Water Soluble Host–Guest Chemistry Involving Aromatic N-Oxides and Sulfonateresorcinarene
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. NMR Spectroscopy
3.2. Isothermal Titration Calorimetry (ITC)
3.3. Computational Studies of the Di-N-Oxides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Timmerman, P.; Verboom, W.; Reinhoudt, D.N. Resorcinarenes. Tetrahedron 1996, 52, 2663–2704. [Google Scholar] [CrossRef]
- Beyeh, N.K.; Díez, I.; Taimoory, S.M.; Meister, D.; Feig, A.I.; Trant, J.F.; Ras, R.H.A.; Rissanen, K. High-Affinity and Selective Detection of Pyrophosphate in Water by a Resorcinarene Salt Receptor. Chem. Sci. 2018, 9, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Beyeh, N.K.; Nonappa; Liljeström, V.; Mikkilä, J.; Korpi, A.; Bochicchio, D.; Pavan, G.M.; Ikkala, O.; Ras, R.H.A.; Kostiainen, M.A. Crystalline Cyclophane-Protein Cage Frameworks. ACS Nano 2018, 12, 8029–8036. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, C.; Papachristou, A.; Ho, T.M.H.; Hine, J.; Curtis, A.D.M. Investigation into Drug Solubilisation Potential of Sulfonated Calix [4] Resorcinarenes. J. Nanomed. Nanotechnol. 2016, 7, 1000370. [Google Scholar] [CrossRef]
- Li, N.; Harrison, R.G.; Lamb, J.D. Application of Resorcinarene Derivatives in Chemical Separations. J. Incl. Phenom. Macrocycl. Chem. 2014, 78, 39–60. [Google Scholar] [CrossRef]
- Kim, B.; Balasubramanian, R.; Pérez-Segarra, W.; Wei, A.; Decker, B.; Mattay, J. Self-Assembly of Resorcinarene-Stabilized Gold Nanoparticles: Influence of the Macrocyclic Headgroup. Supramol. Chem. 2005, 17, 173–180. [Google Scholar] [CrossRef]
- Haines, S.R.; Harrison, R.G. Novel Resorcinarene-Based PH-Triggered Gelator. Chem. Commun. 2002, 2, 2846–2847. [Google Scholar] [CrossRef]
- Schneider, H.J.; Schneider, U. The Host-Guest Chemistry of Resorcinarenes. J. Incl. Phenom. Mol. Recognit. Chem. 1994, 19, 67–83. [Google Scholar] [CrossRef]
- Twum, K.; Rautiainen, J.M.; Yu, S.; Truong, K.N.; Feder, J.; Rissanen, K.; Puttreddy, R.; Beyeh, N.K. Host-Guest Interactions of Sodiumsulfonatomethyleneresorcinarene and Quaternary Ammonium Halides: An Experimental-Computational Analysis of the Guest Inclusion Properties. Cryst. Growth Des. 2020, 20, 2367–2376. [Google Scholar] [CrossRef]
- Andersson, H.; Almqvist, F.; Olsson, R. Synthesis of 2-Substituted Pyridines via a Regiospecific Alkylation, Alkynylation, and Arylation of Pyridine N-Oxides. Org. Lett. 2007, 9, 1335–1337. [Google Scholar] [CrossRef]
- Pignataro, L.; Benaglia, M.; Annunziata, R.; Cinquini, M.; Cozzi, F. Structurally Simple Pyridine N-Oxides as Efficient Organocatalysts for the Enantioselective Allylation of Aromatic Aldehydes. J. Org. Chem. 2006, 71, 1458–1463. [Google Scholar] [CrossRef]
- Bullock, S.J.; Harding, L.P.; Moore, M.P.; Mills, A.; Piela, S.A.F.; Rice, C.R.; Towns-Andrews, L.; Whitehead, M. Synthesis of Ligands Containing N-Oxide Donor Atoms and Their Assembly into Metallosupramolecular Structures. Dalton Trans. 2013, 42, 5805–5811. [Google Scholar] [CrossRef][Green Version]
- Puttreddy, R.; Beyeh, N.K.; Taimoory, S.M.; Meister, D.; Trant, J.F.; Rissanen, K. Host–Guest Complexes of Conformationally Flexible C -Hexyl-2-Bromoresorcinarene and Aromatic N-Oxides: Solid-State, Solution and Computational Studies. Beilstein J. Org. Chem. 2018, 14, 1723–1733. [Google Scholar] [CrossRef] [PubMed]
- Beyeh, N.K.; Puttreddy, R. Methylresorcinarene: A Reaction Vessel to Control the Coordination Geometry of Copper(Ii) in Pyridine N-Oxide Copper(Ii) Complexes. Dalton Trans. 2015, 44, 9881–9886. [Google Scholar] [CrossRef] [PubMed]
- Feely, W.E.; Beavers, E.M. Cyanation of Amine Oxide Salts. A New Synthesis of Cyanopyridines. J. Am. Chem. Soc. 1959, 81, 4004–4007. [Google Scholar] [CrossRef]
- Taimoory, S.M.; Twum, K.; Dashti, M.; Pan, F.; Lahtinen, M.; Rissanen, K.; Puttreddy, R.; Trant, J.F.; Beyeh, N.K. Bringing a Molecular plus One: Synergistic Binding Creates Guest-Mediated Three-Component Complexes. J. Org. Chem. 2020, 85, 5884–5894. [Google Scholar] [CrossRef]
- Cattoni, D.I.; Chara, O.; Kaufman, S.B.; Flecha, F.L.G. Cooperativity in Binding Processes: New Insights from Phenomenological Modeling. PLoS ONE 2015, 10, e0146043. [Google Scholar] [CrossRef] [PubMed]
- Thordarson, P. Determining Association Constants from Titration Experiments in Supramolecular Chemistry. Chem. Soc. Rev. 2011, 40, 1305–1323. [Google Scholar] [CrossRef]
- Hunter, C.A.; Anderson, H.L. What Is Cooperativity? Angew. Chem. Int. Ed. 2009, 48, 7488–7499. [Google Scholar] [CrossRef]
- Szczȩśniak, M.M.; Chałasiński, G. Ab Initio Calculations of Nonadditive Effects. J. Mol. Struct. THEOCHEM 1992, 261, 37–54. [Google Scholar] [CrossRef]
- Szczȩśniak, M.M.; Chałasiński, G.; Piecuch, P. The Nonadditive Interactions in the Ar2HF and Ar2HCl Clusters: An Ab Initio Study. J. Chem. Phys. 1993, 99, 6732–6741. [Google Scholar] [CrossRef]
- Hapka, M.; Rajchel, Ł.; Modrzejewski, M.; Schäffer, R.; Chałasiński, G.; Szczȩśniak, M.M. The Nature of Three-Body Interactions in DFT: Exchange and Polarization Effects. J. Chem. Phys. 2017, 147. [Google Scholar] [CrossRef] [PubMed]
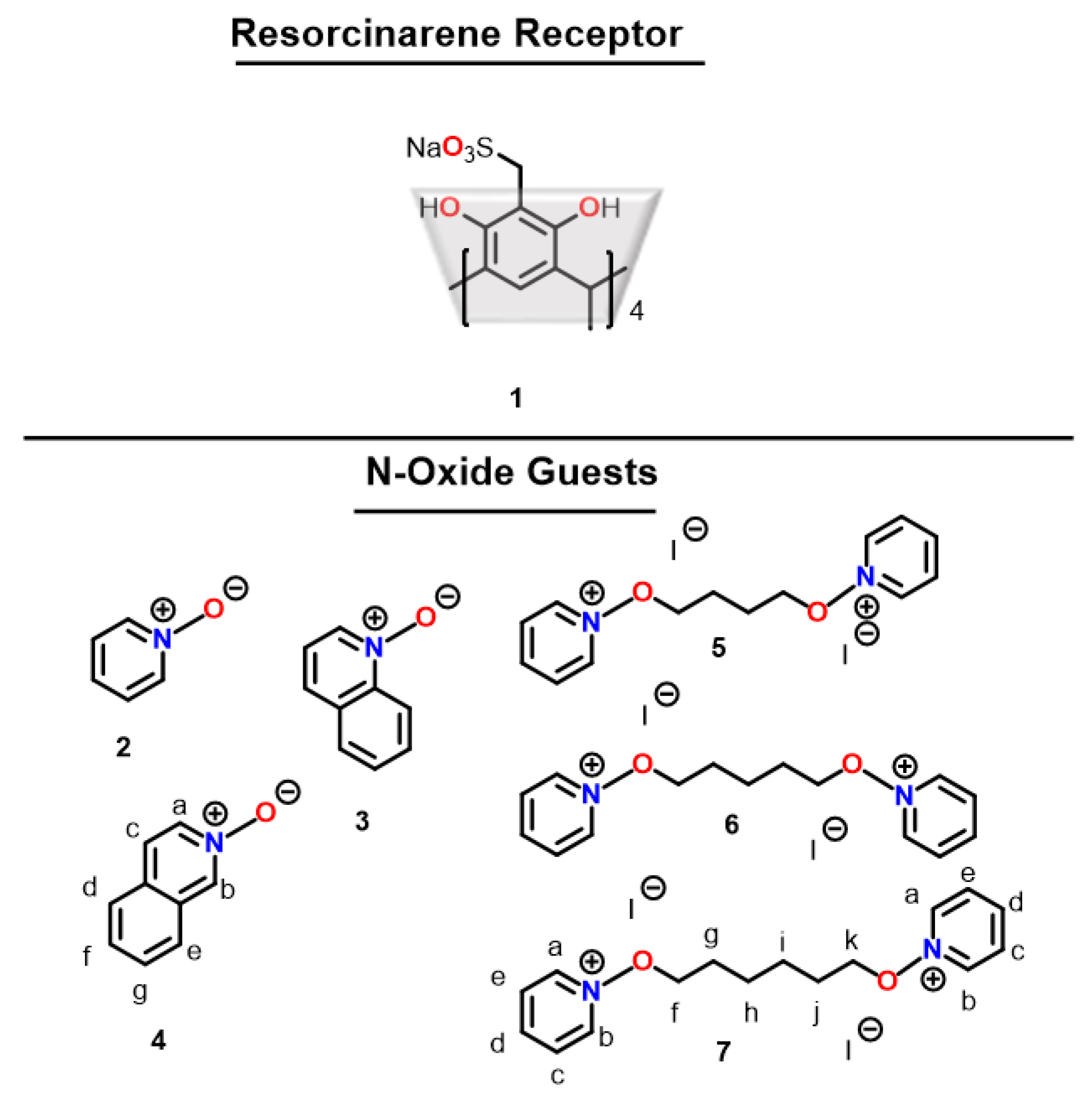
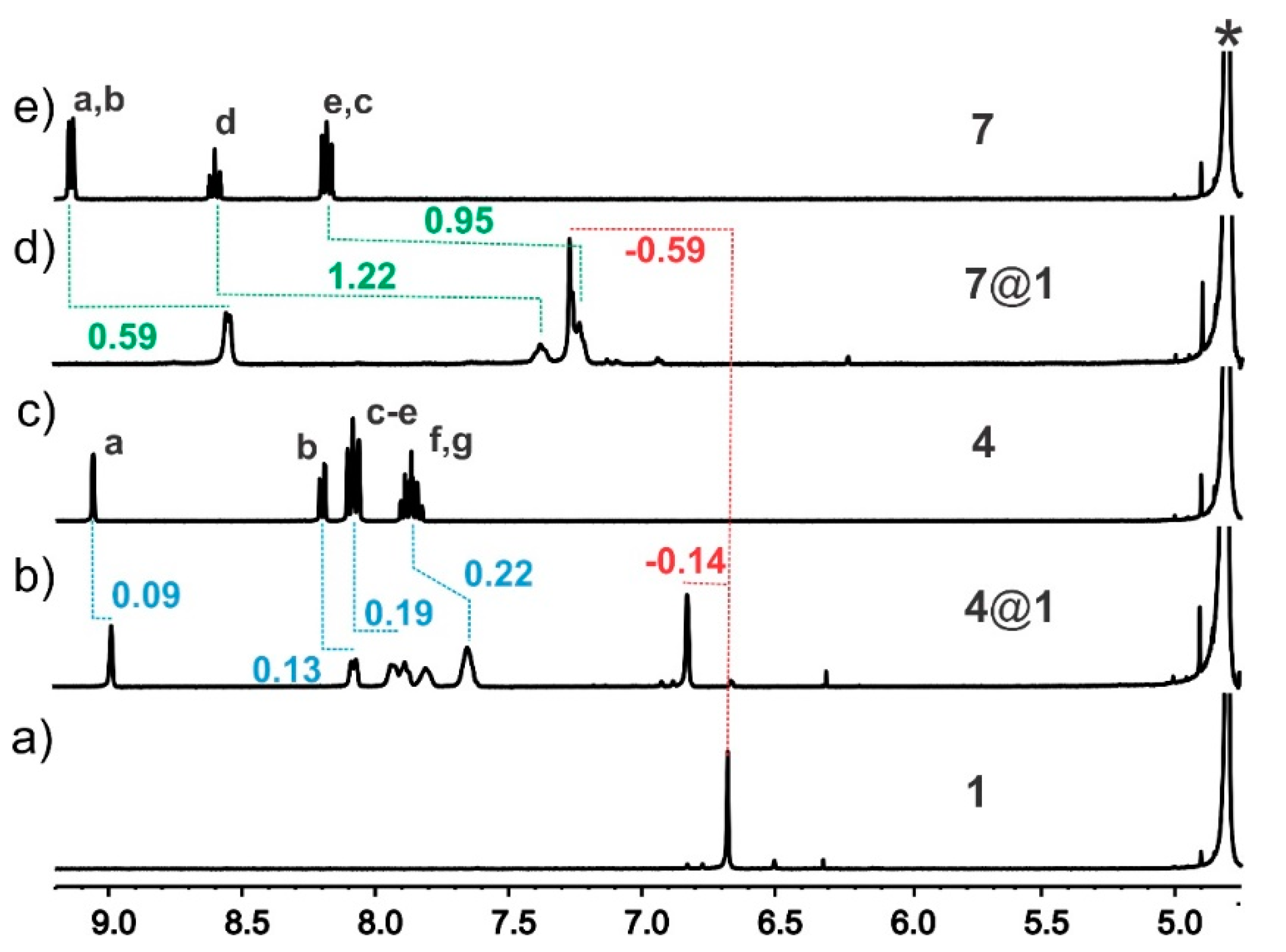
| Complex | K1 (× 104) M−1 | ΔH1 (kcal/mol) | TΔS1 (kcal/mol) | ΔG1 (kcal/mol) |
|---|---|---|---|---|
| 2@1 | - | - | - | - |
| 3@1 | 0.12 ± 0.03 | −1.43 ± 0.18 | 2.77 | −4.20 |
| 4@1 | 0.14 ± 0.03 | −1.42 ± 0.14 | 2.87 | −4.29 |
| 5@1 | 0.17 ± 0.04 | −0.84 ± 0.12 | 3.34 | −4.18 |
| 6@1 | 2.71 ± 0.86 | −10.90 ± 0.56 | −4.86 | −6.06 |
| 7@1 | 3.18 ± 0.79 | −10.00 ± 0.36 | −4.20 | −5.80 |
| Complex | K2 (× 104) M−1 | ΔH2 (kcal/mol) | TΔS2 (kcal/mol) | ΔG2 (kcal/mol) |
| 5@1 | 2.70 ± 0.72 | −0.46 ± 0.19 | 5.20 | −5.66 |
| 6@1 | 0.26 ± 0.02 | −16.00 ± 0.85 | −12.30 | −4.65 |
| 7@1 | 0.30 ± 0.02 | −16.60 ± 0.58 | −11.90 | −4.70 |
| Complex | α = (4K2/K1) a,b |
|---|---|
| 2@1 | - |
| 3@1 | - |
| 4@1 | - |
| 5@1 | 63.53 |
| 6@1 | 0.39 |
| 7@1 | 0.38 |
| Structure | Point Group | Dipole Moment, D | Distance N···N, Å |
|---|---|---|---|
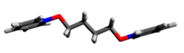 5 | C2h | 0.00 | 8.43 |
 6 | C2v | 4.38 | 9.71 |
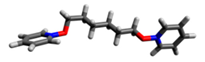 7 | C1 | 0.50 | 10.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Twum, K.; Schileru, N.; Elias, B.; Feder, J.; Yaqoo, L.; Puttreddy, R.; Szczesniak, M.M.; Beyeh, N.K. Water Soluble Host–Guest Chemistry Involving Aromatic N-Oxides and Sulfonateresorcinarene. Symmetry 2020, 12, 1751. https://doi.org/10.3390/sym12111751
Twum K, Schileru N, Elias B, Feder J, Yaqoo L, Puttreddy R, Szczesniak MM, Beyeh NK. Water Soluble Host–Guest Chemistry Involving Aromatic N-Oxides and Sulfonateresorcinarene. Symmetry. 2020; 12(11):1751. https://doi.org/10.3390/sym12111751
Chicago/Turabian StyleTwum, Kwaku, Nicholas Schileru, Bianca Elias, Jordan Feder, Leena Yaqoo, Rakesh Puttreddy, Małgorzata M. Szczesniak, and Ngong Kodiah Beyeh. 2020. "Water Soluble Host–Guest Chemistry Involving Aromatic N-Oxides and Sulfonateresorcinarene" Symmetry 12, no. 11: 1751. https://doi.org/10.3390/sym12111751
APA StyleTwum, K., Schileru, N., Elias, B., Feder, J., Yaqoo, L., Puttreddy, R., Szczesniak, M. M., & Beyeh, N. K. (2020). Water Soluble Host–Guest Chemistry Involving Aromatic N-Oxides and Sulfonateresorcinarene. Symmetry, 12(11), 1751. https://doi.org/10.3390/sym12111751






