3.1. Experimental Error Analysis
To ensure the reliability and repeatability of the experimental results, a preliminary error analysis was conducted prior to formal experimentation, focusing on three key factors that may affect measurement accuracy: (1) the impact of the probe volume on fluid flow behavior; (2) the influence of tracer addition on the overall fluid properties; and (3) the measurement errors associated with dimensionless concentration curves and mixing time.
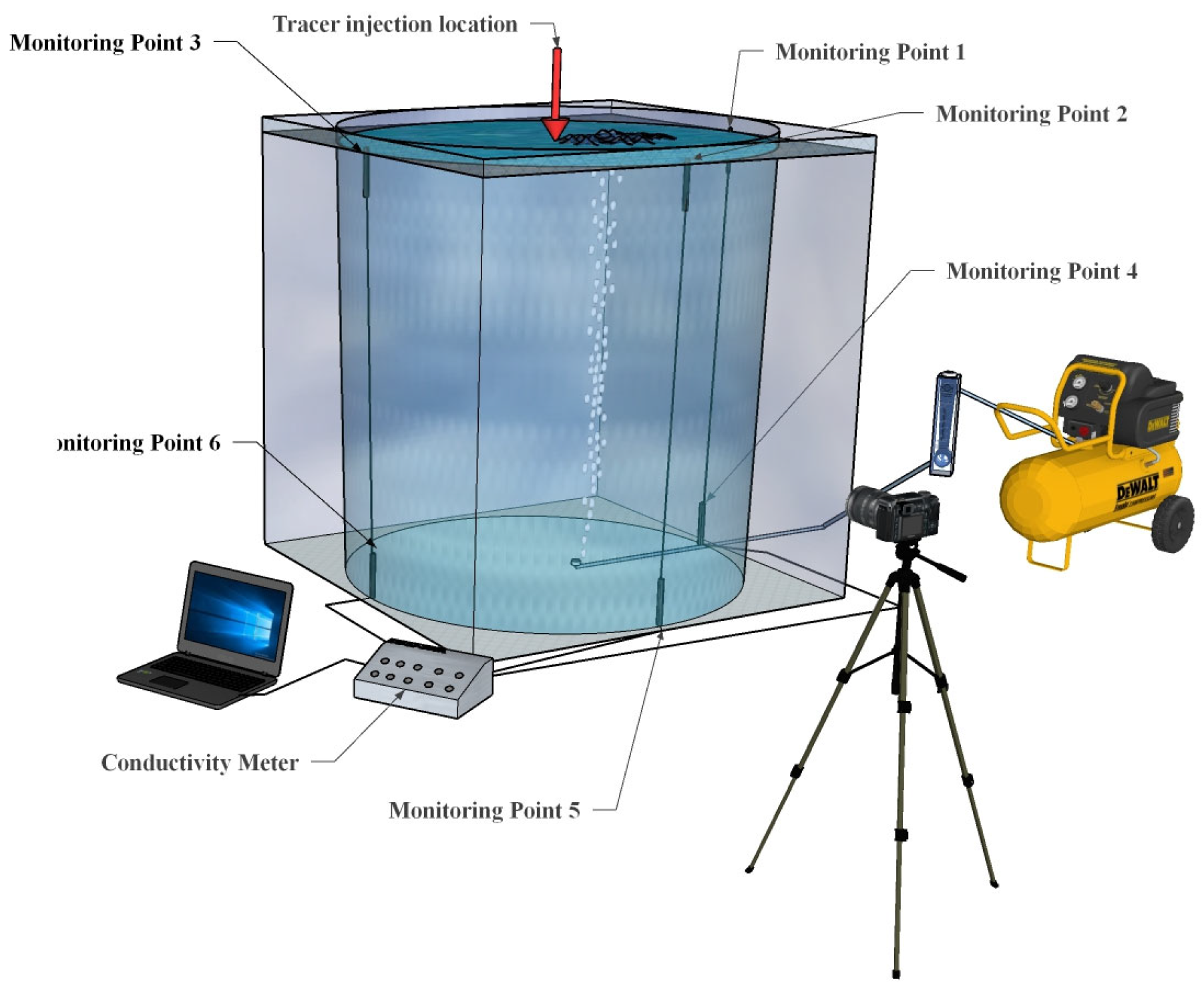
In the experiments, Platinum Black electrodes were employed as conductivity probes. Each probe had a volume of 0.118 cm3, and a total of six probes were installed, resulting in a combined volume of 0.708 cm3. Given that the total volume of the ladle model was 0.53 m3, the volume ratio of probes to the model was only 1.336 × 10⁻6. Therefore, the effect of probe volume on the flow behavior in the ladle water model can be considered negligible.
A saturated NaCl solution was used as the tracer, with a maximum injection volume of 695 mL, which accounted for 0.131% of the total water volume (530 L), corresponding to a mass fraction of 0.047%. At 20 °C, a 7% NaCl solution has a dynamic viscosity of 1.079 mPa·s compared to 1.005 mPa·s for pure water. Additionally, a 1% NaCl solution has a density of 1005 kg/m
3, which is slightly higher than the density of water (998.2 kg/m
3) [
93]. Therefore, in the context of this study, the introduction of the tracer had a minimal effect on the viscosity and density of the fluid, and its impact on flow behavior was deemed insignificant. The water in the ladle was replaced after each experiment to maintain consistency.
To evaluate the repeatability of the experiment, a representative case involving the addition of 185 mL of saturated NaCl solution was selected for ten repeated trials. The resulting dimensionless concentration curves were compared and analyzed. As shown in
Figure 2, the maximum and minimum concentration curves were used to generate a shaded region over time [
90], visually representing the range of variation. These figures indicate that the variation range of the repeated experimental data at these monitoring points was relatively small compared to the average curve.
Table 4 summarizes the mixing times obtained from ten experimental repetitions. The average mixing time from the ten trials was adopted as the representative value for each monitoring point. The standard deviation between the individual mixing times and their average was also calculated. Results showed that the standard deviation at all monitoring points remained within 12%, indicating good repeatability and high reliability of the measured data.
In conclusion, the disturbance caused by the probe volume and tracer properties can be considered negligible. Furthermore, the dimensionless concentration curves and mixing time data demonstrated excellent repeatability. Thus, the experimental results were considered reliable and suitable for subsequent analysis.
3.4. Tracer Transport Path and Analysis
Figure 5 presents the concentration–time curves at Monitoring Point 1 after adding tracer agents of different volumes into the water model of the ladle.
Table 5 summarizes the key time points extracted from the dimensionless concentration curves. The tracer agent volumes were 92 mL, 185 mL, 277 mL, 370 mL, 463 mL, and 695 mL. All dimensionless concentration curves were obtained by averaging the results of ten experimental runs. After introducing 92 mL of saturated NaCl solution into the ladle, the dimensionless concentration at Monitoring Point 1 began to respond at 8 s, which was followed by a rapid increase. At 15 s, the concentration reached a peak value of 2.4, after which it rapidly decreased to 0.69 at 32 s, which represents the lowest concentration observed after the peak. Subsequently, the dimensionless concentration gradually rose and stabilized around 1. For the 185 mL tracer volume, the dimensionless concentration started to rise at 8 s and reached a maximum value of 3.37 at 18 s. Between 18 s and 36 s, the concentration decreased, reaching its lowest value of 0.75 at 36 s, after which it gradually increased and fluctuated around 1. When the tracer volume was 277 mL, the dimensionless concentration began to increase at 5 s, reaching its peak of 3.2 at 18 s. Subsequently, the concentration declined to 0.92, followed by a gradual rise after 35 s. A minor fluctuation was observed at 52 s, after which the curve stabilized around 1. For the 370 mL tracer volume, the dimensionless concentration started to rise after 8 s, reaching a peak value of 2.6 at 19 s. It then declined to 0.81, followed by a gradual increase after 43 s, and then eventually stabilizing within a ±5% standard range. After adding 463 mL of saturated NaCl solution, the concentration variation began at 8 s. The concentration continued to increase between 8 s and 19 s, reaching a peak of 2.76. From 19 s to 38 s, the concentration decreased to its lowest value of 0.75, then slowly rose and stabilized around 1. For the 695 mL tracer volume, the concentration started increasing at 9 s and reached a peak of 1.9 at 21 s. The concentration then declined to its lowest value of 0.64 at 39 s before gradually rising and stabilizing within the ±5% standard range. The initiation time of the concentration variation at Monitoring Point 1 remained around 8 s for all tracer volumes, indicating that changes in tracer volume do not significantly affect the response time at this location. However, the time required to reach the concentration peak varies with volume, recorded as 15 s, 18 s, 18 s, 19 s, 19 s, and 21 s for 92 mL, 185 mL, 277 mL, 370 mL, 463 mL, and 695 mL, respectively. This suggests that increasing the tracer volume delays the peak concentration occurrence. The peak concentration values for increasing tracer volumes were 2.4, 3.37, 3.2, 2.6, 2.76, and 1.9, respectively, indicating an initial increase followed by a decrease. The lower peak concentration at 92 mL was attributed to the small tracer volume, which, upon introduction, was largely carried by strong flow fields to the left side of the ladle, resulting in a lower tracer concentration on the right side. Consequently, the peak concentration at Monitoring Point 1 remained relatively low at 18 s. When the tracer volume exceeded 185 mL, a noticeable decline in peak concentration was observed. The duration from the concentration increase to peak occurrence for each tracer volume was recorded as 7 s, 10 s, 13 s, 11 s, 11 s, and 12 s, respectively, demonstrating that a larger tracer volume extended the time required to reach peak concentration. However, the duration of concentration declined following the peak, which was 17 s, 18 s, 17 s, 24 s, 19 s, and 18 s, respectively. Except for the 370 mL tracer volume, the time interval from peak-to-minimum concentration remained approximately 18 s for all cases, indicating that the tracer volume had minimal influence on the time span from peak-to-minimum concentration.
Figure 6 presents the concentration–time curves at Monitoring Point 2 after adding saturated NaCl solutions of different volumes into the ladle water model.
Table 6 summarizes the key time points extracted from the dimensionless concentration curves. For a tracer volume of 92 mL, the dimensionless concentration began to change at 8 s and reached its peak value of 3.75 at 16 s. Between 16 s and 32 s, the concentration gradually decreased, reaching a minimum value of 0.67 at 32 s. Subsequently, a slight increase was observed, leading to a secondary peak at 51 s with a value of 1.4. When the tracer volume was 185 mL, the concentration started increasing at 8 s and reached a maximum of 2.2 at 18 s. Between 18 s and 33 s, the concentration rapidly decreased to its post-peak minimum of 0.44, after which it gradually stabilized around 1. For the 277 mL tracer volume, the dimensionless concentration began to rise at 9 s, reaching a peak of 2.2 at 19 s. The concentration then gradually decreased, reaching its lowest value of 0.56 at 34 s. After 34 s, the concentration gradually increased and stabilized within a ±5% standard range. When the tracer volume was 370 mL, the concentration response began at 10 s, reaching a peak value of 1.79 at 20 s. Subsequently, the concentration decreased, following a downward trend until reaching its minimum value of 0.45 at 37 s. Afterward, the concentration slowly rose and gradually stabilized around 1. For the 463 mL tracer volume, the conductivity sensor detected a response at 9 s, which was followed by a rapid increase in concentration between 9 s and 18 s. After reaching a maximum value of 2.1, the concentration began to decrease and reached its minimum of 0.51 at 36 s, subsequently stabilizing around 1. When the tracer volume reached 695 mL, the dimensionless concentration started rising at 9 s, reaching its peak of 1.35 at 20 s. It then underwent a sharp decline, reaching its minimum of 0.37 at 38 s, after which it gradually increased and stabilized around 1. For all six tracer volumes, the response time at Monitoring Point 2 was approximately 9 s, indicating that the tracer volume did not significantly affect the electrical conductivity response time at this location. As the tracer volume increased from 92 mL to 695 mL, the times required to reach peak concentration were 16 s, 18 s, 19 s, 20 s, 18 s, and 20 s, respectively. This suggests that a larger tracer volume delays the time to reach the maximum concentration, indicating an influence on the transport velocity of dissolved salts. The peak concentration values for increasing the tracer volumes were recorded as 3.75, 2.2, 2.2, 1.8, 2.1, and 1.35, respectively, demonstrating a general decreasing trend. This trend was attributed to the increased volume reducing the salt concentration at this point, as well as the excessive tracer volumes leading to salt settling at the bottom. By comparing the six dimensionless concentration curves under two different flow rates, it was observed that, at a flow rate of 2.3 L/min, secondary peaks were rarely present, and this phenomenon became even less frequent as the tracer volume increased. This was due to the lower flow rate limiting the movement of dissolved salts along the ladle’s flow field, preventing a substantial amount of salts from re-accumulating and reaching the upper monitoring point, which would otherwise result in a secondary peak. Additionally, the minimum concentration values at 2.3 L/min were consistently lower than those observed at 8.3 L/min. This is because the lower flow rate failed to keep salts suspended in the upper region, which caused them to settle due to buoyancy force, leading to lower minimum values at the upper monitoring point. Furthermore, the recovery from the minimum concentration to the ±5% standard level predominantly exhibited a slow growth trend.
Figure 7 presents the concentration–time curves at Monitoring Point 3 after adding tracer agents of six different volumes into the ladle water model.
Table 7 summarizes the key time points extracted from the dimensionless concentration curves. For a tracer volume of 92 mL, the dimensionless concentration began to rise at 8 s and reached its maximum value of 8 at 15 s. The concentration increased rapidly, with a steep slope observed in the concentration curve between 8 s and 15 s. In the interval from 15 s to 40 s, the concentration exhibited a rapid downward trend, indicating that a large amount of dissolved salt quickly passed through this location. At 50 s, a secondary peak appeared with a value of 1.52, which was followed by a gradual decrease until the concentration stabilized at 1. When the tracer volume was 185 mL, the concentration started increasing at 10 s and reached a maximum value of 3.4 at 21 s. Subsequently, the concentration declined until it stabilized at 1. For the 277 mL tracer volume, the electrical conductivity response was still detected at 8 s, with the concentration reaching a peak value of 2.7 at 19 s. The concentration then continued to decrease until 50 s, reaching a value of 0.76, after which it gradually rose and stabilized at 1. When 370 mL of saturated NaCl solution was introduced, the concentration began to increase slowly at 10 s, which was followed by a rapid rise after 15 s, reaching a peak value of 1.6 at 23 s. Thereafter, the concentration followed a general downward trend, reaching its lowest value of 0.66 at 55 s. After 55 s, the concentration gradually increased until complete mixing was achieved. For the 463 mL tracer volume, the concentration started rising at 8 s, reaching a peak of 2.6 at 20 s. The overall trend of the concentration curve was similar to that observed for the 370 mL tracer volume. When the tracer volume was 695 mL, the concentration increased from 9 s, reaching its peak value of 1.9 at 20 s. From 20 s to 46 s, the concentration followed a decreasing trend, reaching a minimum value of 0.59 at 46 s, after which it slowly increased and eventually stabilized within a ±5% standard range. The response time at Monitoring Point 3 remained around 8 s, indicating that the upper monitoring point’s response time was not significantly affected by the tracer volume. However, at a flow rate of 8.3 L/min, the response time at the upper monitoring point varied noticeably with tracer volume, suggesting a clear relationship between the gas injection rate and the time required for the tracer to reach the upper monitoring point. Since the response times at the three upper monitoring points were similar (all around 8 s), it can be concluded that the gas injection nozzle had little effect on the liquid velocity near the free surface. By comparing the six dimensionless concentration curves in
Figure 7, it was observed that the first peak value generally decreased with increasing tracer volume. This phenomenon occurred because, at smaller tracer volumes, nearly all of the tracer followed the strong recirculating flow to Monitoring Point 3. As the tracer volume increased, the added tracer initially moved downward, temporarily disrupting the flow field in the ladle water model, leading to partial salt deposition. Once the flow field stabilized, only a small amount of salt was transported to the upper Monitoring Point 3 by recirculating flow, resulting in a lower first peak as the tracer volume increased. Furthermore, at Monitoring Point 3, the first peak values under a lower flow rate (2.3 L/min) were consistently lower than those observed at a higher flow rate (8.3 L/min), further confirming that variations in gas flow rate significantly influence the transport behavior of the tracer within the ladle.
Figure 8 presents the concentration–time curves at Monitoring Point 4 after introducing tracer agents of different volumes into the ladle.
Table 8 summarizes the key time points extracted from the dimensionless concentration curves. When 92 mL of saturated NaCl solution was added, two distinct concentration trends, Trend 1 and Trend 2, were observed in the dimensionless concentration curve at Monitoring Point 4. The black curve in the figure represents the average of both trends. The primary difference between these trends lay in their initial concentration behavior: Trend 1 exhibited a gradual increase from zero without pronounced peaks before reaching a dimensionless concentration of 1, whereas Trend 2 showed a rapid increase in concentration with noticeable but relatively small peaks immediately after the response began. A similar phenomenon was observed when the tracer volume increased to 185 mL, with the concentration curve displaying both trends similar to those observed for the 92 mL tracer. This behavior was mainly attributed to the small tracer volume being more significantly affected by the recirculating flow within the ladle, making it highly sensitive to minor variations in the flow field. Consequently, subtle changes in the ladle’s flow conditions altered the tracer transport direction, leading to the emergence of two distinct trends [
90,
91]. Trend 1 closely resembled the previously studied Path 2 curve, indicating that, after injection, the tracer was primarily transported through the left and central regions of the ladle before gradually crossing the gas plume and reaching Monitoring Point 4. In contrast, Trend 2 was similar to the Path 5 curve, suggesting that the tracer predominantly circulated on the right side, leading to the appearance of peak values in the concentration curve. When the tracer volume increased to 277 mL, the dimensionless concentration began to change at 18 s and reached its peak of 1.92 at 40 s. A minor trough appeared at 52 s, with the concentration dropping to 1.36. Between 52 s and 57 s, the concentration increased again, reaching 1.7, before gradually declining after 57 s, until then stabilizing within the range of 0.95–1.05. For a tracer volume of 370 mL, the concentration response began at 13 s, which was followed by a rapid increase, reaching a maximum of 5.4 at 32 s. Subsequently, the concentration declined sharply, reaching its lowest value of 1.39 at 86 s. A temporary increase was observed between 86 s and 103 s, leading to a second peak at 103 s with a value of 1.82. Afterward, the concentration continuously decreased until it stabilized around 1. When 463 mL of saturated NaCl solution was introduced, the concentration started increasing at 14 s, showing a rapid growth phase until 22 s, after which the growth rate slowed. The concentration reached a maximum of 2.27 at 47 s, which was followed by a continuous decline. By 72 s, the concentration decreased to 1.2. Between 72 s and 104 s, fluctuations were observed, with a secondary peak appearing at 104 s with a value of 1.5. Afterward, the concentration gradually decreased with minor oscillations until stabilizing within the required range. For the 695 mL tracer volume, the concentration began to respond at 13 s, which was followed by a rapid increase, reaching a peak of 3.7 at 37 s. After 37 s, the concentration continuously declined with significant fluctuations until complete mixing was achieved. The initial response time of the dimensionless concentration curve for tracer volumes of 92 mL and 185 mL was 24 s and 23 s. For tracer volumes of 277 mL, 370 mL, 463 mL, and 695 mL, the initial response times were 18 s, 13 s, 14 s, and 13 s, respectively. This suggests that, when the tracer volume was small, its transport path was significantly influenced by the flow field, leading to a delayed response at Monitoring Point 4. However, as the tracer volume increased, the influence of the flow field on transport pathways decreased, resulting in a more consistent response time at Monitoring Point 4. Furthermore, with increasing tracer volume, Trend 2 became dominant in the concentration curves at Monitoring Point 4.
Figure 9 presents the concentration–time curves at Monitoring Point 5 after adding tracer agents of different volumes into the ladle.
Table 9 summarizes the key time points extracted from the dimensionless concentration curves. For a tracer volume of 92 mL, the concentration curve began to change at 25 s. Before complete mixing was achieved, the concentration remained below 1, indicating that the dimensionless concentration increased gradually from zero to 1 with minor fluctuations. A relatively faster increase was observed only between 15 s and 25 s. When the tracer volume was 185 mL, the dimensionless concentration started changing at 20 s. However, between 20 s and 27 s, the concentration increased very slowly, indicating that the tracer reached this location at approximately 27 s. Between 27 s and 39 s, the concentration increased rapidly. After 39 s, noticeable fluctuations appear in the concentration curve, which was followed by a gradual decrease until mixing was complete. For the 277 mL tracer volume, the concentration began to change at 20 s. Similar to the 185 mL case, the concentration exhibited minimal variation between 20 s and 28 s, suggesting that only a small amount of salt reached this location initially. Between 28 s and 43 s, the concentration increased rapidly, reaching a maximum value of 1.38 at 49 s. The concentration then declined from 49 s to 61 s, briefly reaching a local minimum of 1.1, which was followed by a slight increase to 1.3. After 69 s, the concentration decreased with minor fluctuations until stabilizing within the range of 0.95–1.05. When the tracer volume increased to 370 mL, the concentration response began at 15 s, which was followed by a rapid increase, reaching 4 at 30 s. Subsequently, the concentration gradually declined, exhibiting significant fluctuations that resulted in a jagged pattern of peaks. Eventually, the concentration stabilized around 1. For the 463 mL tracer volume, the concentration starts increasing at 22 s, with multiple peaks appearing in the curve. The highest peak, reaching 1.9, occurred at 59 s. However, due to significant fluctuations, a clear trend was not observed. When the tracer volume reached 695 mL, the concentration began changing at 15 s, followed by a rapid increase, reaching 3.2 at 26 s. After a brief fluctuation, the concentration continued to rise, reaching a maximum value of 3.7 at 39 s. Beyond 39 s, the concentration exhibited an overall decreasing trend but was accompanied by significant fluctuations, resulting in a jagged concentration curve. These oscillations persisted until approximately 150 s before stabilizing. The response times for different tracer volumes at Monitoring Point 5 were recorded as 25 s, 20–27 s, 20–28 s, 15 s, 22 s, and 15 s, respectively. Most of the response times fell within the 20–25 s range, with the remaining cases occurring at 15 s. This suggests that the first arrival time of the tracer at Monitoring Point 5 was not uniform, indicating the possibility of multiple transport pathways leading to that location. Additionally, the maximum concentrations recorded for different tracer volumes were 1, 1.13, 1.38, 4, 1.9, and 3.7, respectively. The significant variation in both peak concentration values and their occurrence times further confirmed that the tracer reached this point via multiple, possibly non-unique transport pathways.
Figure 10 presents the concentration–time curves at Monitoring Point 6 after introducing saturated NaCl solutions of different volumes (92 mL, 185 mL, 277 mL, 370 mL, 463 mL, and 695 mL) into the ladle water model.
Table 10 summarizes the key time points extracted from the dimensionless concentration curves. For a 92 mL tracer volume, the dimensionless concentration curve began to change at 25 s. However, between 25 s and 35 s, the concentration remained relatively stable, showing no significant increase. After 35 s, a rapid rise was observed, reaching 1.0 at 58 s. From 58 s to 68 s, the concentration decreased to 0.83, which was followed by a gradual increase until it fluctuated around 1.0. When the 185 mL tracer was added, the concentration began to rise at 23 s, showing rapid growth and reaching a maximum of 1.35 at 53 s. After a slight decrease, the concentration increased again to 1.34 at 71 s, which was followed by a gradual decline until complete mixing was achieved. For the 277 mL tracer volume, the concentration started changing at 25 s. Between 25 s and 35 s, the concentration increased rapidly. After 35 s, the concentration curve exhibited a sawtooth pattern, which is characterized by fluctuations with relatively small variations in overall concentration. The peak concentration of 1.37 was recorded at 85 s. When the 370 mL tracer volume was introduced, the concentration began increasing at 18 s, showing a very rapid growth phase. At 35 s, the concentration momentarily stabilized before experiencing a slight decrease, reaching 3.3 at 46 s. The concentration then increased again to 4.5 at 50 s, which was followed by a general downward trend. Throughout the decline, significant fluctuations were observed until the concentration eventually stabilized near 1.0. For the 463 mL tracer volume, the conductivity sensor detected a response at 20 s, which was followed by a rapid increase between 20 s and 40 s. The concentration reached a peak value of 2.6 at 40 s, after which it gradually decreased until complete mixing was achieved. Minor fluctuations were observed throughout the decline. When the 695 mL tracer was added, the concentration started changing at 17 s and exhibited a very rapid increase, reaching 3.7 at 31 s. A short-lived decrease followed, with the concentration dropping to 2.5 at 55 s. After 55 s, the concentration increased slightly again, peaking at 2.9 at 61 s. Subsequently, the concentration followed a general downward trend, which was accompanied by fluctuations. The dimensionless concentration curve at Monitoring Point 6 showed significant fluctuations, with multiple secondary peaks and sawtooth-like oscillations during the concentration decline. This suggests that the tracer, after reaching this location, accumulated and settled before being gradually transported away in batches under the influence of the gas plume, rather than being immediately and entirely dispersed. This stepwise removal process resulted in the observed oscillatory behavior. The sensor response times for different tracer volumes were recorded as 25 s, 23 s, 25 s, 18 s, 20 s, and 17 s, respectively. As the tracer volume increased, the response time of the conductivity sensor gradually shortened, indicating that larger tracer volumes were transported to Monitoring Point 6 more quickly under the influence of the gas plume. Additionally, as the tracer volume increased, sedimentation at Monitoring Point 6 became more pronounced, leading to a prolonged mixing time at this location.
3.5. Analysis of Mixing Time
Figure 11 illustrates the mixing times at various monitoring points after introducing tracer agents of different volumes into the ladle water model. At Monitoring Point 1, the mixing times corresponding to tracer volumes of 92 mL, 185 mL, 277 mL, 370 mL, 463 mL, and 695 mL were 114 s, 81.6 s, 133.6 s, 154.3 s, 156.8 s, and 141.1 s, respectively. The mixing time initially decreased, which was followed by an increase and then another decrease. Two transition points were identified at 185 mL and 463 mL, where the mixing time significantly decreased when the tracer volume increased from 92 mL to 185 mL, which was followed by a continuous increase from 185 mL to 463 mL. However, when the volume increased to 695 mL, the mixing time shortened again. At Monitoring Point 2, the mixing times for increasing tracer volumes were 116.6 s, 116.5 s, 92.6 s, 123 s, 113 s, and 148.8 s, respectively. The mixing time exhibited two increasing phases, occurring between 277 mL to 370 mL and 463 mL to 695 mL. For Monitoring Point 3, the mixing times corresponding to 92 mL, 185 mL, 277 mL, 370 mL, 463 mL, and 695 mL were 129.6 s, 100.8 s, 102.9 s, 125.5 s, 126.1 s, and 143 s, respectively. The mixing time first decreased, then increased, and then remained in an upward trend when the tracer volume exceeded 185 mL. At Monitoring Point 4, the mixing times for increasing tracer volumes were 123.6 s, 103.2 s, 130.8 s, 147.3 s, 151.2 s, and 168.8 s. Notably, when the tracer volume was 185 mL, the mixing time was significantly shorter than for other tracer volumes. For Monitoring Point 5, the mixing times corresponding to different tracer volumes were 141 s, 124 s, 134.4 s, 136.1 s, 142.7 s, and 191.2 s, respectively. A trend of initial reduction followed by an increase was observed. At Monitoring Point 6, the mixing times for increasing tracer volumes were 141.5 s, 115.4 s, 144 s, 162.5 s, 170 s, and 198.3 s. Similar to other locations, the mixing time first decreased and then increased, with the longest mixing time observed at 695 mL, making it the longest among all six monitoring points. As the tracer volume changed, the mixing time at all six monitoring points also varied accordingly, generally exhibiting a trend of initial shortening followed by an increase.
Figure 11 also shows that the mixing times at the bottom monitoring points were relatively concentrated, following similar trends, with a high degree of overlap among different tracer volumes. In contrast, the mixing time variations at the upper monitoring points were less consistent, suggesting that the upper regions were more susceptible to changes in tracer volume.
Figure 12 shows that the mixing times at the upper monitoring points were generally shorter than those at the lower monitoring points. This difference was particularly pronounced when larger tracer volumes were introduced as the mixing times at the lower monitoring points became significantly longer than those at the upper monitoring points. This observation suggests that the gas flow rate was insufficient to fully disperse larger volumes of tracer within the ladle. The three-dimensional visualization further confirmed that when the tracer volume was 185 mL, the mixing time was notably shorter compared to other tracer volumes. Additionally, the mixing time initially decreased and then increased as the tracer volume increased.