Unexpected Encapsulation of Selected Polycyclic Aromatic Hydrocarbons by β-Cyclodextrin Studied Using UV-Vis Spectrophotometry, Micro-Planar Chromatography and Temperature Dependent Inclusion Chromatography
Abstract
:1. Introduction
2. Materials and Method
2.1. Chromatography
2.2. TLC detection and Data Acquisition
2.3. UV-Vis Spectrophotometry and Data Computations
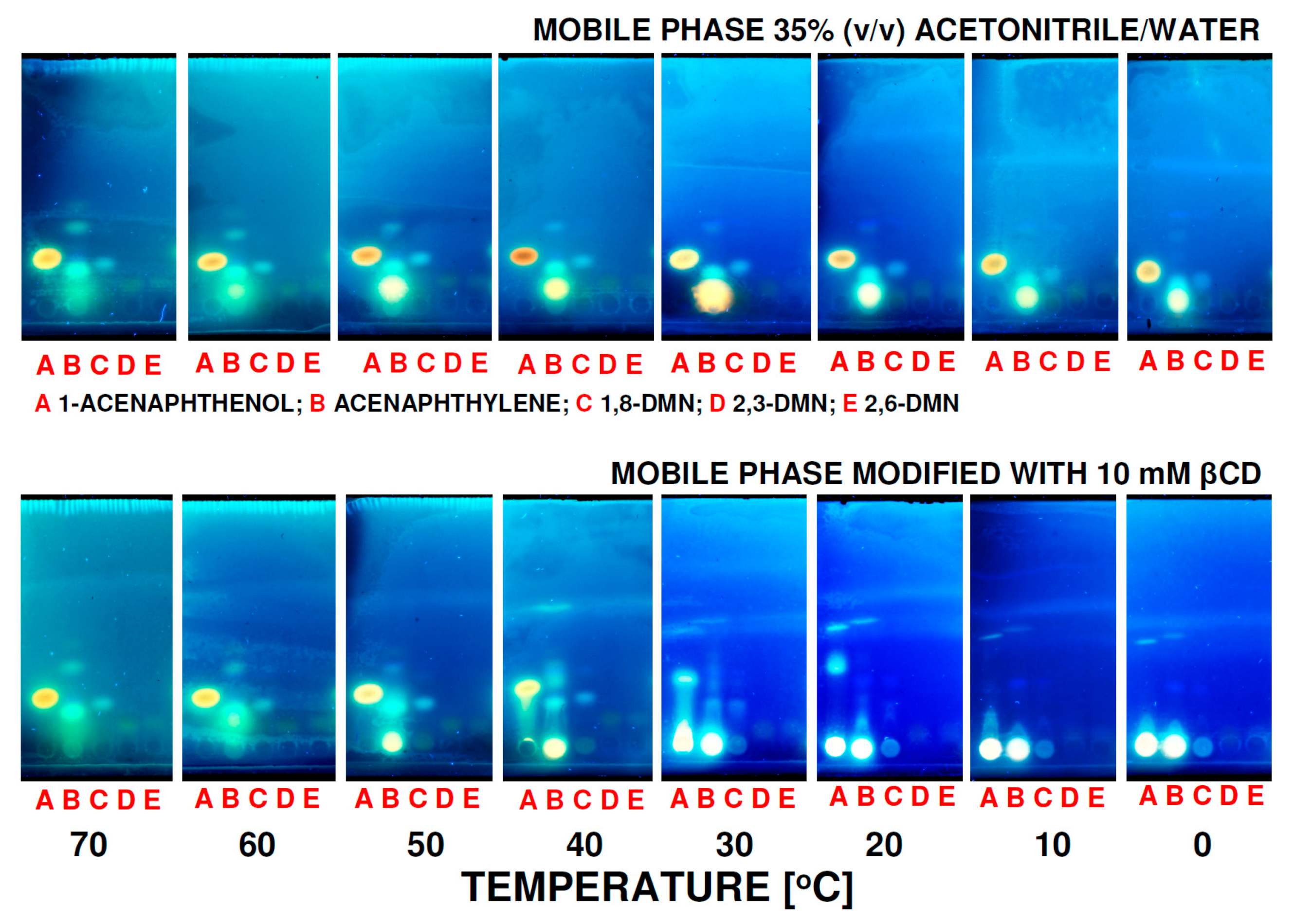
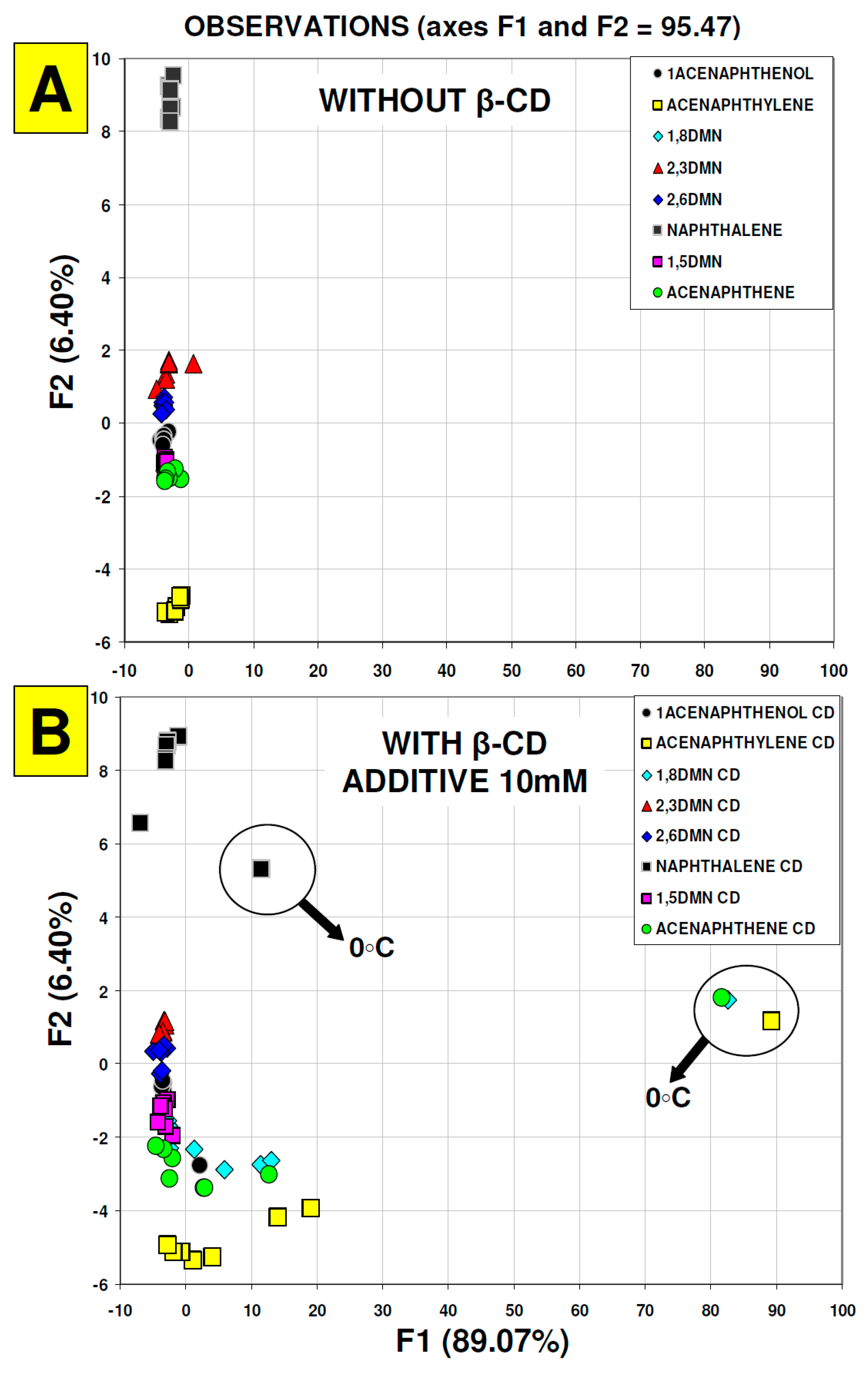
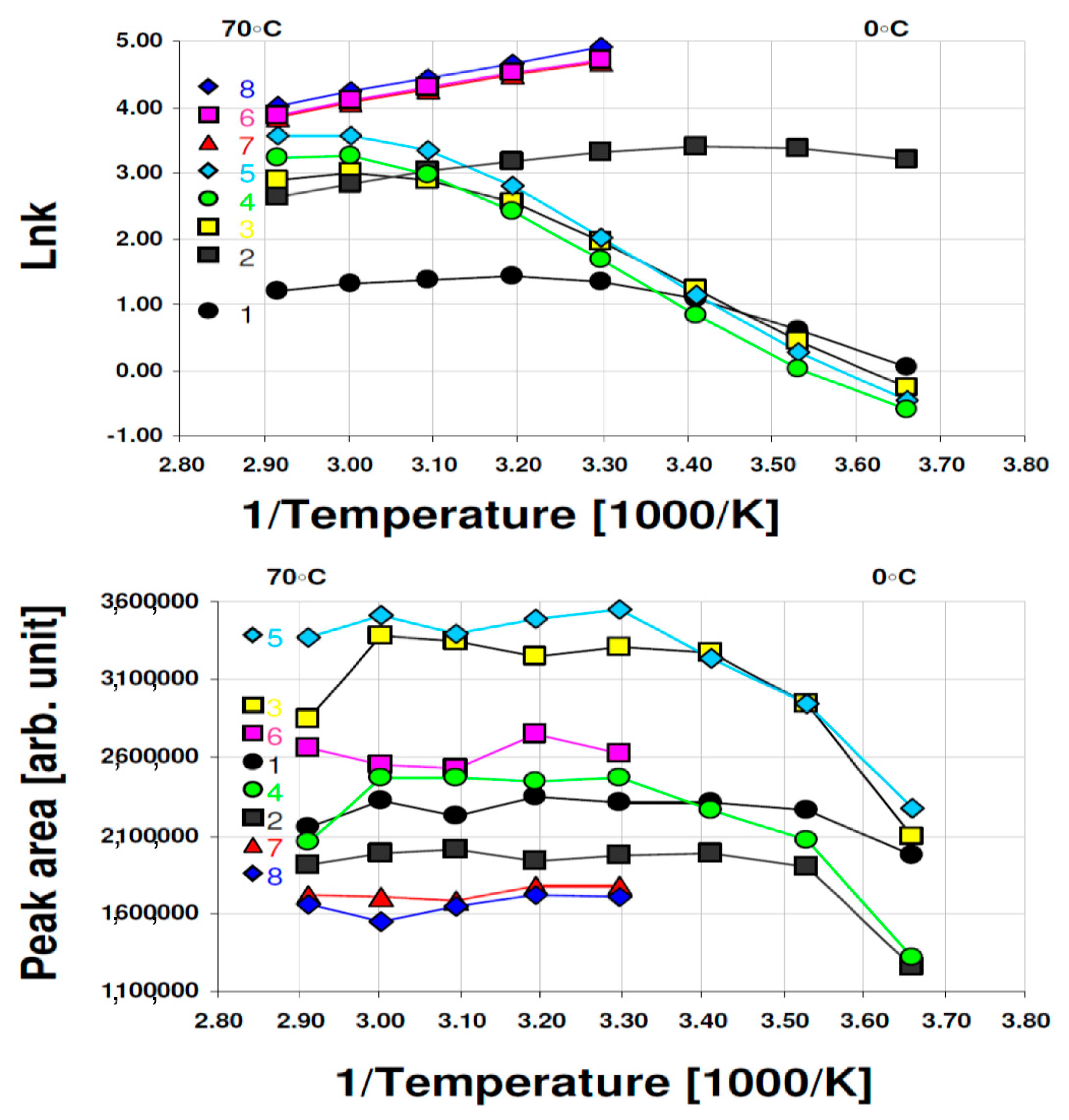
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Connors, K.A. Binding Constats—The Measurement of Molecular Complex Stability; John Wiley & Sons: New York, NY, USA; Chichester, UK; Brisbane, Australia; Toronto, ON, Canada; Singapore, 1987; p. 86. [Google Scholar]
- Lehn, J.M. Supramolecular Chemistry: Concepts and Perspectives; VCH Verlagsgesellschaft: Weinheim, Germany, 1995; p. 2617. [Google Scholar]
- Zarzycki, P.K.; Fenert, B.; Głód, B.K. Cyclodextrins—Based nanocomplexes for encapsulation of bioactive compounds in food, cosmetics, and pharmaceutical products: Principles of supramolecular complexes formation, their influence on the antioxidative properties of target chemicals, and recent advances in selected industrial applications. Encapsul. Nanotechnol. Agri-Food Ind. 2016, 2, 717–767. [Google Scholar]
- Saito, Y.; Tanemura, I.; Sato, T.; Ueda, H. Interaction of Fragrance Materials With 2-Hydroxypropyl-beta-Cyclodextrin by Static and Dynamic Head-space Methods. Int. J. Cosmet. Sci. 1999, 21, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, S.; Sameena, Y.; Enoch, I.V.M.V. Modulation of the interaction of Coumarin 7 with DNA by β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2014, 81, 225–236. [Google Scholar] [CrossRef]
- Stancanelli, R.; Mazzaglia, A.; Tommasini, S.; Calabrò, M.; Villari, V.; Guardo, M.; Ficarra, P.; Ficarra, R. The enhancement of isoflavones water solubility by complexation with modified cyclodextrins: A spectroscopic investigation with implications in the pharmaceutical analysis. J. Pharm. Biomed. Anal. 2007, 44, 980–984. [Google Scholar] [CrossRef]
- Chatjigakis, A.K.; Donze, C.; Coleman, A.W.; Cardot, P. Solubility behavior of. beta.-cyclodextrin in water/cosolvent mixtures. Anal. Chem. 1992, 64, 1632–1634. [Google Scholar] [CrossRef]
- Zarzycki, P.K.; Lamparczyk, H. Evidences for temperature-dependent mechanism of host-guest complexation. Chromatographia 1998, 48, 377–382. [Google Scholar] [CrossRef]
- Zarzycki, P.K.; Ohta, H.; Saito, Y.; Jinno, K. Interaction of native α-cyclodextrin, β-cyclodextrin and γ-cyclodextrin and their hydroxypropyl derivatives with selected organic low molecular mass compounds at elevated and subambient temperature under RP-HPLC conditions. Anal. Bioanal. Chem. 2008, 391, 2793–2801. [Google Scholar] [CrossRef]
- Zarzycki, P.K.; Włodarczyk, E.; Baran, M.J. Determination of endocrine disrupting compounds using temperature-dependent inclusion chromatography: I. Optimization of separation protocol. J. Chromatogr. A 2009, 1216, 7602–7611. [Google Scholar] [CrossRef]
- Kaleniecka, A.; Zarzycki, P.K. Analysis of Selected Endocrine Disrupters Fraction Including Bisphenols Extracted from Daily Products, Food Packaging and Treated Wastewater Using Optimized Solid-Phase Extraction and Temperature-Dependent Inclusion Chromatography. Molocules 2019, 24, 1285. [Google Scholar] [CrossRef] [Green Version]
- Ohta, H.; Włodarczyk, E.; Piaskowski, K.; Kaleniecka, A.; Lewandowska, L.; Baran, M.J.; Wojnicz, M.; Jinno, K.; Saito, Y.; Zarzycki, P.K. Unexpected differences between planar and column liquid chromatographic retention of 1-acenaphthenol enantiomers controlled by supramolecular interactions involving β-cyclodextrin at subambient temperatures. Anal. Bioanal. Chem. 2017, 409, 3695–3706. [Google Scholar] [CrossRef] [Green Version]
- Zarzycki, P.K. Simple horizontal chamber for thermostated micro-thin-layer chromatography. J. Chromatogr. A 2008, 1187, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Zarzycki, P.K.; Baran, M.J.; Harasimiuk, F.B.; Ślączka, M.M. Spectrophotometric study of interaction between selected bile acids and cyclodextrins. Meas. Autom. Monit. 2010, 56, 355–359. [Google Scholar]
- Seidel, V.; Poglits, E.; Schiller, K.; Lindner, W. Simultaneous determination of ochratoxin A and zearalenone in maize by reversed-phase high-performance liquid chromatography with fluorescence detectio. J. Chromatogr. A 1993, 635, 227–235. [Google Scholar] [CrossRef]
- Vazquez, M.; Franco, C.; Cepeda, A.; Prognon, P.; Mahuzier, G. Liquid chromatographic study of the interaction between aflatoxins and β-cyclodextrin. Anal. Chim. Acta 1992, 269, 239–247. [Google Scholar] [CrossRef]
- Bielejewska, A.; Nowakowski, R.; Duszczyk, K.; Sybilska, D. Joint use of cyclodextrin additives in chiral discrimination by reversed-phase high-performance liquid chromatography: Temperature effects. J. Chromatogr. A 1999, 840, 159–170. [Google Scholar] [CrossRef]
- Morin, N.; Guillaume, Y.C.; Rouland, J.-C. A simple model for RPLC retention and selectivity of imidazole enantiomers using β-cyclodextrin as chiral selector. Chromatographia 1998, 48, 388–394. [Google Scholar] [CrossRef]
- Sadlej-Sosnowska, N. Thermodynamic parameters of the formation of a complex between cyclodextrins and steroid hormones. J. Chromatogr. A 1996, 728, 89–95. [Google Scholar] [CrossRef]
- Lepri, L.; Coas, V.; Desideri, P.G.; Checchini, L. Separation of optical and structural isomers by planar chromatography with development by beta-cyclodextrin. JPC-J. Planar. Chromat. 1990, 3, 311. [Google Scholar]
- Lamparczyk, H.; Zarzycki, P.K.; Nowakowska, J. Effect of temperature on separation of norgestrel enantiomers by high-performance liquid chromatography. J. Chromatogr. A 1994, 668, 413–417. [Google Scholar] [CrossRef]
- Zarzycki, P.K.; Nowakowska, J.; Chmielewska, A.; Lamparczyk, H. Retention properties of cyclodextrin in reversed phase HPTLC. JPC-J. Planar. Chromat. 1995, 8, 227–231. [Google Scholar]
- Zarzycki, P.K.; Nowakowska, J.; Chmielewska, A.; Wierzbowska, M.; Lamparczyk, H. Thermodynamic study of the retention behaviour of selected macrocycles using reversed-phase high-performance thin-layer chromatography plates and methanol-water mobile phases. J. Chromatogr. A 1997, 787, 227–233. [Google Scholar] [CrossRef]
- Zarzycki, P.K.; Smith, R. Separation of steroids using temperature-dependent inclusion chromatography. J. Chromatogr. A 2001, 912, 45–52. [Google Scholar] [CrossRef]
- Włodarczyk, E. Znaczeniei Występowanie Wybranych Substancji Typy EDCs w Środowisku. Ph.D. Thesis, Koszalin University of Technology, Koszalin, Poland, 2009. [Google Scholar]
- Zarzycki, P.K.; Włodarczyk, E.; Baran, M.J. Determination of endocrine disrupting compounds using temperature-dependent inclusion chromatography: II. Fast screening of free steroids and related low-molecular-mass compounds fraction in the environmental samples derived from surface waters, treated and untreated sewage waters as well as activated sludge material. J. Chromatogr. A 2009, 1216, 7612–7622. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaleniecka, A.; Włodarczyk, E.; Piaskowski, K.; Lewandowska, L.; Zarzycki, P.K. Unexpected Encapsulation of Selected Polycyclic Aromatic Hydrocarbons by β-Cyclodextrin Studied Using UV-Vis Spectrophotometry, Micro-Planar Chromatography and Temperature Dependent Inclusion Chromatography. Symmetry 2020, 12, 1967. https://doi.org/10.3390/sym12121967
Kaleniecka A, Włodarczyk E, Piaskowski K, Lewandowska L, Zarzycki PK. Unexpected Encapsulation of Selected Polycyclic Aromatic Hydrocarbons by β-Cyclodextrin Studied Using UV-Vis Spectrophotometry, Micro-Planar Chromatography and Temperature Dependent Inclusion Chromatography. Symmetry. 2020; 12(12):1967. https://doi.org/10.3390/sym12121967
Chicago/Turabian StyleKaleniecka, Aleksandra, Elżbieta Włodarczyk, Krzysztof Piaskowski, Lucyna Lewandowska, and Paweł K. Zarzycki. 2020. "Unexpected Encapsulation of Selected Polycyclic Aromatic Hydrocarbons by β-Cyclodextrin Studied Using UV-Vis Spectrophotometry, Micro-Planar Chromatography and Temperature Dependent Inclusion Chromatography" Symmetry 12, no. 12: 1967. https://doi.org/10.3390/sym12121967
APA StyleKaleniecka, A., Włodarczyk, E., Piaskowski, K., Lewandowska, L., & Zarzycki, P. K. (2020). Unexpected Encapsulation of Selected Polycyclic Aromatic Hydrocarbons by β-Cyclodextrin Studied Using UV-Vis Spectrophotometry, Micro-Planar Chromatography and Temperature Dependent Inclusion Chromatography. Symmetry, 12(12), 1967. https://doi.org/10.3390/sym12121967




