Abstract
Cholesterol ester hydroperoxide (CEOOH) is one of the main lipid oxidation products contained in oxidized low-density lipoprotein (LDL). Previous studies suggest that CEOOH in oxidized LDL is closely related to several diseases. Of the oxidation mechanisms of cholesterol ester (CE) in vivo, it has been suggested that enzymatic oxidation induced by lipoxygenase (LOX) plays an important role. Thus, we attempted to develop a method that can evaluate the enzymatic oxidation of CE via the diastereoselective separation of CEOOH bearing 13RS-9Z,11E-hydroperoxy-octadecadienoic acid (13(RS)-HPODE CE). Firstly, we synthesized the standard of 13(RS)-HPODE CE. Using this standard, the screening of analytical conditions (i.e., column, mobile phase, and column temperature) was conducted, and separation of the diastereomers of 13(RS)-HPODE CE was achieved. The diastereoselective separation of 13(RS)-HPODE CE was also confirmed by LC-MS/MS. The developed method (column, CHIRALPAK IB N-3; mobile phase, hexane:ethanol (100:1, v/v); column temperature, 0 °C) can distinguish between enzymatic oxidation and other oxidation mechanisms of CE. Thus, the method can be expected to provide a greater understanding of the biochemical oxidation mechanisms in vivo. Such information will be essential to further elucidate the involvement of CEOOH in various diseases.
1. Introduction
The main lipid peroxidation products contained in oxidized low-density lipoprotein (LDL) are cholesterol ester hydroperoxide (CEOOH) and phosphatidylcholine hydroperoxide (PCOOH), which are the primary products generated by the oxidation of cholesterol ester (CE) and phosphatidylcholine (PC), respectively [1,2,3,4,5]. Increased contents of CEOOH and PCOOH have been detected in the actual blood and organs of patients with diseases such as arteriosclerosis, hyperlipidemia, and diabetes [6,7,8,9,10]. Therefore, CEOOH and PCOOH in oxidized LDL are considered to be closely related to the onset and development of such diseases.
The main lipid peroxidation mechanisms (e.g., CE to CEOOH and PC to PCOOH) that occur in vivo are considered to be enzymatic oxidation, radical oxidation, and singlet-oxygen-induced oxidation (Figure 1a). Of these oxidation mechanisms, previous studies suggest that enzymatic oxidation by lipoxygenase (LOX) plays an important role in the generation of lipid hydroperoxides (i.e., CEOOH and PCOOH) in several diseases [11,12,13,14]. LOX catalyzes the stereo- and regioselective dioxygenation of 1,4-pentadiene of cis-polyunsaturated fatty acids into their corresponding hydroperoxy derivatives [12,15]. For example, when linoleic acid (LA), the main constituent fatty acid of CE and PC in LDL, is oxidized by LOX, 13S-9Z,11E-hydroperoxy-octadecadienoic acid (13(S)-HPODE) is selectively produced. On the other hand, other oxidation mechanisms (i.e., radical oxidation and singlet-oxygen-induced oxidation) afford 13-HPODE as a racemate (13(RS)-HPODE) [16,17] (Figure 1a).
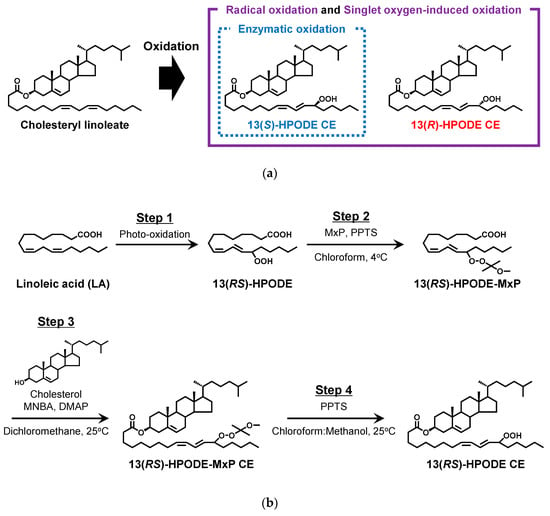
Figure 1.
Chemical structures of CE, 13(R)-HPODE CE, and 13(S)-HPODE CE. LOX-catalyzed oxidation of LA affords 13(S)-HPODE CE, while radical oxidation and singlet-oxygen-induced oxidation of LA afford 13(R)-HPODE CE and 13(S)-HPODE CE (a). The synthesis scheme of CEOOH from LA and cholesterol (b).
Because different lipid hydroperoxide stereoisomers are formed by each oxidation mechanism, an evaluation of enzymatic oxidation in vivo can be made possible through the stereoselective (diastereomeric) analysis of lipid hydroperoxides (CEOOH and PCOOH) bearing 13-HPODE. However, due to analytical challenges, there are no previous reports of successful diastereoselective analysis of such lipid hydroperoxides (i.e., CEOOH and PCOOH). Recently, we achieved the first successful diastereoselective separation of PCOOH bearing 13(RS)-HPODE (13(RS)-HPODE PC) by considering various columns and analytical conditions [18]. Chromatographic separation of 13(R)-HPODE PC and 13(S)-HPODE PC was achieved with a chiral column (CHIRALPAK OP (+) (poly (o-pyridyl diphenylmethacrylate) coated on silica)). However, despite the importance in evaluating the involvement of enzymatic oxidation of CE, diastereoselective analysis of CEOOH isomers has not been achieved to date. Thus in the present study, we sought to develop an analytical method to separate the diastereomers of 13(RS)-HPODE CE by using a chiral column. Using these methods to analyze the diastereomers of lipid hydroperoxides (CEOOH and PCOOH) will greatly contribute to the further understanding of the importance of enzymatic oxidation in vivo.
2. Materials and Methods
2.1. Materials
Cholesteryl linoleate, chloroform-d (CDCl3), pyridinium p-toluenesulfonate (PPTS), and LA were obtained from Sigma (St. Louis, MO, USA). Methylene blue, 2-Methoxypropene (MxP), and 4-(dimethylamino) pyridine (DMAP) were purchased from FUJIFILM Wako Pure Chemical Co. (Osaka, Japan). LOX (from soybean) and 2-methyl-6-nitrobenzoic anhydride (MNBA) were obtained from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Cholesterol was purchased from Olbracht Serdary Research Laboratories (Toronto, ON, Canada). All other reagents were of the highest grade available.
2.2. Synthesis of 13(RS)-HPODE CE
The synthesis of 13(RS)-HPODE CE was accomplished in four steps: (1) preparation of 13(RS)-HPODE via photo-oxidation of LA, (2) treating 13(RS)-HPODE with MxP to protect its hydroperoxyl group (formation of (13(RS)-HPODE-MxP)), (3) esterification of 13(RS)-HPODE-MxP with cholesterol to form 13(RS)-HPODE-MxP CE, and (4) deprotection of 13(RS)-HPODE-MxP CE and purification of 13(RS)-HPODE CE (Figure 1b).
Preparation of 13(RS)-HPODE was performed according to a previously described method with slight modifications [18,19,20,21]. LA (10 g) was dissolved in 2 L of 20 µM methylene blue in chloroform and placed under an LED light (approximately 50,000 lux, 48 h, 15 °C). After the photo-oxidation reaction, the solution was passed through a solid phase extraction (SPE) column (Sep-Pak silica, 10 g; Waters, Milford, MA, USA) which was equilibrated with chloroform. Then, the eluate was evaporated and the residue was dissolved in 4 mL of chloroform. A semipreparative LC-UV system (Shimadzu, Kyoto, Japan) was used to isolate 13(RS)-HPODE from the solution (Condition 1, Table 1). The fraction containing 13(RS)-HPODE was collected, evaporated, and dissolved in chloroform to 15 mg/mL.

Table 1.
Analytical conditions of LC-UV and LC-MS/MS.
Then, to protect the hydroperoxyl group of 13(RS)-HPODE, 250 µL of MxP (1/20 volume equivalent of the 13(RS)-HPODE solution) and 250 µL of 40 mM PPTS in chloroform (1/20 volume equivalent of the 13(RS)-HPODE solution) were added to the crude 13(RS)-HPODE solution. The mixture was incubated for 10 min at 4 °C. After the reaction, the reaction mixture was loaded onto an SPE column (Strata Silica, 200 mg; Phenomenex, Torrance, CA, USA) which was equilibrated with chloroform. The fraction containing 13(RS)-HPODE-MxP was eluted with chloroform, and then the eluate was evaporated. The residue was dissolved in hexane. To isolate 13(RS)-HPODE-MxP, the sample was subjected to semipreparative LC-UV (Condition 1, Table 1). The fraction containing 13(RS)-HPODE-MxP was collected, evaporated, and dissolved in dichloromethane.
To esterify 13(RS)-HPODE-MxP with cholesterol, 13(RS)-HPODE-MxP (20 mg) and cholesterol (25 mg) were mixed in 1.5 mL of dehydrated dichloromethane containing 94.5 mg of MNBA and 67 mg of DMAP. The mixture was allowed to react for 12 h at 25 °C. Then, the reaction mixture was loaded onto an SPE (Sep-Pak silica, 2 g; Waters) that was equilibrated with chloroform. The fraction containing 13(RS)-HPODE-MxP CE was eluted with 16 mL of chloroform, evaporated, dissolved in 2-propanol, and filtrated. To isolate 13(RS)-HPODE-MxP CE, the solution was subjected to semipreparative LC-UV (Condition 2, Table 1). The fraction containing 13(RS)-HPODE-MxP CE was collected, evaporated, and dissolved in chloroform:methanol (1:1, v/v) to 50 mg/mL.
To deprotect 13(RS)-HPODE-MxP CE, 150 µL of 100 mM PPTS in methanol was added to the 13(RS)-HPODE-MxP CE solution, and the reaction mixture was incubated for 1 min at 25 °C. Then, the solution was subjected to semipreparative LC-UV (Condition 2, Table 1) to isolate 13(RS)-HPODE CE. The structure and purity of the obtained 13(RS)-HPODE CE was confirmed by MS and MS/MS using a micrOTOF-Q II mass spectrometer (Bruker Daltonik, Bremen, Germany). The geometric chemical structure was confirmed by 1H NMR using a Varian Unity Plus-600 spectrometer (Varian, Palo Alto, CA, USA) at 600 MHz with CDCl3 as the solvent.
2.3. Screening of Chiral Columns to Separate the Diastereomers of 13(RS)-HPODE CE
The synthesized 13(RS)-HPODE CE was dissolved in hexane to 1 mg/mL. A portion of this sample was introduced to a LC-UV system (Shimadzu) equipped with various chiral columns (CHIRALPAK IA, IB N-5, IC, ID, IE, IF, IG, IH, AD-H and AY-H (4.6 × 250 mm, 5 µm; Daicel, Osaka, Japan) and CHIRALCEL OD-H and OJ-H (4.6 × 250 mm, 5 µm; Daicel)). The details of the analytical conditions are described in Table 2.

Table 2.
The resolution (Rs) and retention times (RT1 and RT2 (min)) of 13(RS)-HPODE CE.
2.4. MS/MS and LC-MS/MS Analysis of 13(RS)-HPODE CE
A portion of the synthesized 13(RS)-HPODE CE was dissolved in 0.1 mM sodium acetate in methanol. To analyze the fragment ions, the sample was directly infused to a microOTOF-Q II mass spectrometer (Bruker Daltonik, Bremen, Germany) at a flow rate of 150 µL/h. MS and MS/MS analyses were performed under the optimized conditions shown in Supplementary Table S1.
The LC-MS/MS analysis system consisted of an Exion LC system coupled with a 4000 QTRAP tandem mass spectrometer (SCIEX, Tokyo, Japan). Multiple reaction monitoring (MRM) of 13(RS)-HPODE CE was performed with the LC-MS/MS system using the identified fragment ions (Condition 3, Table 1).
2.5. Enzymatic Oxidation of CE
Cholesteryl linoleate (1 mg) was dissolved in hexane (1 mL). To prepare a thin lipid film, hexane was evaporated under N2 gas. One milliliter of 50 mM borate buffer (containing 0.25% sodium deoxycholate; pH 9.0) was added to the thin lipid film, which was then sonicated for 5 min. Following sonication, soybean LOX (1.3 × 106 units) in 1 mL of 50 mM borate buffer (pH 9.0) was added. After incubating the reaction mixture in the dark for 30 min at 25 °C, 200 µL of HCl (6 M) was added to stop the reaction. To extract the crude enzymatic oxidation product of cholesteryl linoleate, 2 mL of hexane containing 0.01% butylated hydroxytoluene was added, and the mixture was centrifuged at 1660× g for 10 min at 4 °C. The hexane layer containing the crude enzymatic oxidation product of CE was collected and subjected to LC-MS/MS (Condition 3, Table 1).
3. Results and Discussion
3.1. The Importance of Evaluating Enzymatic Oxidation In Vivo
As described in the introduction, CEOOH and PCOOH in oxidized LDL are closely related to the onset and progression of various diseases. Of all the oxidation mechanisms that afford CEOOH and PCOOH, previous studies have demonstrated the significance of LOX-induced enzymatic oxidation. For instance, Cyrus et al. revealed that the absence of LOX expression decreased lipid peroxidation and atherogenesis in apolipoprotein E-deficient mice [14]. Also, Mabalirajan et al. reported that the overexpression of LOX in non-epithelial cells led to the bronchial epithelial injury of mice [22].
LOX generates 13(S)-HPODE CE and 13(S)-HPODE PC via enzymatic oxidation of CE and PC bearing LA. Such products of enzymatic oxidation differ from the products of other in vivo oxidation mechanisms (i.e., radical oxidation and singlet-oxygen-induced oxidation) in that they are produced in a stereoselective manner. Hence, stereoselective analysis of CEOOH and PCOOH bearing 13-HPODE allows for the identification of enzymatic oxidation. We previously achieved separation of 13(S)-HPODE PC and 13(R)-HPODE PC to evaluate the enzymatic oxidation of PC [18,23]. However, separation of 13(S)-HPODE CE and 13(R)-HPODE CE has not yet been achieved. Because CEOOH is another main oxidation product contained in oxidized LDL, evaluating the involvement of enzymatic oxidation towards the production of CEOOH is also important to evaluate the effect of lipid peroxidation on the onset and development of diseases. In this study, we sought to analyze the diastereomers of 13(RS)-HPODE CE. Utilization of these analytical methods can be expected to clarify the relationship between lipid peroxidation (especially enzymatic oxidation) and the progress of diseases.
3.2. Synthesis of 13(RS)-HPODE CE
The synthesis of 13(RS)-HPODE CE was necessary because it is not commercially available as a standard compound. It is known that 13(RS)-HPODE CE can be synthesized by esterifying 13(RS)-HPODE CE to cholesterol [24]. However, because lipid hydroperoxides (including 13(RS)-HPODE) are relatively unstable compounds, protection of the hydroperoxyl group is necessary when reactions are performed using lipid hydroperoxides as a substrate. Thus, preparation of esterified lipid hydroperoxides has previously been conducted by protecting the hydroperoxyl group of the substrate lipid hydroperoxide with MxP before esterification [18,21,24,25]. Similarly, in this study, 13(RS)-HPODE CE was synthesized by first protecting the hydroperoxyl group of 13(RS)-HPODE with MxP, and then esterifying the resultant 13(RS)-HPODE-MxP with cholesterol using MNBA as a condensing agent. Although previous studies have used N,N’-dicyclohexylcarbodiimide (DCC) as a condensing agent, MNBA was chosen due to the convenience of the purification process [26]. The yield of the condensation reaction was sufficient compared with previous studies, suggesting that not only DCC but also MNBA can be widely applied to the synthesis of other esterified lipid hydroperoxides. The synthesized 13(RS)-HPODE-MxP CE was deprotected, and the resultant 13(RS)-HPODE CE was chromatographically purified. The cis–trans structure of the fatty acid chain was confirmed based on the results of 1H NMR (data not shown), which was in agreement with previous studies [27]. The purity of the obtained 13(RS)-HPODE CE was high enough for use as a standard (>95%), as judged from the MS spectrum (Figure 2a).
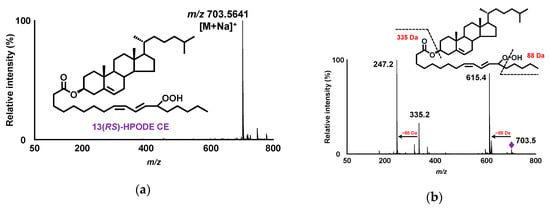
Figure 2.
Q1 mass spectra of 13(RS)-HPODE CE in the presence of Na+ demonstrating sodiated 13(RS)-HPODE CE (m/z 703.5641 [M + Na]+ (calcd. 703.5641)) (a). Product ion mass spectra of 13(RS)-HPODE CE demonstrating product ions formed from sodiated 13(RS)-HPODE CE (m/z 703.5 [M + Na]+) (b).
3.3. Screening of Chiral Columns to Separate the Diastereomers of 13(RS)-HPODE CE
By using the 13(RS)-HPODE CE standard, we then examined LC-UV conditions in order to separate the diastereomers of 13(RS)-HPODE CE. As an initial screening, 12 chiral columns (i.e., CHIRALPAK IA, IB N-5, IC, ID, IE, IF, IG, IH, AD-H, and AY-H and CHIRALCEL OD-H and OJ-H) were tested, each in combination with two types of mobile phases (i.e., hexane:ethanol (100:1, v/v) and hexane:2-propanol (100:1, v/v)). Of the 24 conditions tested, 16 conditions were totally unsuccessful, while 8 conditions enabled partial separation of the diastereomers of 13(RS)-HPODE CE. In particular, CHIRALPAK IB N-5 (cellulose tris (3,5-dimethylphenylcarbamate) immobilized to silica) with hexane:ethanol (100:1, v/v) showed a relatively good partial separation of the diastereomers of 13(RS)-HPODE CE within a short retention time (Table 2). Thus, CHIRALPAK IB N-5 was chosen as a candidate for the following experiments. To achieve the complete separation of 13(RS)-HPODE CE, we tried to improve separation by lowering the column temperature. Lowering the column temperature of 25 °C to 5 °C greatly improved the separation of the diastereomers of 13(RS)-HPODE CE by delaying the elution time (RS, 1.7; RT1, 15.1 min; RT2, 16.6 min). Lowering the column temperature further to 0 °C resulted in a complete separation (RS, 2.0; RT1, 20.0 min; RT2, 22.3 min). Meanwhile, lowering the polarity of the mobile phase from hexane:ethanol (100:1, v/v) to hexane:ethanol (100:0.5, v/v) without changing the column temperature did not improve separation, although the elution time of 13(RS)-HPODE CE was delayed (RS, 1.0; RT1, 25.8 min; RT2, 27.0 min). This suggests that the column temperature is a more important factor than the polarity of the mobile phase. Because diastereoselective separation of 13(RS)-HPODE CE was achieved using LC-UV, we then tried to obtain further confirmation based on structural analysis using MS/MS and LC-MS/MS.
3.4. Confirmation of Diastereoselective Separation of 13(RS)-HPODE CE via LC-MS/MS
The aforementioned LC-UV analysis was conducted based on the detection of the double bonds of 13(RS)-HPODE CE. Thus, to fully confirm the diastereoselective separation of 13(RS)-HPODE CE, detailed structural analysis with MS/MS and LC-MS/MS was conducted using the synthesized 13(RS)-HPODE CE standard. In previous studies, we identified that the use of Na+ during MS/MS analysis affords structure-diagnostic fragment ions that can identify the position of the hydroperoxyl group of various lipid hydroperoxides (e.g., fatty acid hydroperoxide [17,19,27], triacylglycerol hydroperoxide [28], phospholipid hydroperoxide [18,20,23], and squalene hydroperoxide [29]). Hence, in this study, we expected that the method could also be applied to the analysis of CEOOH. To investigate their fragmentations, direct infusion of 13(RS)-HPODE CE into the MS/MS system was performed in the presence of Na+. It was found that 13(RS)-HPODE CE afforded characteristic fragment ions at m/z 615.4 ([M+Na−C5H12O]+) and m/z 247.2 ([M+Na−C5H12O−C27H45]+) corresponding to the neutral losses of 88 and 456 Da, respectively, from the sodiated molecular ion (m/z 703.5 [M + Na]+) (Figure 2b). As we expected, the fragmentation patterns of 13(RS)-HPODE CE observed in this study were consistent with our previous analysis of 13(RS)-HPODE and glycerolipids bearing HPODE (e.g., 13(RS)-HPODE PC and dioleoyl-13(RS)-HPODE triacylglycerol). Therefore, the ability of Na+ to promote ionization of the hydroperoxyl-group-derived fragment ions can be considered effective not only towards the analysis of fatty acid hydroperoxides and glycerolipid hydroperoxides, but also towards the analysis of CEOOH. Based on these results, LC-MS/MS conditions were optimized, and the complete diastereomeric separation of 13(RS)-HPODE CE was achieved (RS, 1.8; RT1, 29 min; RT2, 32 min) (Figure 3a). The optimized conditions were as follows: column, CHIRALPAK IB N-3 (small particle size type of CHIRALPAK IB N-5) (2.1 × 150 mm, 3 µm, Daicel); mobile phase, hexane:ethanol (100:1, v/v); flow rate, 0.2 mL/min; oven, 0 °C; MRM ion pairs; m/z 703 > 247. To the best of our knowledge, this is the first study reporting the direct separation of the diastereomers of CEOOH.
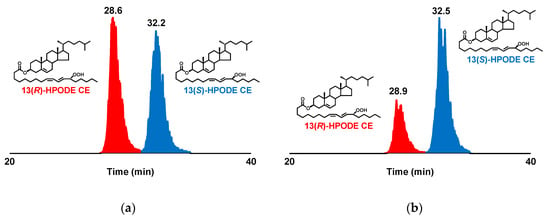
Figure 3.
Diastereomer analysis of synthesized 13(RS)-HPODE CE (a) and LOX-catalyzed oxidation product of CE (b). 13(RS)-HPODE CE was detected by structure-selective MRM (m/z 703 > 247). Detailed analytical conditions are described in the Materials and Methods section as well as Table 1.
In order to characterize 13(R)-HPODE CE and 13(S)-HPODE CE from the two detected peaks, unoxidized CE (cholesterol linoleate) was enzymatically oxidized using LOX to prepare an oxidized CE sample consisting predominantly of 13(S)-HPODE CE. When this sample was analyzed under the optimized conditions, the latter peak was the major peak detected (Figure 3b). Thus, it was confirmed that the order of elution was from 13(R)-HPODE CE to 13(S)-HPODE CE under the developed analytical conditions. The presence of 13(R)-HPODE CE in the LOX-oxidized CE sample was presumably due to the progression of radical oxidation during the emulsification process needed for LOX treatment. In fact, when CE was treated with the same process in the absence of LOX, an increase in the amount of 13(RS)-HPODE CE was confirmed (data not shown). Additionally, it has been previously reported that radical oxidation proceeds simultaneously with LOX oxidation [18]. From the above, we were able to achieve the first chromatographic separation of the diastereomers of 13(RS)-HPODE CE. Currently, we are further analyzing human plasma samples using the present method. It is expected that the results of the analysis of such clinical samples will greatly contribute to elucidate the importance of enzymatic lipid oxidation in vivo.
4. Conclusions
In this study, we achieved diastereoselective separation of 13(RS)-HPODE CE using LC-MS/MS equipped with a chiral column. The present method can differentiate between enzymatic oxidation and other oxidation mechanisms (i.e., radical oxidation and singlet-oxygen-induced oxidation) of CE. Thus, the method can be expected to provide further understanding of the biochemical oxidation mechanisms in vivo. Such information is invaluable for future research to elucidate the involvement of CEOOH in the development of diseases.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-8994/12/7/1127/s1, Supplementary Table S1. Analytical conditions used for MS and MS/MS analysis.
Author Contributions
Conceptualization, J.I., S.K., and K.N.; methodology, J.I.; formal analysis, J.I. and Y.O.; writing—original draft preparation, J.I., N.S., and K.N.; writing—review and editing, J.I., N.S., S.K., Y.O., and K.N.; funding acquisition, K.N. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by JSPS KAKENHI Grant Number 19H02901.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Miyazawa, T.; Yasuda, K.; Fujimoto, K.; Kaneda, T. Presence of phosphatidylcholine hydroperoxide in human plasma. J. Biochem. 1988, 103, 744–746. [Google Scholar] [CrossRef] [PubMed]
- Leitinger, N. Cholesteryl ester oxidation products in atherosclerosis. Mol. Asp. Med. 2003, 24, 239–250. [Google Scholar] [CrossRef]
- Noguchi, N.; Numano, R.; Kaneda, H.; Niki, E. Oxidation of lipids in low density lipoprotein particles. Free Radic. Res. 1998, 29, 43–52. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Niki, E. Presence of cholesteryl ester hydroperoxide in human blood plasma. Biochem. Biophys. Res. Commun. 1989, 165, 988–993. [Google Scholar] [CrossRef]
- Miyazawa, T. Determination of phospholipid hydroperoxides in human blood plasma by a chemiluminescence-HPLC assay. Free Radic. Biol. Med. 1989, 7, 209–217. [Google Scholar] [CrossRef]
- Maor, I.; Hayek, T.; Coleman, R.; Aviram, M. Plasma LDL oxidation leads to its aggregation in the atherosclerotic apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2995–3005. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, T.; Oikawa, S.; Hirayama, Y.; Tokita, Y.; Sekikawa, A.; Ishigaki, Y.; Yamada, R.; Miyazawa, T. Increase of serum phosphatidylcholine hydroperoxide dependent on glycemic control in type 2 diabetic patients. Diabetes Res. Clin. Pract. 2002, 56, 19–25. [Google Scholar] [CrossRef]
- Kinoshita, M.; Oikawa, S.; Hayasaka, K.; Sekikawa, A.; Nagashima, T.; Toyota, T.; Miyazawa, T. Age-related increases in plasma phosphatidylcholine hydroperoxide concentrations in control subjects and patients with hyperlipidemia. Clin. Chem. 2000, 46, 822–828. [Google Scholar] [CrossRef]
- Piotrowski, J.J.; Shah, S.; Alexander, J.J. Mature human atherosclerotic plaque contains peroxidized phosphatidylcholine as a major lipid peroxide. Life Sci. 1996, 58, 735–740. [Google Scholar] [CrossRef]
- Ravandi, A.; Babaei, S.; Leung, R.; Monge, J.C.; Hoppe, G.; Hoff, H.; Kamido, H.; Kuksis, A. Phospholipids and oxophospholipids in atherosclerotic plaques at different stages of plaque development. Lipids 2004, 39, 97–109. [Google Scholar] [CrossRef]
- Funk, C.D.; Cyrus, T. 12/15-lipoxygenase, oxidative modification of LDL and atherogenesis. Trends Cardiovasc. Med. 2001, 11, 116–124. [Google Scholar]
- Singh, N.K.; Rao, G.N. Emerging role of 12/15-Lipoxygenase (ALOX15) in human pathologies. Prog. Lipid Res. 2019, 73, 28–45. [Google Scholar] [CrossRef]
- Belkner, J.; Stender, H.; Kühn, H. The rabbit 15-lipoxygenase preferentially oxygenates LDL cholesterol esters, and this reaction does not require vitamin E. J. Biol. Chem. 1998, 273, 23225–23232. [Google Scholar] [CrossRef]
- Cyrus, T.; Praticò, D.; Zhao, L.; Witztum, J.L.; Rader, D.J.; Rokach, J.; FitzGerald, G.A.; Funk, C.D. Absence of 12/15-lipoxygenase expression decreases lipid peroxidation and atherogenesis in apolipoprotein e-deficient mice. Circulation 2001, 103, 2277–2282. [Google Scholar] [CrossRef] [PubMed]
- Kühn, H.; Wiesner, R.; Lankin, V.Z.; Nekrasov, A.; Alder, L.; Schewe, T. Analysis of the stereochemistry of lipoxygenase-derived hydroxypolyenoic fatty acids by means of chiral phase high-pressure liquid chromatography. Anal. Biochem. 1987, 160, 24–34. [Google Scholar] [CrossRef]
- Frankel, E.N. Chemistry of free radical and singlet oxidation of lipids. Prog. Lipid Res. 1984, 23, 197–221. [Google Scholar] [CrossRef]
- Ito, J.; Shimizu, N.; Kobayashi, E.; Hanzawa, Y.; Otoki, Y.; Kato, S.; Hirokawa, T.; Kuwahara, S.; Miyazawa, T.; Nakagawa, K. A novel chiral stationary phase LC-MS/MS method to evaluate oxidation mechanisms of edible oils. Sci. Rep. 2017, 7, 10026. [Google Scholar] [CrossRef]
- Ito, J.; Nakagawa, K.; Kato, S.; Hirokawa, T.; Kuwahara, S.; Nagai, T.; Miyazawa, T. Direct separation of the diastereomers of phosphatidylcholine hydroperoxide bearing 13-hydroperoxy-9Z,11E-octadecadienoic acid using chiral stationary phase high-performance liquid chromatography. J. Chromatogr. A 2015, 1386, 53–61. [Google Scholar] [CrossRef]
- Ito, J.; Mizuochi, S.; Nakagawa, K.; Kato, S.; Miyazawa, T. Tandem mass spectrometry analysis of linoleic and arachidonic acid hydroperoxides via promotion of alkali metal adduct formation. Anal. Chem. 2015, 87, 4980–4987. [Google Scholar] [CrossRef]
- Kato, S.; Nakagawa, K.; Suzuki, Y.; Asai, A.; Nagao, M.; Nagashima, K.; Oikawa, S.; Miyazawa, T. Liquid chromatography-tandem mass spectrometry determination of human plasma 1-palmitoyl-2-hydroperoxyoctadecadienoyl-phosphatidylcholine isomers via promotion of sodium adduct formation. Anal. Biochem. 2015, 471, 51–60. [Google Scholar] [CrossRef]
- Kato, S.; Nakagawa, K.; Suzuki, Y.; Suzuki, K.; Mizuochi, S.; Miyazawa, T. Preparation of 13 or 9-hydroperoxy-9Z,11E (9E,11E) or 10E,12Z (10E,12E)-octadecadienoic phosphatidylcholine hydroperoxide. J. Oleo Sci. 2014, 63, 431–437. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mabalirajan, U.; Rehman, R.; Ahmad, T.; Kumar, S.; Leishangthem, G.D.; Singh, S.; Dinda, A.K.; Biswal, S.; Agrawal, A.; Ghosh, B. 12/15-lipoxygenase expressed in non-epithelial cells causes airway epithelial injury in asthma. Sci. Rep. 2013, 3, 1540. [Google Scholar] [CrossRef] [PubMed]
- Ito, J.; Nakagawa, K.; Kato, S.; Hirokawa, T.; Kuwahara, S.; Nagai, T.; Miyazawa, T. A novel chiral stationary phase HPLC-MS/MS method to discriminate between enzymatic oxidation and auto-oxidation of phosphatidylcholine. Anal. Bioanal. Chem. 2016, 408, 7785–7793. [Google Scholar] [CrossRef] [PubMed]
- Baba, N.; Tahara, S.; Nakajima, S.; Iwasa, J.; Kaneko, T.; Matsuo, M. Syntheses of Cholesteryl 13-Hydroperoxyoctadecadienoate and Its Derivative with Lipoxygenase. Biosci. Biotechnol. Biochem. 1992, 56, 540. [Google Scholar] [CrossRef]
- Baba, N.; Yoneda, K.; Tahara, S.; Iwasa, J.; Kaneko, T.; Matsuo, M. A regioselective, stereoselective synthesis of a diacylglycerophosphocholine hydroperoxide by use of lipoxygenase and lipase. J. Chem. Soc. Chem. Commun. 1990, 18, 1281–1282. [Google Scholar] [CrossRef]
- Yasuda, T.; Kinoshita, M.; Murata, M.; Matsumori, N. Detailed Comparison of Deuterium Quadrupole Profiles between Sphingomyelin and Phosphatidylcholine Bilayers. Biophys. J. 2014, 106, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Ito, J.; Komuro, M.; Parida, I.S.; Shimizu, N.; Kato, S.; Meguro, Y.; Ogura, Y.; Kuwahara, S.; Miyazawa, T.; Nakagawa, K. Evaluation of lipid oxidation mechanisms in beverages and cosmetics via analysis of lipid hydroperoxide isomers. Sci. Rep. 2019, 9, 7387. [Google Scholar] [CrossRef]
- Kato, S.; Shimizu, N.; Hanzawa, Y.; Otoki, Y.; Ito, J.; Kimura, F.; Takekoshi, S.; Sakaino, M.; Sano, T.; Eitsuka, T.; et al. Determination of triacylglycerol oxidation mechanisms in canola oil using liquid chromatography–tandem mass spectrometry. NPJ Sci. Food 2018, 2, 1. [Google Scholar] [CrossRef]
- Shimizu, N.; Bersabe, H.; Ito, J.; Kato, S.; Towada, R.; Eitsuka, T.; Kuwahara, S.; Miyazawa, T.; Nakagawa, K. Mass Spectrometric Discrimination of Squalene Monohydroperoxide Isomers. J. Oleo Sci. 2017, 66, 227–234. [Google Scholar] [CrossRef][Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).