Simultaneous Quantification of Mixed-Acid Triacylglycerol Positional Isomers and Enantiomers in Palm Oil and Lard by Chiral High-Performance Liquid Chromatography Coupled with Mass Spectrometry
Abstract
1. Introduction
2. Materials and Methods
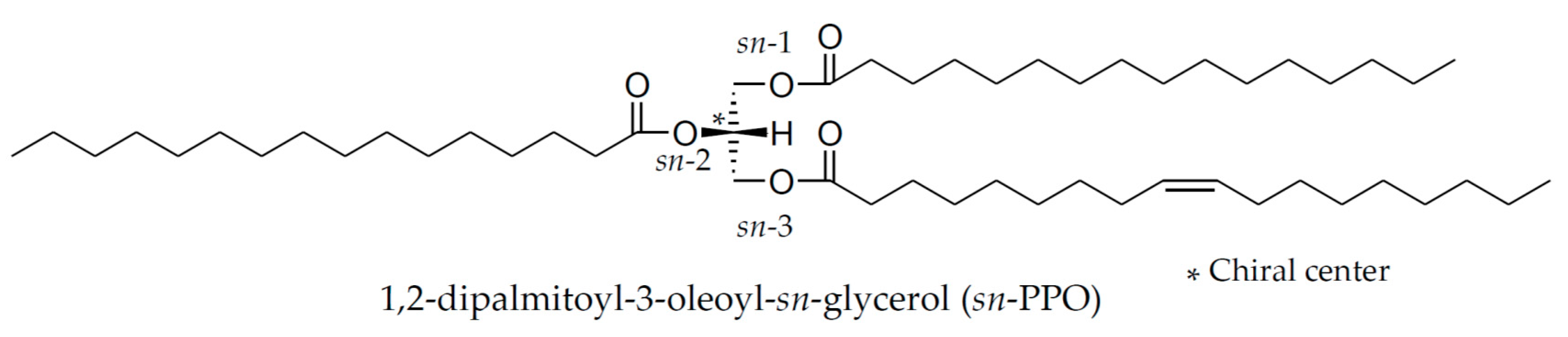
2.1. Materials and Reagents
2.2. Preparation of Standard Solutions
2.3. Preparation of Sample Solution
2.4. LC/MS Analysis Using MRM (Multiple Reaction Monitoring) Mode
2.5. Calculation of the Concentration and Recovery of Each TAG Isomer in Palm Oil and Lard
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Botham, K.M.; Mayes, P.A. Lipids of physiologic significance. In Harper’s Illustrated Biochemistry, 29th ed.; Murray, R.K., Bender, D.A., Botham, K.M., Kennelly, P.J., Rodwell, V.W., Weil, P.A., Eds.; McGraw-Hill Companies: New York, NY, USA, 2012; pp. 140–150. [Google Scholar]
- Nawar, W.W. Lipid. In Food Chemistry, 3rd ed.; Owen, R.F., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1996; pp. 230–231. [Google Scholar]
- OECD-FAO Agricultural Outlook. Available online: http://www.fao.org/3/CA4076EN/CA4076EN_Chapter4_Oilseeds.pdf (accessed on 27 April 2020).
- The Lipid Web. Available online: https://www.lipidhome.co.uk/lipids/simple/tag1/index.htm (accessed on 27 April 2020).
- Renaud, S.C.; Ruf, J.C.; Petithory, D. The positional distribution of fatty acids in palm oil and lard influences their biologic effects in rats. J. Nutr. 1995, 125, 229–237. [Google Scholar] [PubMed]
- Lísa, M.; Holčapek, M. Triacylglycerols profiling in plant oils important in food industry, dietetics and cosmetics using high-performance liquid chromatography–atmospheric pressure chemical ionization mass spectrometry. J. Chromatogr. A 2008, 1198, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Dugo, P.; Kumm, T.; Fazio, A.; Dugo, G.; Mondello, L. Determination of beef tallow in lard through a multidimensional off-line non-aqueous reversed phase–argentation LC method coupled to mass spectrometry. J. Sep. Sci. 2006, 29, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Teh, S.S.; Ong, A.S.H.; Choo, Y.M.; Mah, S.H. Sn-2 hypothesis: A review of the effects of palm oil on blood lipid levels. J. Oleo Sci. 2018, 67, 697–706. [Google Scholar]
- Marikkar, J.M.N.; Lai, O.M.; Ghazali, H.M.; Che Man, Y.B. Detection of lard and randomized lard as adulterants in refined-bleached-deodorized palm oil by differential scanning calorimetry. J. Am. Oil Chem. Soc. 2001, 78, 1113–1119. [Google Scholar] [CrossRef]
- Marikkar, J.M.N.; Lai, O.M.; Ghazali, H.M.; Man, Y.C. Compositional and thermal analysis of RBD palm oil adulterated with lipase-catalyzed interesterified lard. Food Chem. 2002, 76, 249–258. [Google Scholar] [CrossRef]
- Basri, K.N.; Hussain, M.N.; Bakar, J.; Sharif, Z.; Khir, M.F.A.; Zoolfakar, A.S. Classification and quantification of palm oil adulteration via portable NIR spectroscopy. Spectrochim. Acta Part A 2017, 173, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Gotoh, N.; Mizobe, H.; Yoshinaga, K.; Kojima, K.; Matsumoto, Y.; Wada, S. Rapid separation of triacylglycerol positional isomers binding two saturated fatty acids using octacocyl silylation column. J. Oleo Sci. 2011, 60, 345–350. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nagai, T.; Mizobe, H.; Otake, I.; Ichioka, K.; Kojima, K.; Matsumoto, Y.; Gotoh, N.; Kuroda, I.; Wada, S. Enantiomeric separation of asymmetric triacylglycerol by recycle high-performance liquid chromatography with chiral column. J. Chromatogr. A 2011, 1218, 2880–2886. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Matsumoto, Y.; Jiang, Y.; Ishikawa, K.; Wakatabe, T.; Mizobe, H.; Yoshinaga, K.; Kojima, K.; Kuroda, I.; Saito, T.; et al. Actual ratios of triacylglycerol positional isomers and enantiomers comprising saturated fatty acids and highly unsaturated fatty acids in fishes and marine mammals. J. Oleo Sci. 2013, 62, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Watanabe, N.; Yoshinaga, K.; Mizobe, H.; Kojima, K.; Kuroda, I.; Odanaka, Y.; Saito, T.; Beppu, F.; Gotoh, N. Abundances of triacylglycerol positional isomers and enantiomers comprised of a dipalmitoylglycerol backbone and short-or medium-chain fatty acids in bovine milk fat. J. Oleo Sci. 2015, 64, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Ishikawa, K.; Yoshinaga, K.; Yoshida, A.; Beppu, F.; Gotoh, N. Homochiral asymmetric triacylglycerol isomers in egg yolk. J. Oleo Sci. 2017, 12, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Kinoshita, T.; Kasamatsu, E.; Yoshinaga, K.; Mizobe, H.; Yoshida, A.; Itabashi, Y.; Gotoh, N. Simultaneous separation of triacylglycerol enantiomers and positional isomers by chiral high Performance liquid chromatography coupled with mass spectrometry. J. Oleo Sci. 2019, 68, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Botham, K.M.; Mayes, P.A. Metabolism of acylglycerols. In Harper’s Illustrated Biochemistry, 29th ed.; Murray, R.K., Bender, D.A., Botham, K.M., Kennelly, P.J., Rodwell, V.W., Weil, P.A., Eds.; McGraw-Hill Companies: New York, NY, USA, 2012; pp. 229–236. [Google Scholar]

| sn-OPP | sn-PPO | sn-POP | sn-OPO | sn-OOP | sn-POO | |
|---|---|---|---|---|---|---|
| Slope (a) | 0.0206 | 0.0227 | 0.0299 | 0.0335 | 0.0339 | 0.0344 |
| Intercept (b) | −0.0110 | −0.0100 | −0.0112 | −0.0179 | −0.0126 | −0.0156 |
| R2 | 0.9958 | 0.9953 | 0.9956 | 0.9977 | 0.9978 | 0.9976 |
| TAG | sn-OPP | sn-PPO | sn-POP | sn-OPO | sn-OOP | sn-POO |
|---|---|---|---|---|---|---|
| Content (wt%) | 2.1 ± 0.04 | 1.8 ± 0.03 | 19.0 ± 0.36 | 1.2 ± 0.01 | 9.0 ± 0.13 | 6.5 ± 0.03 |
| Recovey (%) | 95 | 97 | 113 | 97 | 98 | 106 |
| RSD(%) of recovery | 7.1 | 8.0 | 2.2 | 19.8 | 25.0 | 4.0 |
| TAG | sn-OPP | sn-PPO | sn-POP | sn-OPO | sn-OOP | sn-POO |
|---|---|---|---|---|---|---|
| Content (wt%) | 1.2 ± 0.01 | 2.6 ± 0.01 | 1.4 ± 0.00 | 12.8 ± 0.15 | 2.6 ± 0.01 | 4.2 ± 0.02 |
| Recovey (%) | 95 | 103 | 102 | 120 | 96 | 102 |
| RSD(%) of recovery | 6.4 | 4.4 | 2.9 | 15.3 | 3.4 | 5.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagai, T.; Kinoshita, T.; Kasamatsu, E.; Yoshinaga, K.; Mizobe, H.; Yoshida, A.; Itabashi, Y.; Gotoh, N. Simultaneous Quantification of Mixed-Acid Triacylglycerol Positional Isomers and Enantiomers in Palm Oil and Lard by Chiral High-Performance Liquid Chromatography Coupled with Mass Spectrometry. Symmetry 2020, 12, 1385. https://doi.org/10.3390/sym12091385
Nagai T, Kinoshita T, Kasamatsu E, Yoshinaga K, Mizobe H, Yoshida A, Itabashi Y, Gotoh N. Simultaneous Quantification of Mixed-Acid Triacylglycerol Positional Isomers and Enantiomers in Palm Oil and Lard by Chiral High-Performance Liquid Chromatography Coupled with Mass Spectrometry. Symmetry. 2020; 12(9):1385. https://doi.org/10.3390/sym12091385
Chicago/Turabian StyleNagai, Toshiharu, Tetsuaki Kinoshita, Erika Kasamatsu, Kazuaki Yoshinaga, Hoyo Mizobe, Akihiko Yoshida, Yutaka Itabashi, and Naohiro Gotoh. 2020. "Simultaneous Quantification of Mixed-Acid Triacylglycerol Positional Isomers and Enantiomers in Palm Oil and Lard by Chiral High-Performance Liquid Chromatography Coupled with Mass Spectrometry" Symmetry 12, no. 9: 1385. https://doi.org/10.3390/sym12091385
APA StyleNagai, T., Kinoshita, T., Kasamatsu, E., Yoshinaga, K., Mizobe, H., Yoshida, A., Itabashi, Y., & Gotoh, N. (2020). Simultaneous Quantification of Mixed-Acid Triacylglycerol Positional Isomers and Enantiomers in Palm Oil and Lard by Chiral High-Performance Liquid Chromatography Coupled with Mass Spectrometry. Symmetry, 12(9), 1385. https://doi.org/10.3390/sym12091385




