Possibility of Using Astaxanthin-Rich Dried Cell Powder from Paracoccus carotinifaciens to Improve Egg Yolk Pigmentation of Laying Hens
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Feeding Experiments
2.3. HPLC Analysis
2.4. Carotenoid Extraction from Egg Yolk
2.5. Statistical Analysis
3. Results and Discussion
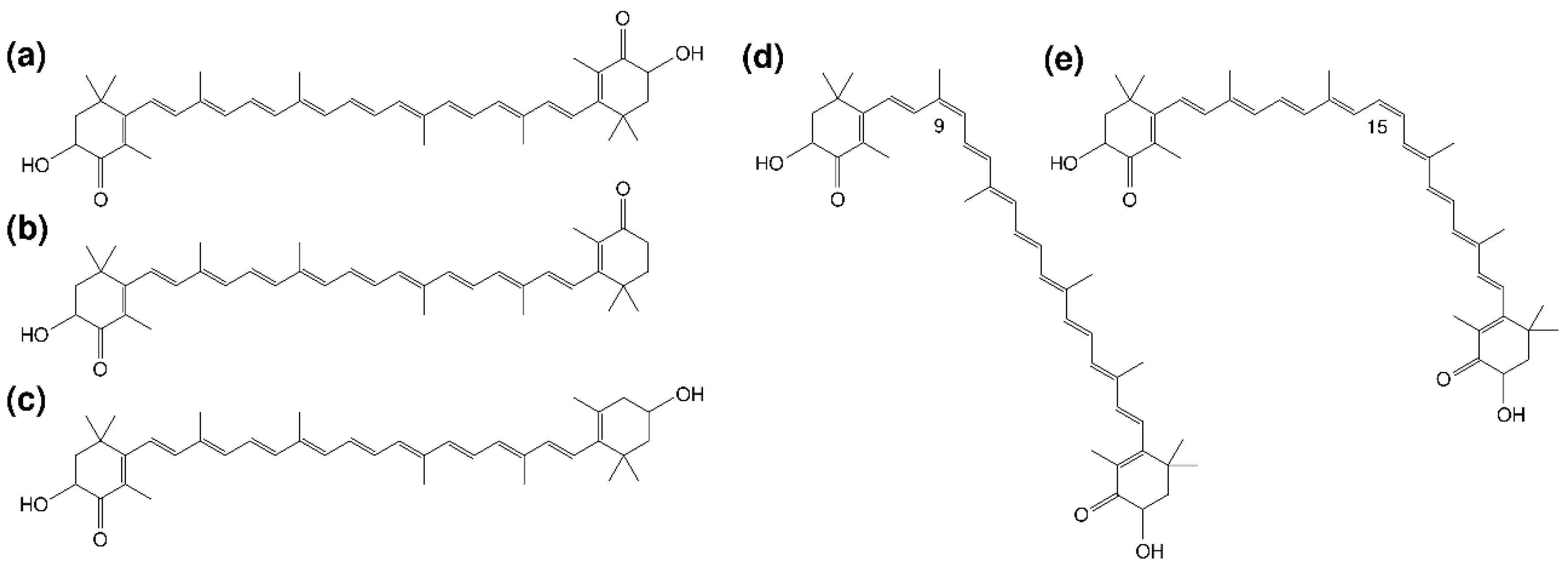
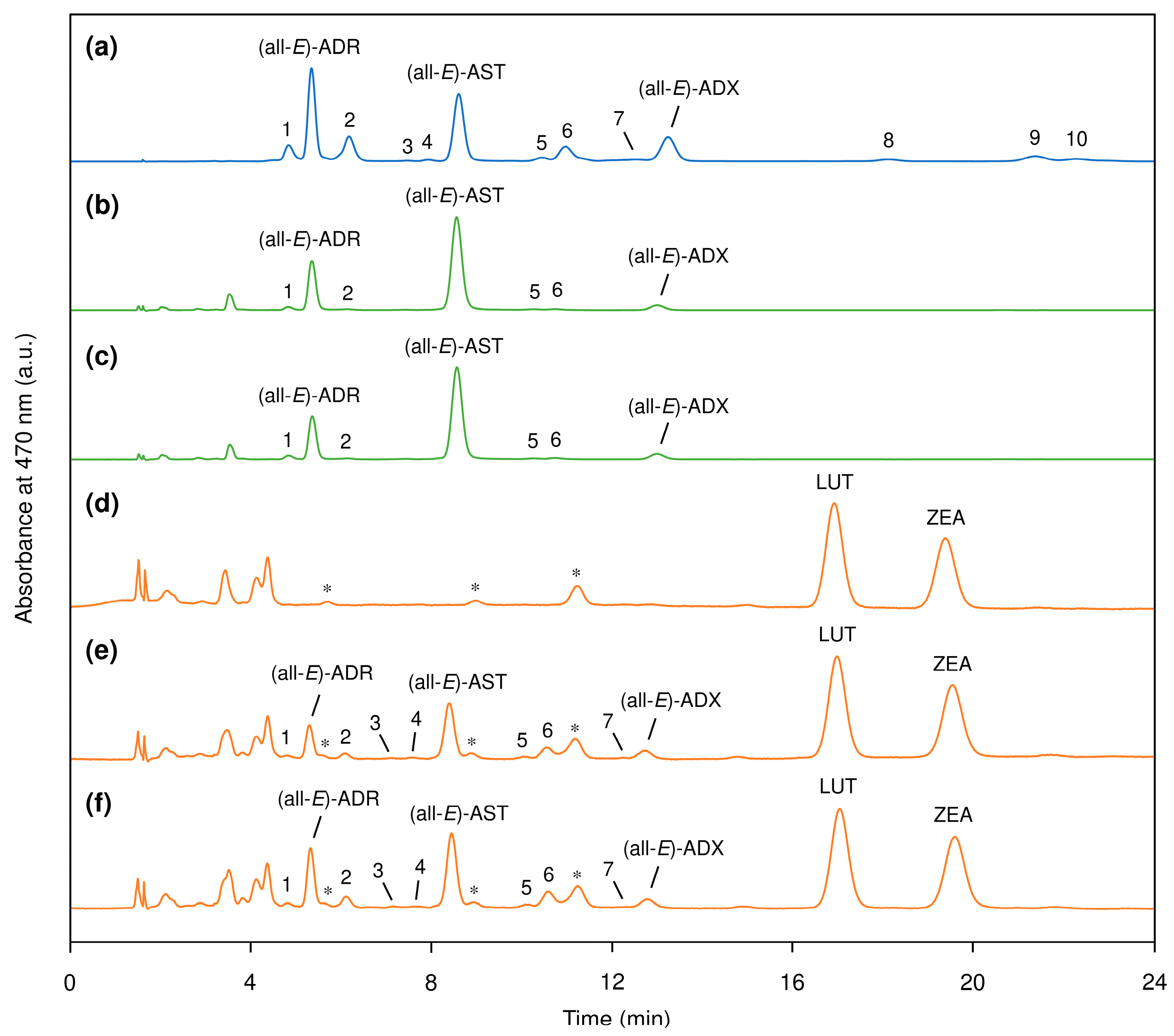
3.1. Profile of Carotenoid Isomers in Egg Yolk
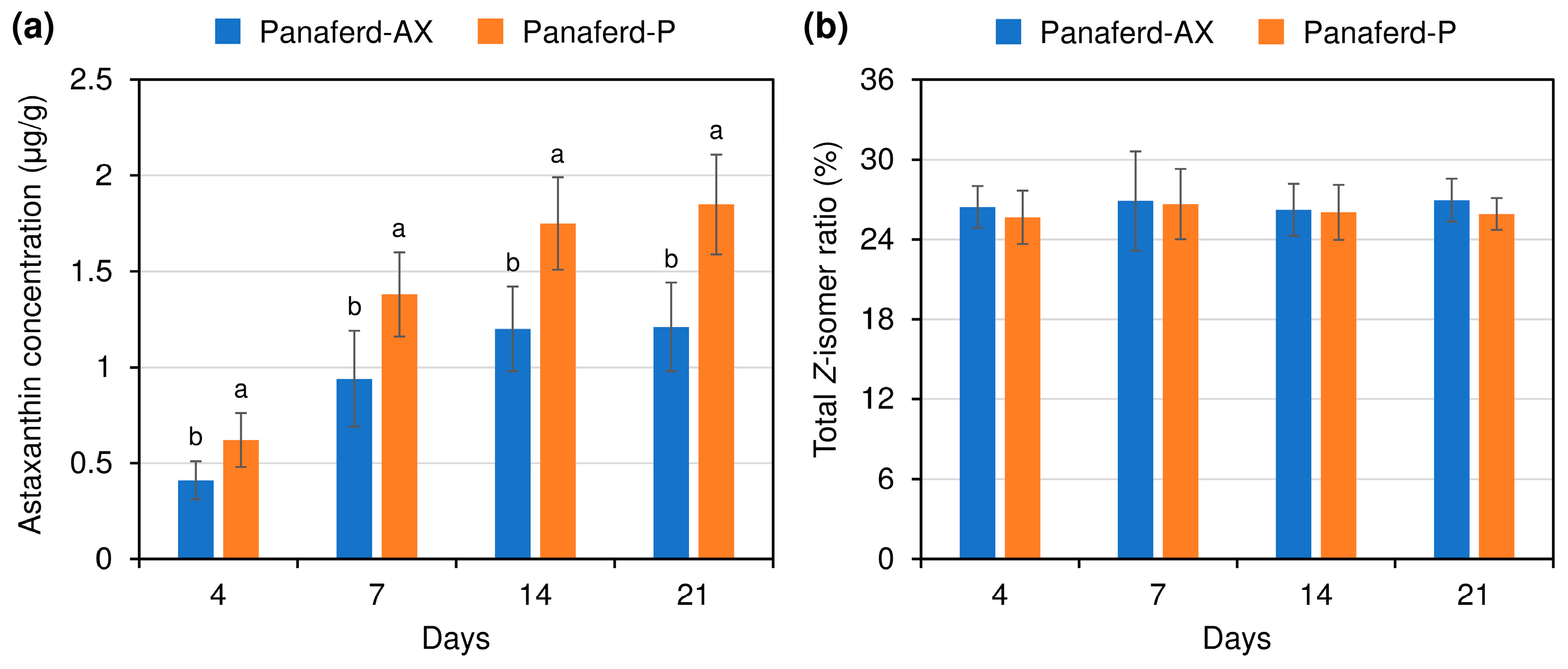
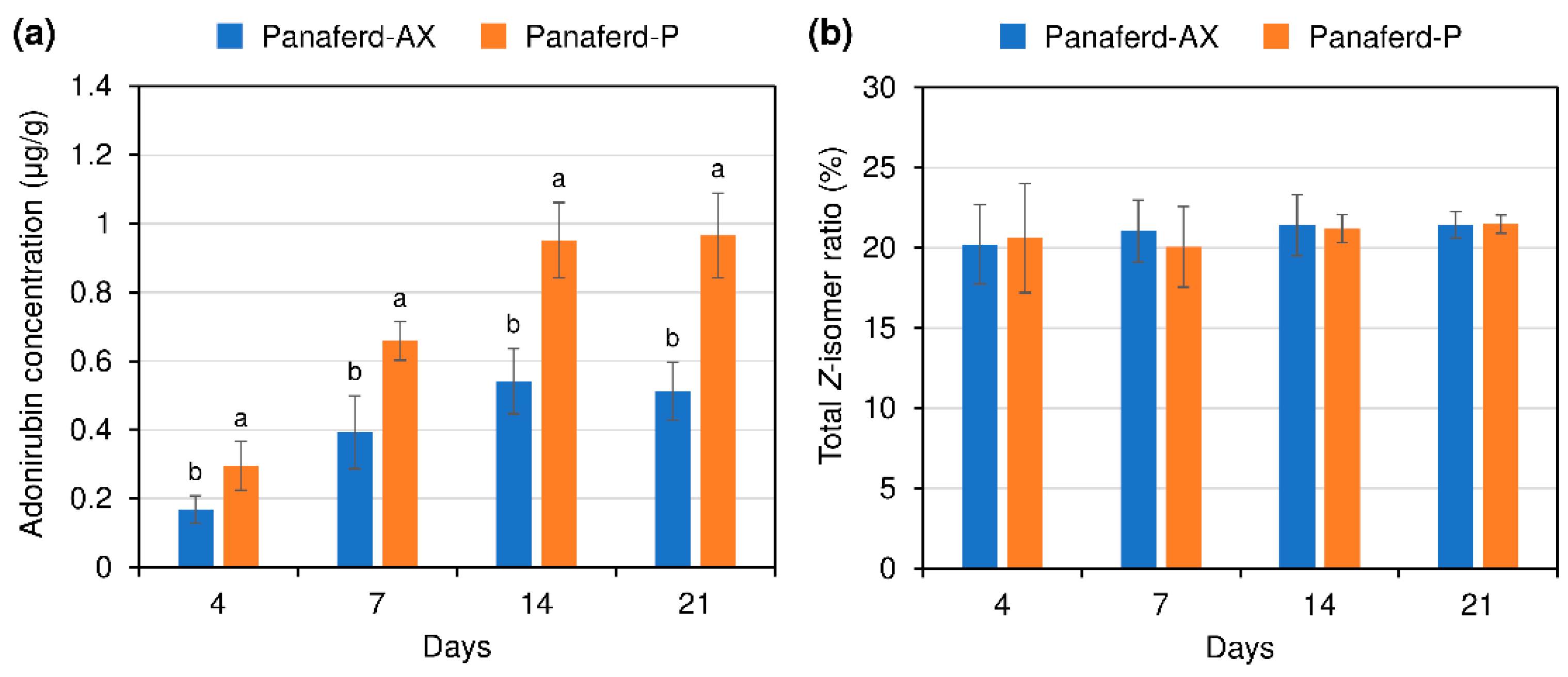
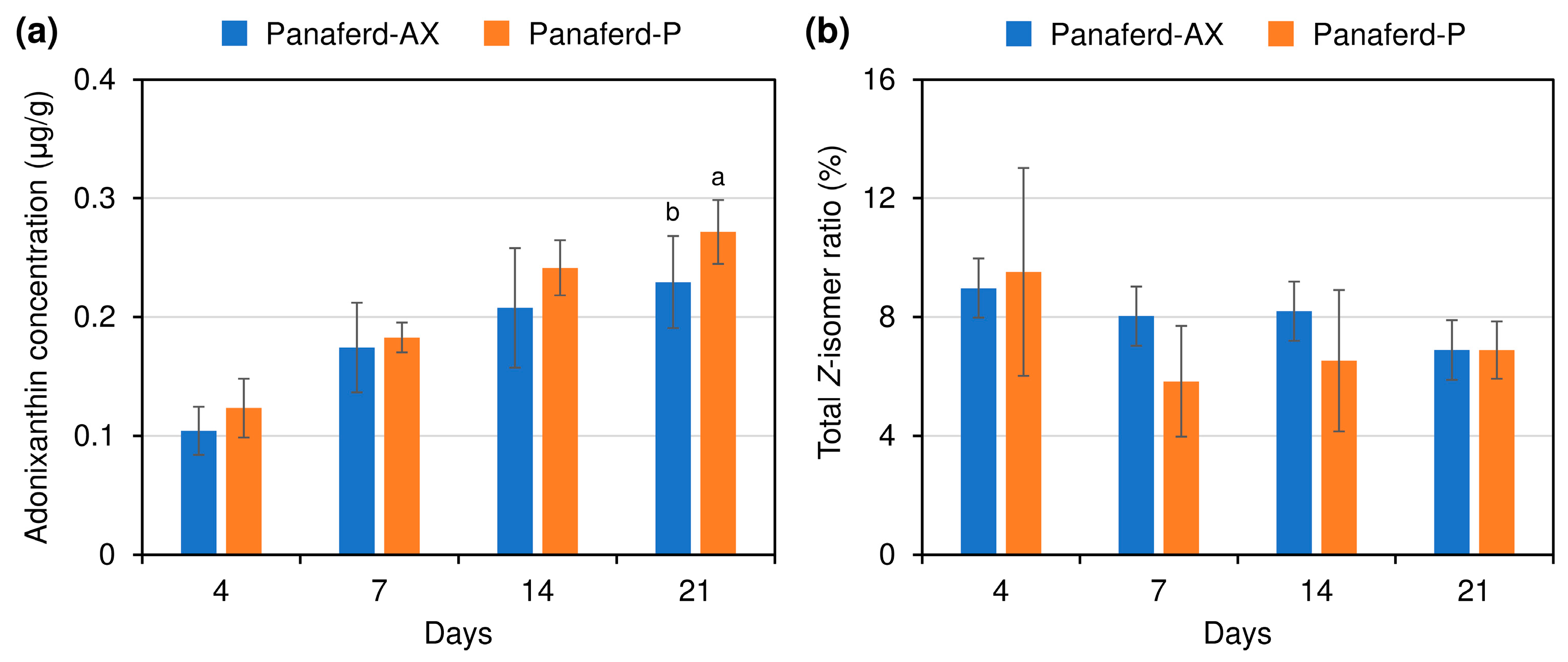
3.2. Evaluation of Carotenoid Concentration and Z-isomer Ratio in Egg Yolk
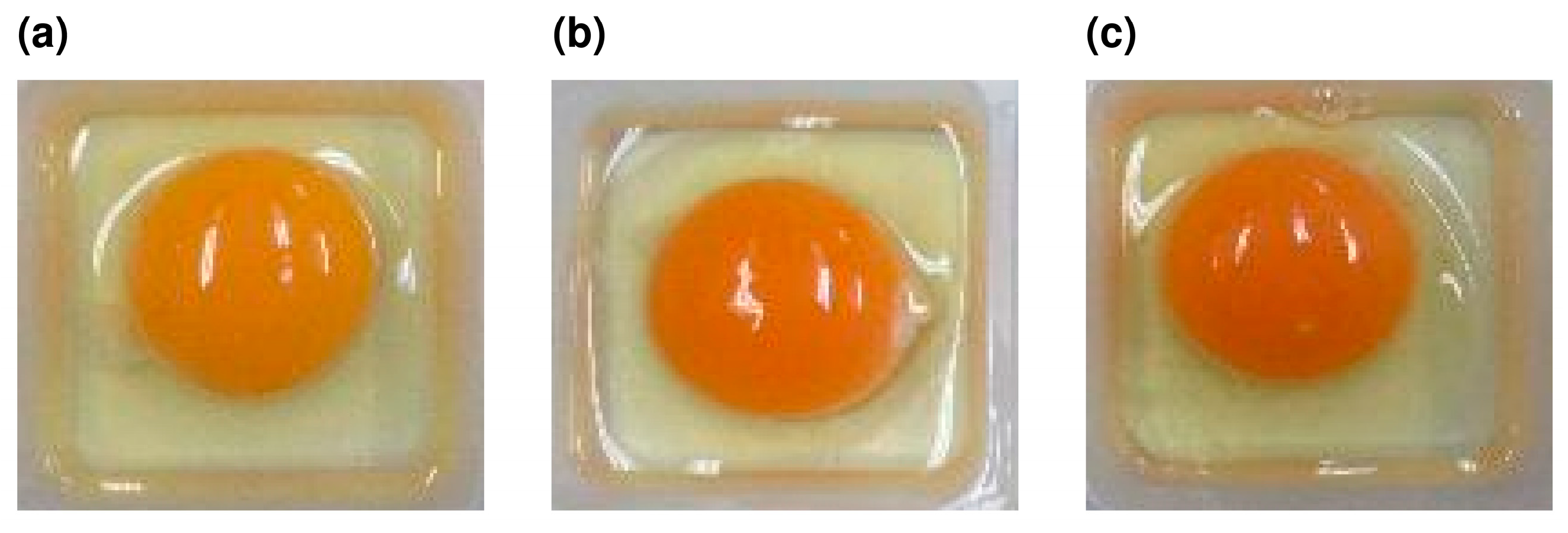
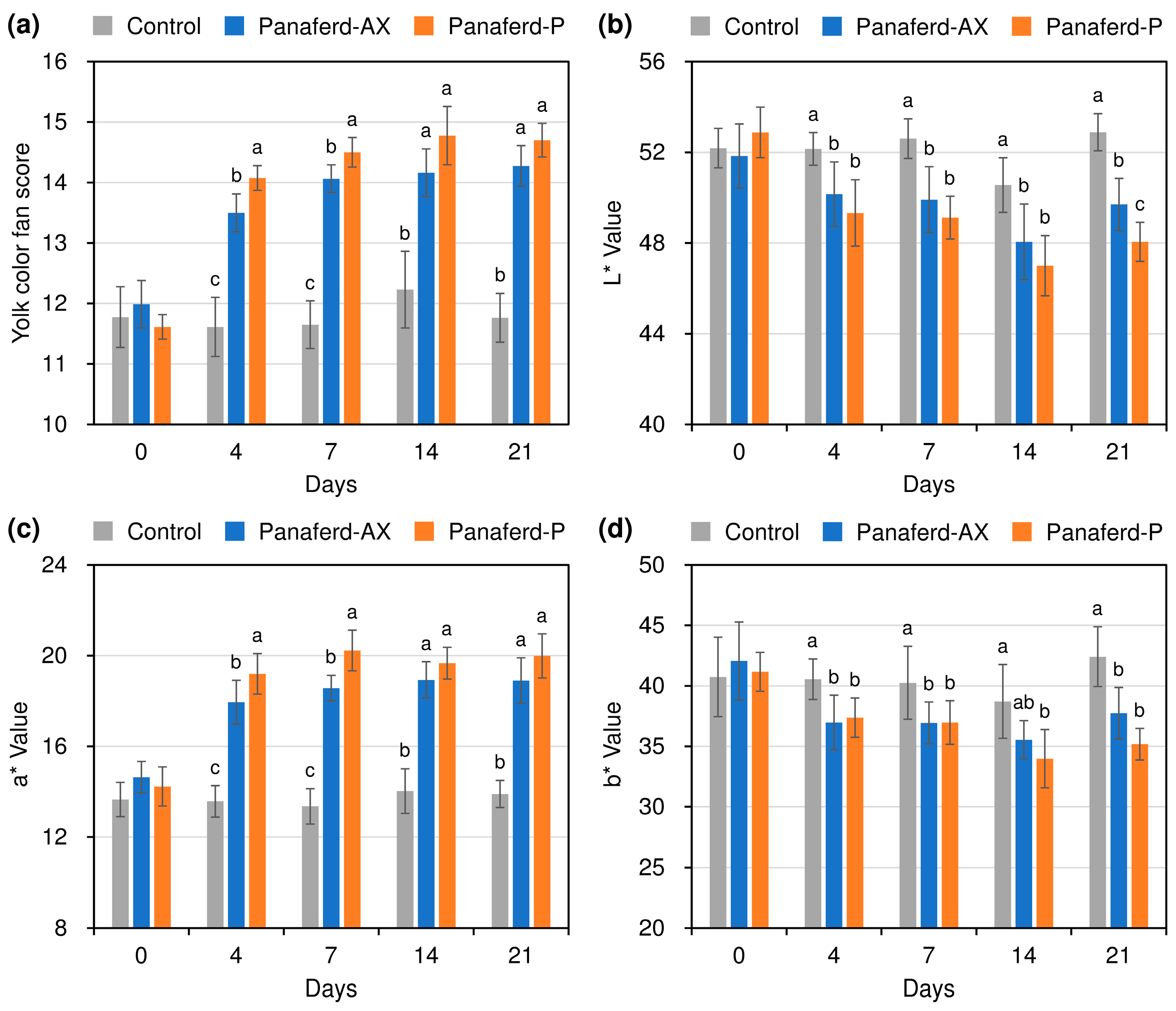
3.3. Evaluation of Egg Yolk Pigmentation
3.4. Evaluation of Other Egg Qualities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nimalaratne, C.; Wu, J. Hen egg as an antioxidant food commodity: A review. Nutrients 2015, 7, 8274–8293. [Google Scholar] [CrossRef] [PubMed]
- Karadas, F.; Grammenidis, E.; Surai, P.F.; Acamovic, T.; Sparks, N.H.C. Effects of carotenoids from lucerne, marigold and tomato on egg yolk pigmentation and carotenoid composition. Br. Poult. Sci. 2006, 47, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Karadas, F.; Surai, P.F.; Sparks, N.H.; Grammenidis, E. Effects of maternal dietary supplementation with three sources of carotenoids on the retinyl esters of egg yolk and developing quail liver. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2005, 140, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Kim, J.W.; Kim, J.H.; Lee, D.G.; Lee, S.; Kil, D.Y. Effect of feeding duration of diets containing corn distillers dried grains with solubles on productive performance, egg quality, and lutein and zeaxanthin concentrations of egg yolk in laying hens. Poult. Sci. 2016, 95, 2366–2371. [Google Scholar] [CrossRef]
- Skřivan, M.; Englmaierová, M.; Skřivanová, E.; Bubancová, I. Increase in lutein and zeaxanthin content in the eggs of hens fed marigold flower extract. Czech J. Anim. Sci. 2015, 60, 89–96. [Google Scholar] [CrossRef]
- Walker, L.A.; Wang, T.; Xin, H.; Dolde, D. Supplementation of laying-hen feed with palm tocos and algae astaxanthin for egg yolk nutrient enrichment. J. Agric. Food Chem. 2012, 60, 1989–1999. [Google Scholar] [CrossRef]
- Magnuson, A.D.; Sun, T.; Yin, R.; Liu, G.; Tolba, S.; Shinde, S.; Lei, X.G. Supplemental microalgal astaxanthin produced coordinated changes in intrinsic antioxidant systems of layer hens exposed to heat stress. Algal Res. 2018, 33, 84–90. [Google Scholar] [CrossRef]
- Sato, W.; Nagai, H.; Kawashima, Y.; Ikarashi, M.; Sakai, Y. Formula Feed for Poultry. U.S. Patent 15,575,965, 31 May 2018. [Google Scholar]
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Fakhri, S.; Abbaszadeh, F.; Dargahi, L.; Jorjani, M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharm. Res. 2018, 136, 1–20. [Google Scholar] [CrossRef]
- Akiba, Y.; Sato, K.; Takahashi, K.; Takahashi, Y.; Furuki, A.; Konashi, S.; Nishida, H.; Tsunekawa, H.; Hayasaka, Y.; Nagao, H. Pigmentation of egg yolk with yeast Phaffia rhodozyma containing high concentration of astaxanthin in laying hens fed on a low-carotenoid diet. Jpn. Poult. Sci. 2000, 37, 77–85. [Google Scholar] [CrossRef][Green Version]
- Akiba, Y.; Sato, K.; Takahashi, K.; Toyomizu, M.; Takahashi, Y.; Konashi, S.; Nishida, H.; Tsunekawa, H.; Hayasaka, Y.; Nagao, H. Improved pigmentation of egg yolk by feeding of yeast Phaffia rhodozyma containing high concentration of astaxanthin in laying hens. Jpn. Poult. Sci. 2000, 37, 162–170. [Google Scholar] [CrossRef]
- Nagai, H.; Sato, W.; Takahashi, T.; Sato, H. Carotenoid Production Method. European Patent 3,441,464, 13 February 2019. [Google Scholar]
- Hirasawa, K.; Satoh, H.; Yoneda, H.; Yata, T.; Azuma, M. Method for Producing Astaxanthin by Fermentation. United States Patent 10,240,215, 26 March 2019. [Google Scholar]
- Honda, M.; Kageyama, H.; Hibino, T.; Sowa, T.; Kawashima, Y. Efficient and environmentally friendly method for carotenoid extraction from Paracoccus carotinifaciens utilizing naturally occurring Z-isomerization-accelerating catalysts. Proc. Biochem. 2020, 89, 146–154. [Google Scholar] [CrossRef]
- Maoka, T.; Yasui, H.; Ohmori, A.; Tokuda, H.; Suzuki, N.; Osawa, A.; Shindo, K.; Ishibashi, T. Anti-oxidative, anti-tumor-promoting, and anti-carcinogenic activities of adonirubin and adonixanthin. J. Oleo Sci. 2013, 62, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, K.; Tsubokura, A. Method for Separating Carotenoid. United States Patent 8,853,460, 7 October 2014. [Google Scholar]
- Li, H.; Jin, L.; Wu, F.; Thacker, P.; Li, X.; You, J.; Wang, X.; Liu, S.; Li, S.; Xu, Y. Effect of red pepper (Capsicum frutescens) powder or red pepper pigment on the performance and egg yolk color of laying hens. Asian Australas. J. Anim. Sci. 2012, 25, 1605–1610. [Google Scholar] [CrossRef]
- Hayasaka, Y.; Tsunekawa, H. Color-Improving Feed for Laying Hen. Japan Patent 07-115915, 9 May 1995. [Google Scholar]
- Yu, W.; Liu, J. Astaxanthin isomers: Selective distribution and isomerization in aquatic animals. Aquaculture 2020, 520, 734915. [Google Scholar] [CrossRef]
- Honda, M.; Murakami, K.; Watanabe, Y.; Higashiura, T.; Fukaya, T.; Kanda, H.; Goto, M. The E/Z isomer ratio of lycopene in foods and effect of heating with edible oils and fats on isomerization of (all-E)-lycopene. Eur. J. Lipid Sci. Technol. 2017, 119, 1600389. [Google Scholar] [CrossRef]
- Humphries, J.M.; Khachik, F. Distribution of lutein, zeaxanthin, and related geometrical isomers in fruit, vegetables, wheat, and pasta products. J. Agric. Food Chem. 2003, 51, 1322–1327. [Google Scholar] [CrossRef]
- Boileau, T.W.M.; Boileau, A.C.; Erdman, J.W., Jr. Bioavailability of all-trans and cis-isomers of lycopene. Exp. Biol. Med. 2002, 227, 914–919. [Google Scholar] [CrossRef]
- Honda, M.; Nakayama, Y.; Nishikawa, S.; Tsuda, T. Z-Isomers of lycopene exhibit greater liver accumulation than the all-E-isomer in mice. Biosci. Biotechnol. Biochem. 2020, 84, 428–431. [Google Scholar] [CrossRef]
- Coral-Hinostroza, G.N.; Ytrestøyl, T.; Ruyter, B.; Bjerkeng, B. Plasma appearance of unesterified astaxanthin geometrical E/Z and optical R/S isomers in men given single doses of a mixture of optical 3 and 3’ R/S isomers of astaxanthin fatty acyl diesters. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2004, 139, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Ishikawa, H.; Hayashi, Y. Alterations in lycopene concentration and Z-isomer content in egg yolk of hens fed all-E-isomer-rich and Z-isomer-rich lycopene. Anim. Sci. J. 2019, 90, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.K.; Kim, S.I.; Cho, C.H.; Yim, Y.H.; Kim, H.S. Use of lycopene, an antioxidant carotinoid, in laying hens for egg yolk pigmentation. Asian Australas. J. Anim. Sci. 2003, 16, 1799–1803. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, H.; Liu, R.; Zhu, H.; Zhang, L.; Tsao, R. Bioaccessibility, cellular uptake, and transport of astaxanthin isomers and their antioxidative effects in human intestinal epithelial Caco-2 cells. J. Agric. Food Chem. 2017, 65, 10223–10232. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, L.; Zhang, H.; Sun, Q.; Liu, R.; Li, J.; Wu, L.; Tsao, R. Rapid and efficient conversion of all-E-astaxanthin to 9Z-and 13Z-isomers and assessment of their stability and antioxidant activities. J. Agric. Food Chem. 2017, 65, 818–826. [Google Scholar] [CrossRef]
- Yang, C.; Hassan, Y.I.; Liu, R.; Zhang, H.; Chen, Y.; Zhang, L.; Tsao, R. Anti-inflammatory effects of different astaxanthin isomers and the roles of lipid transporters in the cellular transport of astaxanthin isomers in Caco-2 cell monolayers. J. Agric. Food Chem. 2019, 67, 6222–6231. [Google Scholar] [CrossRef]
- Su, F.; Xu, H.; Yang, N.; Liu, W.; Liu, J. Hydrolytic efficiency and isomerization during de-esterification of natural astaxanthin esters by saponification and enzymolysis. Elec. J. Biotechnol. 2018, 34, 37–42. [Google Scholar] [CrossRef]
- Honda, M.; Sowa, T.; Kawashima, Y. Thermal-and photo-induced isomerization of all-E-and Z-isomer-rich xanthophylls: Astaxanthin and its structurally related xanthophylls, adonirubin and adonixanthin. Eur. J. Lipid Sci. Technol. 2020, 122, 1900462. [Google Scholar] [CrossRef]
- LaFountain, A.M.; Frank, H.A.; Prum, R.O. Carotenoids from the crimson and maroon plumages of Old World orioles (Oriolidae). Arch. Biochem. Biophys. 2013, 539, 126–132. [Google Scholar] [CrossRef]
- Kaga, K.; Honda, M.; Adachi, T.; Honjo, M.; Kanda, H.; Goto, M. Nanoparticle formation of PVP/astaxanthin inclusion complex by solution-enhanced dispersion by supercritical fluids (SEDS): Effect of PVP and astaxanthin Z-isomer content. J. Supercrit. Fluids 2018, 136, 44–51. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, F.; Zhao, G.; Wang, Z.; Liao, X.; Hu, X. Isomerization of trans-astaxanthin induced by copper (II) ion in ethanol. J. Agric. Food Chem. 2005, 53, 9620–9623. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D.; Wu, Y.C.; Zhu, W.L.; Yin, H.; Yi, L.T. Identification of geometrical isomers and comparison of different isomeric samples of astaxanthin. J. Food Sci. 2012, 77, C934–C940. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.P.; Chen, F. Isomerization of trans-astaxanthin to cis-isomers in organic solvents. J. Agric. Food Chem. 1999, 47, 365–3660. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Kodama, T.; Kageyama, H.; Hibino, T.; Kanda, H.; Goto, M. Enhanced solubility and reduced crystallinity of carotenoids, β-carotene and astaxanthin, by Z-isomerization. Eur. J. Lipid Sci. Technol. 2018, 120, 1800191. [Google Scholar] [CrossRef]
- Honda, M.; Kageyama, H.; Hibino, T.; Ichihashi, K.; Takada, W.; Goto, M. Isomerization of commercially important carotenoids (lycopene, β-carotene, astaxanthin) by natural catalysts: Isothiocyanates and polysulfides. J. Agric. Food Chem. 2020, 68, 3228–3237. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhashi, K.; Someya, T.; Hayashi, M.; Yamada, M.; Uchizawa, S.; Hirasawa, K.; Kawashima, Y. Method for producing carotenoid-containing composition, and carotenoid-containing composition. U.S. Patent 9,687,422, 27 June 2017. [Google Scholar]
- Vishwanathan, R.; Wilson, T.A.; Nicolosi, R.J. Bioavailability of a nanoemulsion of lutein is greater than a lutein supplement. Nano. Biomed. Eng. 2009, 1, 38–49. [Google Scholar] [CrossRef]
- Affandi, M.M.M.; Julianto, T.; Majeed, A.B.A. Enhanced oral bioavailability of astaxanthin with droplet size reduction. Food Sci. Technol. Res. 2012, 18, 549–554. [Google Scholar] [CrossRef]
- Yonekura, L.; Nagao, A. Intestinal absorption of dietary carotenoids. Mol. Nutr. Food Res. 2007, 51, 107–115. [Google Scholar] [CrossRef]
- Yang, Y.X.; Kim, Y.J.; Jin, Z.; Lohakare, J.D.; Kim, C.H.; Ohh, S.H.; Lee, S.H.; Choi, J.Y.; Chae, B.J. Effects of dietary supplementation of astaxanthin on production performance, egg quality in layers and meat quality in finishing pigs. Asia Australas. J. Anim. Sci. 2006, 19, 1019–1025. [Google Scholar] [CrossRef]
| Ingredient | g/kg |
| Corn | 566.0 |
| Soybean meal | 174.0 |
| Limestone | 93.4 |
| Corn gluten meal | 50.0 |
| Rice bran | 30.0 |
| Vegetable oil | 27.9 |
| Fish meal | 25.0 |
| Corn gluten feed | 20.7 |
| Calcium phosphate | 8.60 |
| Sodium chloride | 4.40 |
| Chemical Composition (Dry Matter Basis) | g/kg |
| Metabolizable energy (kcal/kg) | 2720 |
| Dry matter | 898.4 |
| Organic matter | 850.3 |
| Crude protein | 183.4 |
| Ether extract | 69.2 |
| Crude fiber | 39.3 |
| Neutral detergent fiber | 797.2 |
| Acid detergent fiber | 297.6 |
| Nitrogen free extract | 558.4 |
| Crude ash | 149.7 |
| Calcium | 15.5 |
| Phosphorus | 9.6 |
| Diet | ||||||
|---|---|---|---|---|---|---|
| Items | Days | Control 1 | Panaferd-AX | Panaferd-P | SEM | p-Value |
| Egg weight (g) | 0 | 57.3 | 57.7 | 55.3 | 4.8 | 0.576 |
| 4 | 58.0 | 55.8 | 59.2 | 5.8 | 0.517 | |
| 7 | 58.8 | 58.4 | 59.0 | 3.6 | 0.947 | |
| 14 | 60.4 | 58.6 | 59.9 | 3.0 | 0.503 | |
| 21 | 59.8 | 59.7 | 61.0 | 3.6 | 0.701 | |
| Yolk weight (g) | 0 | 13.8 | 14.6 | 13.0 | 2.6 | 0.459 |
| 4 | 14.2 | 13.7 | 15.2 | 2.8 | 0.578 | |
| 7 | 14.9 | 14.5 | 14.6 | 1.1 | 0.794 | |
| 14 | 15.5 | 14.9 | 15.3 | 1.0 | 0.442 | |
| 21 | 16.0 | 15.7 | 15.6 | 0.8 | 0.626 | |
| Albumen height (mm) | 0 | 7.0 | 7.1 | 7.9 | 1.0 | 0.121 |
| 4 | 7.7 | 8.1 | 7.4 | 1.6 | 0.706 | |
| 7 | 7.4 | 7.7 | 7.7 | 1.2 | 0.885 | |
| 14 | 8.3 | 7.6 | 8.5 | 1.2 | 0.250 | |
| 21 | 7.1 | 7.5 | 6.8 | 1.3 | 0.598 | |
| Haugh unit 2 | 0 | 84.7 | 84.3 | 89.9 | 10.1 | 0.468 |
| 4 | 91.6 | 93.4 | 86.2 | 10.6 | 0.384 | |
| 7 | 86.6 | 91.5 | 89.1 | 7.5 | 0.441 | |
| 14 | 94.6 | 90.4 | 95.7 | 6.4 | 0.240 | |
| 21 | 83.2 | 87.0 | 82.8 | 10.1 | 0.664 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honda, M.; Kawashima, Y.; Hirasawa, K.; Uemura, T.; Jinkun, S.; Hayashi, Y. Possibility of Using Astaxanthin-Rich Dried Cell Powder from Paracoccus carotinifaciens to Improve Egg Yolk Pigmentation of Laying Hens. Symmetry 2020, 12, 923. https://doi.org/10.3390/sym12060923
Honda M, Kawashima Y, Hirasawa K, Uemura T, Jinkun S, Hayashi Y. Possibility of Using Astaxanthin-Rich Dried Cell Powder from Paracoccus carotinifaciens to Improve Egg Yolk Pigmentation of Laying Hens. Symmetry. 2020; 12(6):923. https://doi.org/10.3390/sym12060923
Chicago/Turabian StyleHonda, Masaki, Yuki Kawashima, Kazuaki Hirasawa, Takeshi Uemura, Sun Jinkun, and Yoshiaki Hayashi. 2020. "Possibility of Using Astaxanthin-Rich Dried Cell Powder from Paracoccus carotinifaciens to Improve Egg Yolk Pigmentation of Laying Hens" Symmetry 12, no. 6: 923. https://doi.org/10.3390/sym12060923
APA StyleHonda, M., Kawashima, Y., Hirasawa, K., Uemura, T., Jinkun, S., & Hayashi, Y. (2020). Possibility of Using Astaxanthin-Rich Dried Cell Powder from Paracoccus carotinifaciens to Improve Egg Yolk Pigmentation of Laying Hens. Symmetry, 12(6), 923. https://doi.org/10.3390/sym12060923





