A Systematic Review of Non-Pharmacological Interventions to Improve Gait Asymmetries in Neurological Populations
Abstract
:1. Introduction
1.1. Rationale
1.2. Objective
2. Methods
2.1. Systematic Review Protocol
2.2. Inclusion/Exclusion Criteria
2.3. Search Strategy
2.4. Study Selection
2.5. Data Extraction
3. Results
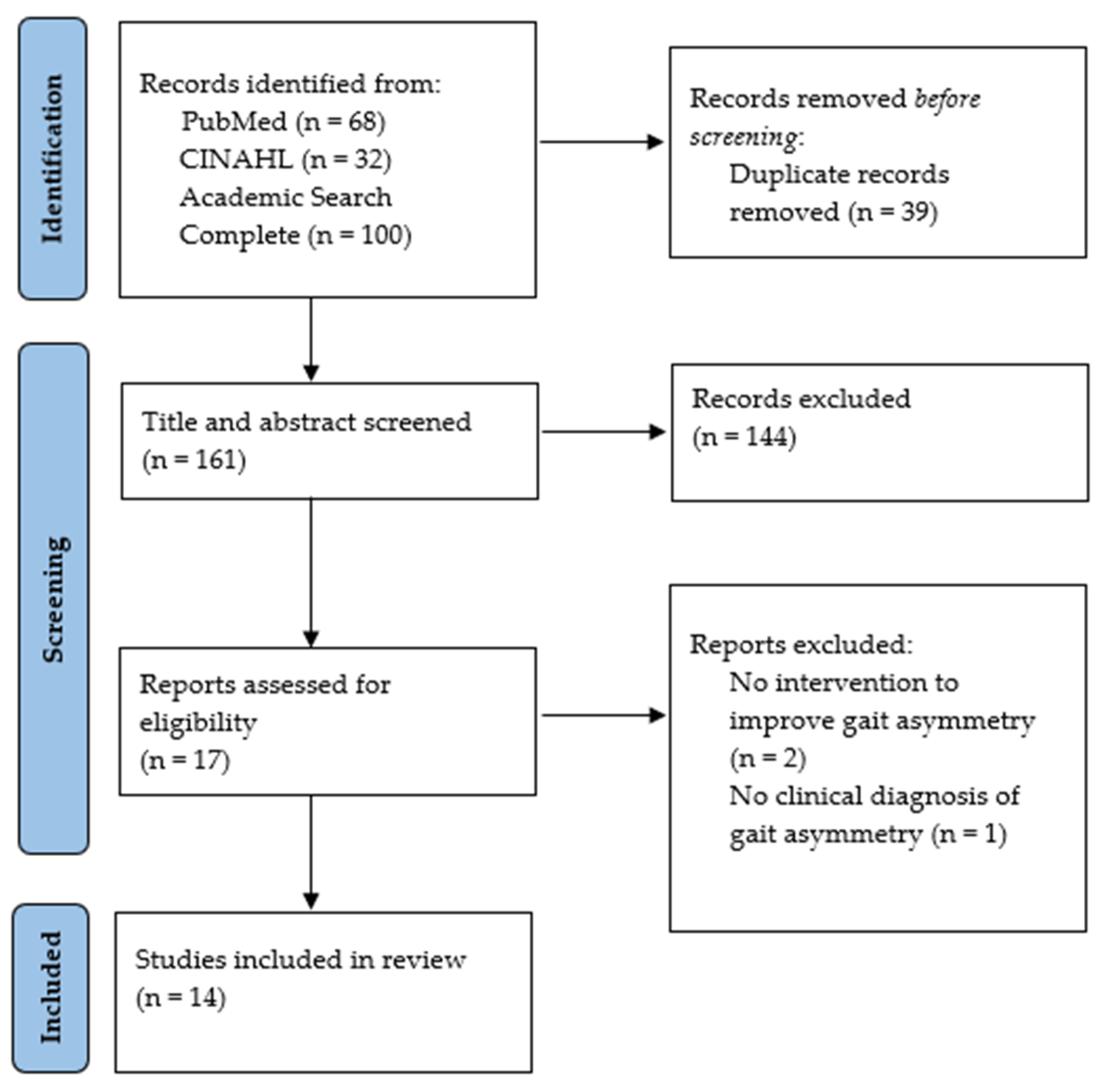
3.1. Study Search Selection
3.2. Demographic Information
3.3. Interventions and Assessments of Gait Asymmetry
3.4. Gait Symmetry Equations and Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Protocol Registration
References
- LaRoche, D.P.; Cook, S.B.; Mackala, K. Strength Asymmetry Increases Gait Asymmetry and Variability in Older Women. Med. Sci. Sports Exerc. 2012, 44, 2172–2181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patterson, K.K.; Parafianowicz, I.; Danells, C.J.; Closson, V.; Verrier, M.C.; Staines, W.R.; Black, S.E.; McIlroy, W.E. Gait Asymmetry in Community-Ambulating Stroke Survivors. Arch. Phys. Med. Rehabil. 2008, 89, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Yogev, G.; Plotnik, M.; Peretz, C.; Giladi, N.; Hausdorff, J.M. Gait Asymmetry in Patients with Parkinson’s Disease and Elderly Fallers: When Does the Bilateral Coordination of Gait Require Attention? Exp. Brain Res. Heidelb. 2007, 177, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Lewek, M.D.; Bradley, C.E.; Wutzke, C.J.; Zinder, S.M. The Relationship between Spatiotemporal Gait Asymmetry and Balance in Individuals With Chronic Stroke. J. Appl. Biomech. 2014, 30, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Jørgensen, L.; Crabtree, N.J.; Reeve, J.; Jacobsen, B.K. Ambulatory Level and Asymmetrical Weight Bearing after Stroke Affects Bone Loss in the Upper and Lower Part of the Femoral Neck Differently: Bone Adaptation after Decreased Mechanical Loading. Bone 2000, 27, 701–707. [Google Scholar] [CrossRef]
- Awad, L.N.; Palmer, J.A.; Pohlig, R.T.; Binder-Macleod, S.A.; Reisman, D.S. Walking Speed and Step Length Asymmetry Modify the Energy Cost of Walking After Stroke. Neurorehabil. Neural. Repair. 2015, 29, 416–423. [Google Scholar] [CrossRef] [Green Version]
- Finley, J.M.; Bastian, A.J. Associations between Foot Placement Asymmetries and Metabolic Cost of Transport in Hemiparetic Gait. Neurorehabil. Neural. Repair. 2017, 31, 168–177. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, N.; Finley, J.M. Individual Differences in Locomotor Function Predict the Capacity to Reduce Asymmetry and Modify the Energetic Cost of Walking Poststroke. Neurorehabil. Neural. Repair. 2018, 32, 701–713. [Google Scholar] [CrossRef] [Green Version]
- Wei, T.-S.; Liu, P.-T.; Chang, L.-W.; Liu, S.-Y. Gait Asymmetry, Ankle Spasticity, and Depression as Independent Predictors of Falls in Ambulatory Stroke Patients. PLoS ONE 2017, 12, e0177136. [Google Scholar] [CrossRef]
- Bautmans, I.; Jansen, B.; Van Keymolen, B.; Mets, T. Reliability and Clinical Correlates of 3D-Accelerometry Based Gait Analysis Outcomes According to Age and Fall-Risk. Gait Posture 2011, 33, 366–372. [Google Scholar] [CrossRef]
- Mahlknecht, P.; Kiechl, S.; Bloem, B.R.; Willeit, J.; Scherfler, C.; Gasperi, A.; Rungger, G.; Poewe, W.; Seppi, K. Prevalence and Burden of Gait Disorders in Elderly Men and Women Aged 60–97 Years: A Population-Based Study. PLoS ONE 2013, 8, e69627. [Google Scholar] [CrossRef] [PubMed]
- Roemmich, R.T.; Hack, N.; Akbar, U.; Hass, C.J. Effects of Dopaminergic Therapy on Locomotor Adaptation and Adaptive Learning in Persons with Parkinson’s Disease. Behav. Brain Res. 2014, 268, 31–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaafsma, J.D.; Giladi, N.; Balash, Y.; Bartels, A.L.; Gurevich, T.; Hausdorff, J.M. Gait Dynamics in Parkinson’s Disease: Relationship to Parkinsonian Features, Falls and Response to Levodopa. J. Neurol. Sci. 2003, 212, 47–53. [Google Scholar] [CrossRef]
- Nonnekes, J.; Snijders, A.H.; Nutt, J.G.; Deuschl, G.; Giladi, N.; Bloem, B.R. Freezing of Gait: A Practical Approach to Management. Lancet Neurol. 2015, 14, 768–778. [Google Scholar] [CrossRef]
- Wu, Z.; Xu, H.; Zhu, S.; Gu, R.; Zhong, M.; Jiang, X.; Shen, B.; Zhu, J.; Pan, Y.; Dong, J.; et al. Gait Analysis of Old Individuals with Mild Parkinsonian Signs and Those Individuals’ Gait Performance Benefits Little from Levodopa. Risk Manag. Healthc. Policy 2021, 14, 1109–1118. [Google Scholar] [CrossRef]
- Suppa, A.; Kita, A.; Leodori, G.; Zampogna, A.; Nicolini, E.; Lorenzi, P.; Rao, R.; Irrera, F. L-DOPA and Freezing of Gait in Parkinson’s Disease: Objective Assessment through a Wearable Wireless System. Front. Neurol. 2017, 8, 406. [Google Scholar] [CrossRef]
- Plotnik, M.; Hausdorff, J.M. The Role of Gait Rhythmicity and Bilateral Coordination of Stepping in the Pathophysiology of Freezing of Gait in Parkinson’s Disease. Mov. Disord. 2008, 23, S444–S450. [Google Scholar] [CrossRef] [PubMed]
- Nonnekes, J.; Nieuwboer, A. Towards Personalized Rehabilitation for Gait Impairments in Parkinson’s Disease. J. Parkinsons Dis. 2018, 8, S101–S106. [Google Scholar] [CrossRef] [Green Version]
- Panero, E.; Digo, E.; Dimanico, U.; Artusi, C.A.; Zibetti, M.; Gastaldi, L. Effect of Deep Brain Stimulation Frequency on Gait Symmetry, Smoothness and Variability Using IMU. In Proceedings of the 2021 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Lausanne, Switzerland, 23–25 June 2021; pp. 1–6. [Google Scholar]
- Reisman, D.S.; Wityk, R.; Silver, K.; Bastian, A.J. Locomotor Adaptation on a Split-Belt Treadmill Can Improve Walking Symmetry Post-Stroke. Brain 2007, 130, 1861–1872. [Google Scholar] [CrossRef] [Green Version]
- Reisman, D.S.; Wityk, R.; Silver, K.; Bastian, A.J. Split-Belt Treadmill Adaptation Transfers to Overground Walking in Persons Poststroke. Neurorehabil. Neural. Repair 2009, 23, 735–744. [Google Scholar] [CrossRef] [Green Version]
- Roemmich, R.T.; Nocera, J.R.; Stegemöller, E.L.; Hassan, A.; Okun, M.S.; Hass, C.J. Locomotor Adaptation and Locomotor Adaptive Learning in Parkinson’s Disease and Normal Aging. Clin. Neurophysiol. 2014, 125, 313–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Combs, S.A.; Dugan, E.L.; Ozimek, E.N.; Curtis, A.B. Bilateral Coordination and Gait Symmetry after Body-Weight Supported Treadmill Training for Persons with Chronic Stroke. Clin. Biomech. 2013, 28, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Lin, K.-H.; Lu, T.-W.; Chai, H.-M.; Chen, H.-L.; Tang, P.-F.; Hu, M.-H. Immediate Effect of Lateral-Wedged Insole on Stance and Ambulation after Stroke. Am. J. Phys. Med. Rehabil. 2010, 89, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Aruin, A.S.; Rao, N. The Effect of a Single Textured Insole in Gait Rehabilitation of Individuals with Stroke. Int. J. Rehabil. Res. 2018, 41, 218–223. [Google Scholar] [CrossRef]
- Esquenazi, A.; Ofluoglu, D.; Hirai, B.; Kim, S. The Effect of an Ankle-Foot Orthosis on Temporal Spatial Parameters and Asymmetry of Gait in Hemiparetic Patients. PMR 2009, 1, 1014–1018. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef]
- Afzal, M.R.; Oh, M.-K.; Lee, C.-H.; Park, Y.S.; Yoon, J. A Portable Gait Asymmetry Rehabilitation System for Individuals with Stroke Using a Vibrotactile Feedback. BioMed Res. Int. 2015, 2015, 375638. [Google Scholar] [CrossRef]
- Arazpour, M.; Ahmadi, F.; Bahramizadeh, M.; Samadian, M.; Mousavi, M.E.; Bani, M.A.; Hutchins, S.W. Evaluation of Gait Symmetry in Poliomyelitis Subjects: Comparison of a Conventional Knee–Ankle–Foot Orthosis and a New Powered Knee–Ankle–Foot Orthosis. Prosthet. Orthot. Int. 2016, 40, 689–695. [Google Scholar] [CrossRef]
- Beauchamp, M.K.; Skrela, M.; Southmayd, D.; Trick, J.; Van Kessel, M.; Brunton, K.; Inness, E.; McIlroy, W.E. Immediate Effects of Cane Use on Gait Symmetry in Individuals with Subacute Stroke. Physiother. Can. 2009, 61, 154–160. [Google Scholar] [CrossRef]
- Brodie, M.A.D.; Dean, R.T.; Beijer, T.R.; Canning, C.G.; Smith, S.T.; Menant, J.C.; Lord, S.R. Symmetry Matched Auditory Cues Improve Gait Steadiness in Most People with Parkinson’s Disease but Not in Healthy Older People. J. Parkinson’s Dis. 2015, 5, 105–116. [Google Scholar] [CrossRef]
- Fasano, A.; Schlenstedt, C.; Herzog, J.; Plotnik, M.; Rose, F.E.M.; Volkmann, J.; Deuschl, G. Split-Belt Locomotion in Parkinson’s Disease Links Asymmetry, Dyscoordination and Sequence Effect. Gait Posture 2016, 48, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Kahn, J.H.; Hornby, T.G. Rapid and Long-Term Adaptations in Gait Symmetry Following Unilateral Step Training in People with Hemiparesis. Phys. Ther. 2009, 89, 474–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewek, M.D.; Feasel, J.; Wentz, E.; Brooks, F.P.; Whitton, M.C. Use of Visual and Proprioceptive Feedback to Improve Gait Speed and Spatiotemporal Symmetry Following Chronic Stroke: A Case Series. Phys. Ther. 2012, 92, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Lewek, M.D.; Braun, C.H.; Wutzke, C.; Giuliani, C. The Role of Movement Errors in Modifying Spatiotemporal Gait Asymmetry Post Stroke: A Randomized Controlled Trial. Clin. Rehabil. 2018, 32, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Little, V.L.; Perry, L.A.; Mercado, M.W.V.; Kautz, S.A.; Patten, C. Gait Asymmetry Pattern Following Stroke Determines Acute Response to Locomotor Task. Gait Posture 2020, 77, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.C.; Rao, N.; Muthukrishnan, S.; Aruin, A.S. A Textured Insole Improves Gait Symmetry in Individuals with Stroke. Disabil. Rehabil. 2018, 40, 2798–2802. [Google Scholar] [CrossRef] [PubMed]
- Miéville, C.; Lauzière, S.; Betschart, M.; Nadeau, S.; Duclos, C. More Symmetrical Gait after Split-Belt Treadmill Walking Does Not Modify Dynamic and Postural Balance in Individuals Post-Stroke. J. Electromyogr. Kinesiol. 2018, 41, 41–49. [Google Scholar] [CrossRef]
- Padmanabhan, P.; Rao, K.S.; Gulhar, S.; Cherry-Allen, K.M.; Leech, K.A.; Roemmich, R.T. Persons Post-Stroke Improve Step Length Symmetry by Walking Asymmetrically. J. NeuroEng. Rehabil. 2020, 17, 105. [Google Scholar] [CrossRef]
- Yen, S.-C.; Schmit, B.D.; Wu, M. Using Swing Resistance and Assistance to Improve Gait Symmetry in Individuals Post-Stroke. Hum. Mov. Sci. 2015, 42, 212–224. [Google Scholar] [CrossRef] [Green Version]
- Zanardi, A.P.J.; Martinez, F.G.; da Silva, E.S.; Casal, M.Z.; Martins, V.F.; Passos-Monteiro, E.; Haas, A.N.; Peyré-Tartaruga, L.A. Effects of Nordic Walking on Gait Symmetry in Mild Parkinson’s Disease. Symmetry 2019, 11, 1481. [Google Scholar] [CrossRef] [Green Version]
- Błażkiewicz, M.; Wiszomirska, I.; Wit, A. Comparison of Four Methods of Calculating the Symmetry of Spatial-Temporal Parameters of Gait. Acta Bioeng. Biomech. 2014, 16, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.K.; Gage, W.H.; Brooks, D.; Black, S.E.; McIlroy, W.E. Evaluation of Gait Symmetry after Stroke: A Comparison of Current Methods and Recommendations for Standardization. Gait Posture 2010, 31, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Viteckova, S.; Kutilek, P.; Svoboda, Z.; Krupicka, R.; Kauler, J.; Szabo, Z. Gait Symmetry Measures: A Review of Current and Prospective Methods. Biomed. Signal. Process. Control. 2018, 42, 89–100. [Google Scholar] [CrossRef]
- Queen, R.; Dickerson, L.; Ranganathan, S.; Schmitt, D. A Novel Method for Measuring Asymmetry in Kinematic and Kinetic Variables: The Normalized Symmetry Index. J. Biomech. 2020, 99, 109531. [Google Scholar] [CrossRef] [PubMed]
- Alves, S.A.; Ehrig, R.M.; Raffalt, P.C.; Bender, A.; Duda, G.N.; Agres, A.N. Quantifying Asymmetry in Gait: The Weighted Universal Symmetry Index to Evaluate 3D Ground Reaction Forces. Front. Bioeng. Biotechnol. 2020, 8, 579511. [Google Scholar] [CrossRef]
- Helm, E.E.; Reisman, D.S. The Split-Belt Walking Paradigm: Exploring Motor Learning and Spatiotemporal Asymmetry Poststroke. Phys. Med. Rehabil. Clin. N. Am. 2015, 26, 703–713. [Google Scholar] [CrossRef] [Green Version]
- Chang, M.D.; Shaikh, S.; Chau, T. Effect of Treadmill Walking on the Stride Interval Dynamics of Human Gait. Gait Posture 2009, 30, 431–435. [Google Scholar] [CrossRef]
- Watkins, C.L.; Leathley, M.J.; Gregson, J.M.; Moore, A.P.; Smith, T.L.; Sharma, A.K. Prevalence of Spasticity Post Stroke. Clin. Rehabil. 2002, 16, 515–522. [Google Scholar] [CrossRef]
- Arene, N.; Hidler, J. Understanding Motor Impairment in the Paretic Lower Limb after a Stroke: A Review of the Literature. Top. Stroke Rehabil. 2009, 16, 346–356. [Google Scholar] [CrossRef]
- Giladi, N.; Treves, T.A.; Simon, E.S.; Shabtai, H.; Orlov, Y.; Kandinov, B.; Paleacu, D.; Korczyn, A.D. Freezing of Gait in Patients with Advanced Parkinson’s Disease. J. Neural. Transm. 2001, 108, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Ploeger, H.E.; Bus, S.A.; Nollet, F.; Brehm, M.-A. Gait Patterns in Association with Underlying Impairments in Polio Survivors with Calf Muscle Weakness. Gait Posture 2017, 58, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Laffont, I.; Julia, M.; Tiffreau, V.; Yelnik, A.; Herisson, C.; Pelissier, J. Aging and Sequelae of Poliomyelitis. Ann. Phys. Rehabil. Med. 2010, 53, 24–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, H.P.; Husted, C.; Lewek, M.D. Improving Spatiotemporal Gait Asymmetry Has Limited Functional Benefit for Individuals Poststroke. J. Neurol. Phys. Ther. 2020, 44, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Creaby, M.W.; Cole, M.H. Gait Characteristics and Falls in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Parkinsonism. Relat. Disord. 2018, 57, 1–8. [Google Scholar] [CrossRef]
- Mirelman, A.; Bonato, P.; Camicioli, R.; Ellis, T.D.; Giladi, N.; Hamilton, J.L.; Hass, C.J.; Hausdorff, J.M.; Pelosin, E.; Almeida, Q.J. Gait Impairments in Parkinson’s Disease. Lancet Neurol. 2019, 18, 697–708. [Google Scholar] [CrossRef]
- Farrokhi, S.; O’Connell, M.; Gil, A.B.; Sparto, P.J.; Fitzgerald, G.K. Altered Gait Characteristics in Individuals With Knee Osteoarthritis and Self-Reported Knee Instability. J. Orthop. Sports Phys. Ther. 2015, 45, 351–359. [Google Scholar] [CrossRef] [Green Version]
- Son, S.J.; Kim, H.; Seeley, M.K.; Hopkins, J.T. Altered Walking Neuromechanics in Patients with Chronic Ankle Instability. J. Athl. Train. 2019, 54, 684–697. [Google Scholar] [CrossRef] [Green Version]
- Schmid, A.; Duncan, P.W.; Studenski, S.; Lai, S.M.; Richards, L.; Perera, S.; Wu, S.S. Improvements in Speed-Based Gait Classifications Are Meaningful. Stroke 2007, 38, 2096–2100. [Google Scholar] [CrossRef] [Green Version]
| Database | Search Index | Search Terms |
|---|---|---|
| PubMed | Title and Other Term | (gait or ambulation or walking or mobility or locomotor or locomotion) AND (symmetry or asymmetry or symmetries or asymmetries or symmetrical or asymmetrical) |
| CINAHL | Title and Abstract | (gait or ambulation or walking or mobility or locomotor or locomotion) AND (symmetry or asymmetry or symmetries or asymmetries or symmetrical or asymmetrical) |
| Academic Search Complete | Title and Abstract | (gait or ambulation or walking or mobility or locomotor or locomotion) AND (symmetry or asymmetry or symmetries or asymmetries or symmetrical or asymmetrical) |
| References | N (Clinical/Control) | Population | Age (Years) |
|---|---|---|---|
| Afzal et al., 2015 | 4/5 | Stroke | NR/26.2 ± 3.27 |
| Arazapour et al., 2016 | 7 | Poliomyelitis | 47–57 |
| Beauchamp et al., 2009 | 14 | Stroke | 61 ± 10 |
| Brodie et al., 2015 | 10/11, 9 | Parkinson’s Disease | 67/66, 30 |
| Fasano et al., 2016 | 20 | Parkinson’s Disease | 60.5 ± 8.8 |
| Kahn et al., 2009 | 18 | Stroke | 34–74 |
| Lewek et al., 2012 | 2 | Stroke | 53, 60 |
| Lewek et al., 2018 | 48 | Stroke | 59 ± 12 |
| Little et al., 2020 | 39 | Stroke | 61.3 ± 11.4 |
| Ma et al., 2018 | 17 | Stroke | 56.2 ± 7.3 |
| Mieville et al., 2018 | 20 | Stroke | 49.4 ± 13.2 |
| Padmanabhan et al., 2020 | 9 | Stroke | 54 ± 4 |
| Yen et al., 2015 | 10 | Stroke | 36–67 |
| Zanardi et al., 2019 | 14 | Parkinson’s Disease | 66.8 ± 9.6 |
| References | Gait Variables | Gait Assessment | Intervention |
|---|---|---|---|
| Afzal et al., 2015 | ST | Force-sensitive resistors | Insoles with vibrotactors to provide feedback. Healthy group walked 10 m in seven scenarios. Stroke group walked 6 m in three scenarios. Both groups performed two trials per scenario. |
| Arazapour et al., 2016 | SW, SL, ST, SWT, double-limb support time, stance phase, walking speed, knee flexion | 3D Motion Capture (VICON) | Compared drop-locked orthosis to new powered Knee Ankle Foot Orthosis (KAFO). Three trials of 6 m walking at preferred speed for each orthosis. |
| Beauchamp et al., 2009 | ST, SWT | Pressure-Sensitive Mat (GAITRite) | Walked 6 m in all three conditions (1) without cane, (2) single-point cane, and (3) quad cane. |
| Brodie et al., 2015 | SL | Triaxial accelerometers (Opal) | Auditory cues matched to each person’s cadence. Five baseline walks no cues, then five conditions of five repeat walks cued at person’s cadence and cued to various paired-step asymmetries of −10%, −5%, 0%, +5%, and +10%. |
| Fasano et al., 2016 | SL, SW, ST, SWT, joint ROM, double support time, speed | 3D Motion Capture (Qualisys), split-belt treadmill | Walking task: (1) Belts at same speed (tied) 5 min; (2) split w/worst side reduction (i.e., worst leg/shortest SL on slower belt), 10 min; (3) split w/best side reduction (i.e., best leg on slower belt), 10 min. |
| Kahn et al., 2009 | SL, stride length, ST, SWT | Pressure-Sensitive Mat (Gait Mat II), treadmill | Stepping task: Step w/impaired limb on treadmill while unimpaired held off treadmill, phase 1—single session unilateral stepping (UST), phase 2—repeated UST. UST for 20 min, 1 session in phase 1, 10 sessions in phase 2 over 2–3 weeks. |
| Lewek et al., 2012 | SL, step time | Split-belt treadmill | (1) 20 min treadmill walking followed by (2) 10–15 min overground training using the IVERT system to provide symmetry feedback in virtual reality. A total of 18 sessions over 6 weeks. |
| Lewek et al., 2018 | SL, step time | Pressure-sensitive mat (GAITRite), 3D motion capture (VICON), split-belt treadmill | Walking task: (1) 2 min belts tied, (2) 18 min training phase, (3) 10–15 min overground walking; 18 sessions; three groups: augmentation, minimization, or control. |
| Little et al., 2020 | SL, stride length, SLS (single limb support) %, gait speed | Pressure-sensitive mat (GAITRite), 3D motion capture (VICON), split-belt treadmill | Three treadmill conditions: Thera stride, 30% body weight support, and guidance of non-paretic limb. Maximum of 40 s for each condition. |
| Ma et al., 2018 | SL, SW, stance phase, single support phase | Pressure-sensitive mat (GAITRite) | Walked with and without textured insole. Two trials each condition. |
| Mieville et al., 2018 | ST, SL, trunk progression, forward foot placement | 3D motion capture (Optotrak Certus), split-belt treadmill | Walking task: (1) Baseline, tied belt at self-selected speed, 3 min; (2) perturbation, split belt w/slow belt at self-selected speed and fast belt at double the self-selected speed, 6 min; (3) post-perturbation, tied belt at self-selected speed, 3 min. Protocol completed twice with non-paretic (NP) on fast belt first. |
| Padmanabhan et al., 2020 | SL | 3D motion capture (VICON), split-belt treadmill | Three walking treadmill trials, each 4 min: (1) Without feedback (baseline), (2) participants preferred gait pattern with visual feedback, and (3) symmetry step lengths with visual feedback and instructed to hit same target. |
| Yen et al., 2015 | SL, SWT | Custom 3D position sensors | Ankle position signals triggered swing assistance or resistance to the affected leg via a cable-driven robot. Swing assistance and resistance sessions were 2 weeks apart. |
| Zanardi et al., 2019 | Max flexion (hip, knee, ankle), hip abduction ROM, ST, relative ST(%) | 3D motion capture (VICON), treadmill | Eleven weeks of Nordic Walking (NW): 4 sessions NW technique adaptation, 18 sessions NW training. |
| References | Symmetry Equation | Findings |
|---|---|---|
| Afzal et al., 2015 | Increased stance time and reduced gait asymmetry. | |
| Arazapour et al., 2016 | New powered KAFO decreases asymmetries in base width, swing time, stance phase %, and knee flexion during the swing phase. | |
| Beauchamp et al., 2009 | Single cane showed improvement in gait symmetry in subjects with baseline asymmetry. | |
| Brodie et al., 2015 | Auditory cues improved gait steadiness in most subjects with PD. Gait symmetry unaffected by symmetry matched auditory cues. | |
| Fasano et al., 2016 | NR | Best reduction side led to worsening interlimb coordination, but improved spatial symmetry. Worst reduction side led to improved interlimb coordination, but decreased spatial symmetry. |
| Kahn et al., 2009 | Phase 1: SLA improved by 9–13% and was maintained up to 24 h post-training, and ~12% improved single limb stance time of impaired limb. Phase 2: SLA decreased at 1 and 2 weeks post-training. | |
| Lewek et al., 2012 | Improved step length and step time asymmetries. | |
| Lewek et al., 2018 | All groups improved step length asymmetries from pre- to post-testing. No improvement in stance time with temporal training. | |
| Little et al., 2020 | Guidance of non-paretic leg induced temporal symmetry by increasing paretic and decreasing nonparetic SLS% concurrently. Guidance of non-paretic leg induced spatial symmetry, but was not statistically significant. | |
| Ma et al., 2018 | Decreased stance phase and single support phase asymmetries | |
| Mieville et al., 2018 | Reduced asymmetries in at least one spatiotemporal parameter in non-paretic fast and paretic fast conditions. | |
| Padmanabhan et al., 2020 | Improved step length asymmetries with symmetric stepping condition. | |
| Yen et al., 2015 | Improved step length symmetry after induced swing resistance in post-adaptation phase. | |
| Zanardi et al., 2019 | Generalized Estimating Equation (GEE); p < 0.05 considered asymmetric | Improved degree of maximum knee flexion in less-affected limb and improved hip abduction range of motion. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meder, K.G.; LoJacono, C.T.; Rhea, C.K. A Systematic Review of Non-Pharmacological Interventions to Improve Gait Asymmetries in Neurological Populations. Symmetry 2022, 14, 281. https://doi.org/10.3390/sym14020281
Meder KG, LoJacono CT, Rhea CK. A Systematic Review of Non-Pharmacological Interventions to Improve Gait Asymmetries in Neurological Populations. Symmetry. 2022; 14(2):281. https://doi.org/10.3390/sym14020281
Chicago/Turabian StyleMeder, Krista G., Chanel T. LoJacono, and Christopher K. Rhea. 2022. "A Systematic Review of Non-Pharmacological Interventions to Improve Gait Asymmetries in Neurological Populations" Symmetry 14, no. 2: 281. https://doi.org/10.3390/sym14020281
APA StyleMeder, K. G., LoJacono, C. T., & Rhea, C. K. (2022). A Systematic Review of Non-Pharmacological Interventions to Improve Gait Asymmetries in Neurological Populations. Symmetry, 14(2), 281. https://doi.org/10.3390/sym14020281






