- Pan, C.-Y.; Tsai, C.-L.; Chu, C.-H.; Sung, M.-C.; Ma, W.-Y.; Huang, C.-Y. Objectively Measured Physical Activity and Health-Related Physical Fitness in Secondary School-Aged Male Students with Autism Spectrum Disorders. Phys. Ther. 2016, 96, 511–520. [Google Scholar] [CrossRef]
- Tyler, K.; MacDonald, M.; Menear, K. Physical Activity and Physical Fitness of School-Aged Children and Youth with Autism Spectrum Disorders. Autism Res. Treat. 2014, 2014, e312163. [Google Scholar] [CrossRef] [PubMed]
- Bandini, L.G.; Gleason, J.; Curtin, C.; Lividini, K.; Anderson, S.E.; Cermak, S.A.; Maslin, M.; Must, A. Comparison of Physical Activity between Children with Autism Spectrum Disorders and Typically Developing Children. Autism 2013, 17, 44–54. [Google Scholar] [CrossRef]
- Pan, C.-Y. The Efficacy of an Aquatic Program on Physical Fitness and Aquatic Skills in Children with and without Autism Spectrum Disorders. Res. Autism Spectr. Disord. 2011, 5, 657–665. [Google Scholar] [CrossRef]
- Curtin, C.; Jojic, M.; Bandini, L.G. Obesity in Children with Autism Spectrum Disorders. Harv. Rev. Psychiatry 2014, 22, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Cairney, J.; Hay, J.; Faught, B.E.; Corna, L.M.; Flouris, A.D. Developmental Coordination Disorder, Age and Play: A Test of the Divergence in Activity-Deficit with Age Hypothesis. Adapt. Phys. Act. Q. 2006, 23, 261–276. [Google Scholar] [CrossRef]
- Haga, M. Physical Fitness in Children with High Motor Competence Is Different from That in Children with Low Motor Competence. Phys. Ther. 2009, 89, 1089–1097. [Google Scholar] [CrossRef]
- Hands, B. Changes in Motor Skill and Fitness Measures among Children with High and Low Motor Competence: A Five-Year Longitudinal Study. J. Sci. Med. Sport 2008, 11, 155–162. [Google Scholar] [CrossRef]
- Sutapa, P.; Pratama, K.W.; Rosly, M.M.; Ali, S.K.S.; Karakauki, M. Improving Motor Skills in Early Childhood through Goal-Oriented Play Activity. Children 2021, 8, 994. [Google Scholar] [CrossRef]
- Donnelly, F.C.; Mueller, S.S.; Gallahue, D.L. Developmental Physical Education for All Children: Theory into Practice; Human Kinetics: Champaign, IL, USA, 2016; ISBN 978-1-4925-8565-7. [Google Scholar]
- Barnett, L.M.; van Beurden, E.; Morgan, P.J.; Brooks, L.O.; Beard, J.R. Childhood Motor Skill Proficiency as a Predictor of Adolescent Physical Activity. J. Adolesc. Health 2009, 44, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.N.; Landa, R.J.; Galloway, J.C. Current Perspectives on Motor Functioning in Infants, Children, and Adults with Autism Spectrum Disorders. Phys. Ther. 2011, 91, 1116–1129. [Google Scholar] [CrossRef] [PubMed]
- Bodison, S.C. Developmental Dyspraxia and the Play Skills of Children with Autism. Am. J. Occup. Ther. 2015, 69, 6905185060. [Google Scholar] [CrossRef] [PubMed]
- Bouffard, M.; Watkinson, E.J.; Thompson, L.P.; Dunn, J.L.C.; Romanow, S.K.E. A Test of the Activity Deficit Hypothesis with Children with Movement Difficulties. Adapt. Phys. Act. Q. 1996, 13, 61–73. [Google Scholar] [CrossRef]
- Gong, L.; Liu, Y.; Yi, L.; Fang, J.; Yang, Y.; Wei, K. Abnormal Gait Patterns in Autism Spectrum Disorder and Their Correlations with Social Impairments. Autism Res. 2020, 13, 1215–1226. [Google Scholar] [CrossRef]
- Piek, J.; Bradbury, G.; Elsley, S.; Tate, L. Motor Coordination and Social–Emotional Behaviour in Preschool-aged Children. Int. J. Disabil. Dev. Educ.-Int. J. Disabil Dev. Educ. 2008, 55, 143–151. [Google Scholar] [CrossRef]
- Skinner, R.A.; Piek, J.P. Psychosocial Implications of Poor Motor Coordination in Children and Adolescents. Hum. Mov. Sci. 2001, 20, 73–94. [Google Scholar] [CrossRef]
- MacNeil, L.K.; Mostofsky, S.H. Specificity of Dyspraxia in Children with Autism. Neuropsychology 2012, 26, 165–171. [Google Scholar] [CrossRef]
- Delcour, M.; Russier, M.; Castets, F.; Turle-Lorenzo, N.; Canu, M.-H.; Cayetanot, F.; Barbe, M.F.; Coq, J.-O. Early Movement Restriction Leads to Maladaptive Plasticity in the Sensorimotor Cortex and to Movement Disorders. Sci. Rep. 2018, 8, 16328. [Google Scholar] [CrossRef]
- Mawson, A.R. Toward a Theory of Childhood Learning Disorders, Hyperactivity, and Aggression. ISRN Psychiatry 2012, 2012, 589792. [Google Scholar] [CrossRef] [PubMed]
- Hilton, C.L.; Cumpata, K.; Klohr, C.; Gaetke, S.; Artner, A.; Johnson, H.; Dobbs, S. Effects of Exergaming on Executive Function and Motor Skills in Children with Autism Spectrum Disorder: A Pilot Study. Am. J. Occup. Ther. 2014, 68, 57–65. [Google Scholar] [CrossRef]
- Lochbaum, M.; Crews, D. Viability of Cardiorespiratory and Muscular Strength Programs for the Adolescent with Autism. Complement. Health Pract. Rev. 2003, 8, 225–233. [Google Scholar] [CrossRef]
- Todd, T.; Reid, G. Increasing Physical Activity in Individuals with Autism. Focus Autism Other Dev. Disabil.-Focus Autism Dev. Disabil. 2006, 21, 167–176. [Google Scholar] [CrossRef]
- Gibala, M.J. High-Intensity Interval Training: A Time-Efficient Strategy for Health Promotion? Curr. Sports Med. Rep. 2007, 6, 211. [Google Scholar] [CrossRef]
- Koral, J.; Oranchuk, D.J.; Herrera, R.; Millet, G.Y. Six Sessions of Sprint Interval Training Improves Running Performance in Trained Athletes. J. Strength Cond. Res. 2018, 32, 617–623. [Google Scholar] [CrossRef]
- Rodríguez-Barbero, S.; González-Mohíno, F.; Rodrigo-Carranza, V.; Santos-Garcia, D.J.; Boullosa, D.; González-Ravé, J.M. Acute Effects of Interval Training on Running Kinematics in Runners: A Systematic Review. Gait Posture 2023, 103, 19–26. [Google Scholar] [CrossRef]
- Taylor, J.; Macpherson, T.; Spears, I.; Weston, M. The Effects of Repeated-Sprint Training on Field-Based Fitness Measures: A Meta-Analysis of Controlled and Non-Controlled Trials. Sports Med. 2015, 45, 881–891. [Google Scholar] [CrossRef]
- Bond, B.; Weston, K.L.; Williams, C.A.; Barker, A.R. Perspectives on High-Intensity Interval Exercise for Health Promotion in Children and Adolescents. Open Access J. Sports Med. 2017, 8, 243–265. [Google Scholar] [CrossRef]
- Lau, P.W.C.; Wong, D.P.; Ngo, J.K.; Liang, Y.; Kim, C.G.; Kim, H.S. Effects of High-Intensity Intermittent Running Exercise in Overweight Children. Eur. J. Sport Sci. 2015, 15, 182–190. [Google Scholar] [CrossRef]
- Nourry, C.; Deruelle, F.; Guinhouya, C.; Baquet, G.; Fabre, C.; Bart, F.; Berthoin, S.; Mucci, P. High-Intensity Intermittent Running Training Improves Pulmonary Function and Alters Exercise Breathing Pattern in Children. Eur. J. Appl. Physiol. 2005, 94, 415–423. [Google Scholar] [CrossRef]
- Kozlowski, K.F.; Lopata, C.; Donnelly, J.P.; Thomeer, M.L.; Rodgers, J.D.; Seymour, C. Feasibility and Associated Physical Performance Outcomes of a High-Intensity Exercise Program for Children with Autism. Res. Q. Exerc. Sport 2021, 92, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Olin, S.S.; McFadden, B.A.; Golem, D.L.; Pellegrino, J.K.; Walker, A.J.; Sanders, D.J.; Arent, S.M. The Effects of Exercise Dose on Stereotypical Behavior in Children with Autism. Med. Sci. Sports Exerc. 2017, 49, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Poon, E.T.-C.; Wongpipit, W.; Sun, F.; Tse, A.C.-Y.; Sit, C.H.-P. High-Intensity Interval Training in Children and Adolescents with Special Educational Needs: A Systematic Review and Narrative Synthesis. Int. J. Behav. Nutr. Phys. Act. 2023, 20, 13. [Google Scholar] [CrossRef] [PubMed]
- Leahy, A.A.; Kennedy, S.G.; Smith, J.J.; Eather, N.; Boyer, J.; Thomas, M.; Shields, N.; Dascombe, B.; Lubans, D.R. Feasibility of a School-Based Physical Activity Intervention for Adolescents with Disability. Pilot Feasibility Stud. 2021, 7, 120. [Google Scholar] [CrossRef]
- Granatosky, M.C.; Amanat, S.; Panyutina, A.A.; Youlatos, D. Gait Mechanics of a Blind Echolocating Rodent: Implications for the Locomotion of Small Arboreal Mammals and Proto-Bats. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2021, 335, 436–453. [Google Scholar] [CrossRef]
- Granatosky, M.C.; McElroy, E.J.; Laird, M.F.; Iriarte-Diaz, J.; Reilly, S.M.; Taylor, A.B.; Ross, C.F. Joint Angular Excursions during Cyclical Behaviors Differ between Tetrapod Feeding and Locomotor Systems. J. Exp. Biol. 2019, 222, jeb200451. [Google Scholar] [CrossRef]
- Granatosky, M.C.; McElroy, E.J. Stride Frequency or Length? A Phylogenetic Approach to Understand How Animals Regulate Locomotor Speed. J. Exp. Biol. 2022, 225, jeb243231. [Google Scholar] [CrossRef]
- Chester, V.L.; Calhoun, M. Gait Symmetry in Children with Autism. Autism Res. Treat. 2012, 2012, 576478. [Google Scholar] [CrossRef]
- Delattre, N.; Lafortune, M.A.; Moretto, P. Dynamic Similarity during Human Running: About Froude and Strouhal Dimensionless Numbers. J. Biomech. 2009, 42, 312–318. [Google Scholar] [CrossRef]
- Alexander, R.M. Walking and Running. Math. Gaz. 1996, 80, 262–266. [Google Scholar] [CrossRef]
- Granatosky, M.C.; Bryce, C.M.; Hanna, J.; Fitzsimons, A.; Laird, M.F.; Stilson, K.; Wall, C.E.; Ross, C.F. Inter-Stride Variability Triggers Gait Transitions in Mammals and Birds. Proc. R. Soc. B 2018, 285. [Google Scholar] [CrossRef] [PubMed]
- Heglund, N.C.; Taylor, C.R. Speed, Stride Frequency and Energy Cost per Stride: How Do They Change with Body Size and Gait? J. Exp. Biol. 1988, 138, 301–318. [Google Scholar] [CrossRef] [PubMed]
- Halsey, L.G.; White, C.R. Terrestrial Locomotion Energy Costs Vary Considerably between Species: No Evidence That This Is Explained by Rate of Leg Force Production or Ecology. Sci. Rep. 2019, 9, 656. [Google Scholar] [CrossRef]
- Lighton, J.R. Measuring Metabolic Rates: A Manual for Scientists; Oxford University Press: Oxford, UK, 2008. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Nieuwenhuys, A.; Papageorgiou, E.; Desloovere, K.; Molenaers, G.; De Laet, T. Statistical Parametric Mapping to Identify Differences between Consensus-Based Joint Patterns during Gait in Children with Cerebral Palsy. PLoS ONE 2017, 12, e0169834. [Google Scholar] [CrossRef]
- Park, J.; Han, K. Quantifying Gait Asymmetry in Stroke Patients: A Statistical Parametric Mapping (SPM) Approach. Med. Sci. Monit. 2025, 31, e946754. [Google Scholar] [CrossRef]
- Bar-Haim, Y.; Bart, O. Motor Function and Social Participation in Kindergarten Children. Soc. Dev. 2006, 15, 296–310. [Google Scholar] [CrossRef]
- Danion, F.; Varraine, E.; Bonnard, M.; Pailhous, J. Stride Variability in Human Gait: The Effect of Stride Frequency and Stride Length. Gait Posture 2003, 18, 69–77. [Google Scholar] [CrossRef]
- Hausdorff, J.M.; Nelson, M.E.; Kaliton, D.; Layne, J.E.; Bernstein, M.J.; Nuernberger, A.; Singh, M.A.F. Etiology and Modification of Gait Instability in Older Adults: A Randomized Controlled Trial of Exercise. J. Appl. Physiol. 2001, 90, 2117–2129. [Google Scholar] [CrossRef]
- Mo, S.; Lau, F.O.Y.; Lok, A.K.Y.; Chan, Z.Y.S.; Zhang, J.H.; Shum, G.; Cheung, R.T.H. Bilateral Asymmetry of Running Gait in Competitive, Recreational and Novice Runners at Different Speeds. Hum. Mov. Sci. 2020, 71, 102600. [Google Scholar] [CrossRef]
- Li, Y.; Koldenhoven, R.M.; Liu, T.; Venuti, C.E. Age-Related Gait Development in Children with Autism Spectrum Disorder. Gait Posture 2021, 84, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Arslan, E.; Ince, G.; Akyüz, M. Effects of a 12-Week Structured Circuit Exercise Program on Physical Fitness Levels of Children with Autism Spectrum Condition and Typically Developing Children. Int. J. Dev. Disabil. 2022, 68, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Berryman, N.; Mujika, I.; Arvisais, D.; Roubeix, M.; Binet, C.; Bosquet, L. Strength Training for Middle- and Long-Distance Performance: A Meta-Analysis. Int. J. Sports Physiol. Perform. 2018, 13, 57–64. [Google Scholar] [CrossRef]
- Kram, R.; Taylor, C.R. Energetics of Running: A New Perspective. Nature 1990, 346, 265. [Google Scholar] [CrossRef]
- Pontzer, H. A Unified Theory for the Energy Cost of Legged Locomotion. Biol. Lett. 2016, 12, 20150935. [Google Scholar] [CrossRef] [PubMed]
- Arellano, C.J.; Kram, R. The Effects of Step Width and Arm Swing on Energetic Cost and Lateral Balance during Running. J. Biomech. 2011, 44, 1291–1295. [Google Scholar] [CrossRef]
- Arellano, C.J.; Kram, R. The Metabolic Cost of Human Running: Is Swinging the Arms Worth It? J. Exp. Biol. 2014, 217, 2456–2461. [Google Scholar] [CrossRef]
- Collins, S.H.; Adamczyk, P.G.; Kuo, A.D. Dynamic Arm Swinging in Human Walking. Proc. R. Soc. B Biol. Sci. 2009, 276, 3679–3688. [Google Scholar] [CrossRef]
- Castaño, P.R.L.; Suárez, D.P.M.; González, E.R.; Robledo-Castro, C.; Hederich-Martínez, C.; Cadena, H.P.G.; Vargas, P.A.S.; Montenegro, L.C.G. Effects of Physical Exercise on Gross Motor Skills in Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2023, 54, 2816–2825. [Google Scholar] [CrossRef]
- Hynes, J.; Block, M. Effects of Physical Activity on Social, Behavioral, and Cognitive Skills in Children and Young Adults with Autism Spectrum Disorder: A Systematic Review of the Literature. Rev. J. Autism Dev. Disord. 2023, 10, 749–770. [Google Scholar] [CrossRef]
- Pan, C.-Y.; Chu, C.-H.; Tsai, C.-L.; Sung, M.-C.; Huang, C.-Y.; Ma, W.-Y. The Impacts of Physical Activity Intervention on Physical and Cognitive Outcomes in Children with Autism Spectrum Disorder. Autism 2017, 21, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.L.J.; Abel, E.A.; Reimer, S.; McPartland, J.C. Brief Report: A Specialized Fitness Program for Individuals with Autism Spectrum Disorder Benefits Physical, Behavioral, and Emotional Outcomes. J. Autism Dev. Disord. 2024, 54, 2402–2410. [Google Scholar] [CrossRef] [PubMed]
- Toscano, C.V.A.; Carvalho, H.M.; Ferreira, J.P. Exercise Effects for Children with Autism Spectrum Disorder: Metabolic Health, Autistic Traits, and Quality of Life. Percept. Mot. Ski. 2018, 125, 126–146. [Google Scholar] [CrossRef]
- Potdevin, F.; Gillet, C.; Barbier, F.; Coello, Y.; Moretto, P. Propulsion and Braking in the Study of Asymmetry in Able-Bodied Men’s Gaits. Percept. Mot. Ski. 2008, 107, 849–861. [Google Scholar] [CrossRef]
- Mostofsky, S.H.; Dubey, P.; Jerath, V.K.; Jansiewicz, E.M.; Goldberg, M.C.; Denckla, M.B. Developmental Dyspraxia Is Not Limited to Imitation in Children with Autism Spectrum Disorders. J. Int. Neuropsychol. Soc. 2006, 12, 314–326. [Google Scholar] [CrossRef]
| Development | Gender | n | Age (Mean ± Std) | Leg Length (Mean ± Std) | Training Duration (Mean ± Std) |
|---|---|---|---|---|---|
| Neurodivergent (ND) | Male | 12 | 12.08 ± 1.44 | 0.68 ± 0.09 | 12.50 ± 7.15 |
| Neurotypical (NT) | Female | 5 | 10.60 ± 4.04 | 0.72 ± 0.19 | NA |
| Male | 10 | 12.70 ± 1.34 | 0.79 ± 0.16 | NA |
| Response Variable (Dimensionless) | Fixed Effect | Estimate | Standard Error | df | t Value | p-Value | |
|---|---|---|---|---|---|---|---|
| Pre-Program Comparison (a) | Velocity | Development | 0.33 | 0.12 | 19.03 | 2.68 | 0.015 |
| Age | −0.02 | 0.03 | 19.10 | −0.87 | 0.393 | ||
| Foot | 0.00 | 0.02 | 191.04 | 0.00 | 1.000 | ||
| Stride Duration | Development | −0.23 | 0.09 | 19.03 | −2.64 | 0.016 | |
| Age | −0.02 | 0.02 | 19.08 | −1.22 | 0.238 | ||
| Foot | 0.00 | 0.01 | 191.03 | −0.03 | 0.978 | ||
| Stride Length | Development | 0.35 | 0.23 | 18.97 | 1.53 | 0.144 | |
| Age | −0.10 | 0.05 | 19.06 | −2.09 | 0.051 | ||
| Foot | 0.00 | 0.05 | 190.98 | −0.03 | 0.977 | ||
| Duty Factor | Development | −1.59 | 1.33 | 18.92 | −1.19 | 0.247 | |
| Age | 0.40 | 0.28 | 19.03 | 1.43 | 0.168 | ||
| Foot | −1.03 | 0.31 | 190.93 | −3.34 | 0.001 | ||
| Neurodivergent Change (b) | Velocity | Session | 0.38 | 0.03 | 196.34 | 14.26 | <0.001 |
| Age | −0.08 | 0.05 | 10.03 | −1.54 | 0.155 | ||
| Duration | 0.01 | 0.01 | 9.98 | 0.47 | 0.646 | ||
| Foot | 0.00 | 0.03 | 195.98 | 0.00 | 1.000 | ||
| Stride Duration | Session | −0.02 | 0.02 | 196.59 | −1.27 | 0.205 | |
| Age | 0.00 | 0.03 | 9.97 | 0.16 | 0.878 | ||
| Duration | 0.01 | 0.01 | 9.87 | 1.21 | 0.255 | ||
| Foot | −0.01 | 0.02 | 195.87 | −0.76 | 0.450 | ||
| Stride Length | Session | 0.86 | 0.06 | 196.37 | 13.99 | <0.001 | |
| Age | −0.17 | 0.10 | 9.99 | −1.59 | 0.142 | ||
| Duration | 0.02 | 0.02 | 9.93 | 0.99 | 0.345 | ||
| Foot | −0.03 | 0.06 | 195.93 | −0.45 | 0.654 | ||
| Duty Factor | Session | −0.44 | 0.40 | 196.32 | −1.10 | 0.272 | |
| Age | 0.49 | 0.71 | 9.96 | 0.69 | 0.507 | ||
| Duration | −0.09 | 0.16 | 9.90 | −0.55 | 0.592 | ||
| Foot | −1.16 | 0.39 | 195.91 | −2.95 | 0.004 | ||
| Post-Program Comparison (c) | Velocity | Development | −0.04 | 0.14 | 19.07 | −0.30 | 0.766 |
| Age | −0.04 | 0.03 | 19.12 | −1.24 | 0.229 | ||
| Foot | 0.00 | 0.02 | 181.09 | 0.00 | 1.000 | ||
| Stride Duration | Development | −0.19 | 0.08 | 19.19 | −2.48 | 0.023 | |
| Age | −0.03 | 0.02 | 19.27 | −1.62 | 0.122 | ||
| Foot | −0.02 | 0.02 | 181.22 | −1.21 | 0.226 | ||
| Stride Length | Development | −0.47 | 0.27 | 18.99 | −1.72 | 0.101 | |
| Age | −0.14 | 0.06 | 19.05 | −2.47 | 0.023 | ||
| Foot | −0.03 | 0.05 | 181.01 | −0.69 | 0.490 | ||
| Duty Factor | Development | −1.24 | 1.24 | 18.98 | −1.00 | 0.331 | |
| Age | 0.47 | 0.26 | 19.12 | 1.82 | 0.084 | ||
| Foot | −2.16 | 0.35 | 181.04 | −6.16 | <0.001 |
| Response Variable (Dimensionless) | Fixed Effect | Estimate | Standard Error | df | t Value | p-Value | |
|---|---|---|---|---|---|---|---|
| Pre-Program Comparison (a) | Ratio: Stride Duration | Development | 0.00 | 0.01 | 105.00 | −0.43 | 0.670 |
| Age | 0.00 | 0.00 | 105.00 | −0.61 | 0.543 | ||
| Ratio: Stride Length | Development | 0.00 | 0.01 | 105.00 | −0.43 | 0.670 | |
| Age | 0.00 | 0.00 | 105.00 | −0.61 | 0.543 | ||
| Ratio: Swing Time | Development | 0.02 | 0.01 | 15.85 | 2.11 | 0.051 | |
| Age | 0.00 | 0.00 | 19.51 | 1.05 | 0.304 | ||
| Ratio: Stance Time | Development | −0.05 | 0.03 | 20.25 | −1.93 | 0.067 | |
| Age | −0.01 | 0.01 | 22.02 | −1.02 | 0.319 | ||
| Neurodivergent Change (b) | Ratio: Stride Duration | Session | −0.02 | 0.01 | 103.00 | −2.89 | 0.005 |
| Age | 0.00 | 0.00 | 103.00 | 0.14 | 0.888 | ||
| Duration | 0.00 | 0.00 | 103.00 | 0.72 | 0.476 | ||
| Ratio: Stride Length | Session | −0.02 | 0.01 | 103.00 | −2.89 | 0.005 | |
| Age | 0.00 | 0.00 | 103.00 | 0.14 | 0.888 | ||
| Duration | 0.00 | 0.00 | 103.00 | 0.72 | 0.476 | ||
| Ratio: Swing Time | Session | 0.02 | 0.01 | 95.10 | 1.40 | 0.164 | |
| Age | 0.00 | 0.01 | 10.19 | 0.80 | 0.442 | ||
| Duration | 0.00 | 0.00 | 9.57 | 1.35 | 0.209 | ||
| Ratio: Stance Time | Session | −0.08 | 0.03 | 95.70 | −3.20 | 0.002 | |
| Age | 0.00 | 0.01 | 9.76 | −0.32 | 0.753 | ||
| Duration | 0.00 | 0.00 | 8.97 | −0.88 | 0.402 | ||
| Post-Program Comparison (c) | Ratio: Stride Duration | Development | 0.01 | 0.01 | 100.00 | 1.90 | 0.060 |
| Age | 0.00 | 0.00 | 100.00 | −1.02 | 0.309 | ||
| Ratio: Stride Length | Development | 0.01 | 0.01 | 100.00 | 1.90 | 0.060 | |
| Age | 0.00 | 0.00 | 100.00 | −1.02 | 0.309 | ||
| Ratio: Swing Time | Development | 0.01 | 0.01 | 19.10 | 0.63 | 0.535 | |
| Age | 0.00 | 0.00 | 21.88 | 0.71 | 0.488 | ||
| Ratio: Stance Time | Development | 0.02 | 0.03 | 20.06 | 0.72 | 0.479 | |
| Age | −0.01 | 0.01 | 21.80 | −1.01 | 0.324 |
| Joint Angle | Fixed Effect | Estimate | Standard Error | df | t Value | p-Value | |
|---|---|---|---|---|---|---|---|
| Pre-Program Comparison (a) | Shoulder | Development | 14.94 | 9.49 | 19.07 | 1.57 | 0.132 |
| Age | −0.76 | 1.98 | 19.04 | −0.38 | 0.706 | ||
| Elbow | Development | 4.28 | 9.97 | 19.12 | 0.43 | 0.673 | |
| Age | −2.08 | 2.08 | 19.08 | −1.00 | 0.330 | ||
| Hip | Development | 12.33 | 5.98 | 18.95 | 2.06 | 0.053 | |
| Age | −2.31 | 1.25 | 18.93 | −1.85 | 0.080 | ||
| Knee | Development | 2.43 | 8.85 | 18.93 | 0.27 | 0.787 | |
| Age | −3.22 | 1.85 | 18.92 | −1.74 | 0.098 | ||
| Ankle | Development | 5.64 | 4.75 | 18.98 | 1.19 | 0.249 | |
| Age | −3.27 | 0.99 | 18.97 | −3.30 | 0.004 | ||
| Neurodivergent Change (b) | Shoulder | Session | 7.00 | 4.60 | 83.94 | 1.52 | 0.132 |
| Duration | −0.65 | 0.64 | 9.35 | −1.01 | 0.340 | ||
| Age | −2.08 | 2.80 | 9.82 | −0.74 | 0.475 | ||
| Elbow | Session | −2.29 | 5.23 | 94.00 | −0.44 | 0.663 | |
| Duration | −0.95 | 0.41 | 94.00 | −2.31 | 0.023 | ||
| Age | −2.78 | 1.83 | 94.00 | −1.52 | 0.133 | ||
| Hip | Session | −3.24 | 2.07 | 83.84 | −1.57 | 0.121 | |
| Duration | −0.28 | 0.62 | 9.70 | −0.45 | 0.660 | ||
| Age | −5.60 | 2.68 | 9.83 | −2.09 | 0.064 | ||
| Knee | Session | −3.79 | 2.38 | 83.87 | −1.60 | 0.114 | |
| Duration | −0.14 | 0.90 | 9.78 | −0.16 | 0.880 | ||
| Age | −6.12 | 3.88 | 9.86 | −1.58 | 0.146 | ||
| Ankle | Session | −2.23 | 1.82 | 84.28 | −1.23 | 0.224 | |
| Duration | −0.19 | 0.39 | 10.00 | −0.50 | 0.627 | ||
| Age | −2.48 | 1.67 | 10.25 | −1.49 | 0.167 | ||
| Post-Program Comparison (c) | Shoulder | Development | 7.16 | 10.60 | 18.89 | 0.68 | 0.508 |
| Age | −0.99 | 2.21 | 18.88 | −0.45 | 0.661 | ||
| Elbow | Development | 5.74 | 9.25 | 19.12 | 0.62 | 0.542 | |
| Age | −1.97 | 1.93 | 19.12 | −1.02 | 0.320 | ||
| Hip | Development | 16.30 | 6.11 | 18.99 | 2.67 | 0.015 | |
| Age | −3.22 | 1.28 | 18.98 | −2.52 | 0.021 | ||
| Knee | Development | 7.95 | 6.06 | 18.82 | 1.31 | 0.206 | |
| Age | −3.49 | 1.27 | 18.81 | −2.76 | 0.013 | ||
| Ankle | Development | 8.32 | 3.69 | 19.11 | 2.25 | 0.036 | |
| Age | −3.29 | 0.77 | 19.10 | −4.27 | <0.001 |
| Measurement | Fixed Effect | Estimate | Standard Error | t Value | p-Value | |
|---|---|---|---|---|---|---|
| Pre-Program Comparison (a) | Cost of Locomotion | Development | −0.62 | 0.79 | −0.78 | 0.454 |
| Age | −0.06 | 0.35 | −0.18 | 0.863 | ||
| Cost of Transport | Development | −0.57 | 0.30 | −1.91 | 0.089 | |
| Age | −0.04 | 0.13 | −0.29 | 0.777 | ||
| Cost of Locomotion Per Stride | Development | 5.78 | 3.54 | 1.63 | 0.137 | |
| Age | −0.05 | 0.69 | −0.07 | 0.942 | ||
| Neurodivergent Change (b) | Cost of Locomotion | Session | −1.37 | 0.41 | −3.35 | 0.008 |
| Age | −0.60 | 0.27 | −2.18 | 0.057 | ||
| Cost of Transport | Session | −0.60 | 0.21 | −2.82 | 0.020 | |
| Age | 0.04 | 0.14 | 0.28 | 0.785 | ||
| Cost of Locomotion Per Stride | Session | −0.78 | 0.35 | −2.22 | 0.053 | |
| Age | −0.41 | 0.24 | −1.75 | 0.114 | ||
| Post-Program Comparison (c) | Cost of Locomotion | Development | 0.71 | 0.75 | 0.94 | 0.372 |
| Age | −0.02 | 0.33 | −0.06 | 0.955 | ||
| Cost of Transport | Development | 0.03 | 0.30 | 0.10 | 0.925 | |
| Age | −0.03 | 0.13 | −0.21 | 0.836 | ||
| Cost of Locomotion Per Stride | Development | 0.81 | 0.71 | 1.14 | 0.283 | |
| Age | 0.13 | 0.31 | 0.41 | 0.693 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chernik, N.D.; Young, M.W.; Jacobson, R.N.; Kantounis, S.J.; Lynch, S.K.; Virga, J.Q.; Cannata, M.J.; English, H.M.; Krish, P.; Kanumuru, A.; et al. Pilot Study: Effects of High-Intensity Training on Gait Symmetry and Locomotor Performance in Neurodivergent Children. Symmetry 2025, 17, 1073. https://doi.org/10.3390/sym17071073
Chernik ND, Young MW, Jacobson RN, Kantounis SJ, Lynch SK, Virga JQ, Cannata MJ, English HM, Krish P, Kanumuru A, et al. Pilot Study: Effects of High-Intensity Training on Gait Symmetry and Locomotor Performance in Neurodivergent Children. Symmetry. 2025; 17(7):1073. https://doi.org/10.3390/sym17071073
Chicago/Turabian StyleChernik, Noah D., Melody W. Young, Reuben N. Jacobson, Stratos J. Kantounis, Samantha K. Lynch, James Q. Virga, Matthew J. Cannata, Hannah M. English, Pranav Krish, Anand Kanumuru, and et al. 2025. "Pilot Study: Effects of High-Intensity Training on Gait Symmetry and Locomotor Performance in Neurodivergent Children" Symmetry 17, no. 7: 1073. https://doi.org/10.3390/sym17071073
APA StyleChernik, N. D., Young, M. W., Jacobson, R. N., Kantounis, S. J., Lynch, S. K., Virga, J. Q., Cannata, M. J., English, H. M., Krish, P., Kanumuru, A., Lopez, A., & Granatosky, M. C. (2025). Pilot Study: Effects of High-Intensity Training on Gait Symmetry and Locomotor Performance in Neurodivergent Children. Symmetry, 17(7), 1073. https://doi.org/10.3390/sym17071073





