Abstract
The near elimination of inland salt marshes in Central Europe occurred throughout the 19th and 20th centuries, and the currently remaining marshes exist in a degraded condition. This work examines the impact of groundwater level on the growth of plants from a seed bank obtained from a degraded salt marsh in proximity to still existing one through an ex-situ experiment. An experimental tank was set up with the sample seed bank experiencing differing levels of water level. There were 1233 specimens of 44 taxa grown from the seed bank, of which 5 species were abundant, and 10 species are considered as halophytes. Only Lotus tenuis from halophytes was more abundant, and only five species of halophytes were represented by more than three individuals. The water level has a significant impact on the number of species (based on linear regression analysis) as well as species distribution among different water level treatments (a non-metric multidimensional analysis (nMDS) followed by linear regression). The results show a strong negative relationship between the average water level and the number of species. The water level did not affect the species composition of halophytes, but differences in individual species abundances were found among the halophytes. The species Bupleurum tenuissimum, Crypsis schoenoides, Melilotus dentatus, and Plantago maritima grew on the drier and non-inundated soils. Tripolium pannonicum, Spergularia maritima, and Lotus tenuis grew on both wet and dry soils. Trifolium fragiferum and Bolboschoenus maritimus were found in places with water stagnant at the soil level. Pulicaria dysenterica grew in inundated soil.
1. Introduction
Temperate climate inland salt marshes belong to a set of ecologically extreme habitats due to their high concentration of soluble salts in the soil and a strong water level fluctuation throughout a year [1]. These salt marshes are typically found in generally arid areas where the soil water vapor amounts are higher than absorption rates. This imbalance results in salt ions rising through the soil profile and accumulating near the top. Frequently these favorable for salt marsh conditions occur primarily near mineral springs or on plains with mineralized groundwater [2,3]. Additionally, these salt marshes experience considerable water level changes throughout a year; the soil is inundated in winter, but water levels decrease over the spring, summer, and autumn months, wherein the soil may dry out completely [4]. These Central and Eastern European biotopes are predominantly located on the eastern steppes of the Pontic-Pannonian region [5,6,7] with a lobe extending into the eastern parts of the Czech Republic [8,9]. However previously abundant these salt marshes were in the Czech Republic, they now occupy very small and fragmented patches [1,2].
Inland salt marshes gradually disappeared from the rural landscape starting in the 18th century and culminated in the destruction of the majority of their original native areas. The Central European annual halophytic grass vegetation biotopes occupy only 10% of their former 18th-century range and have largely disappeared from rural landscape [5,10]. Even though some patches remain, the surviving salt marsh, halophytic reed grass, and sedge vegetation patches experienced abiotic and biotic degradation that then caused the extinction of some species and the extirpation of entire halophytic habitats [10,11]. This degradation and loss are mostly attributed to the fact that all Czech salt marshes are located in intensively farmed areas; salt marshes were systematically removed from the landscape in this region until 1989 [12], which is similar in other Central European regions [5]. The removal of salt marsh wetland biotopes was primarily achieved through draining and their subsequent transformation to arable land [13]. If not removed from the landscape entirely, salt marsh habitats also encountered lowered water tables and the general disruption of the natural hydrological regime that produced decreasing soil salinity [10]. When livestock grazing and frequent mowing abated, the former extensively used salt marshes then experienced the retreat of halophytes and the subsequent replacement by plant species with a greater competitive ability [11,14]. As a result, some habitats, such as inland salt marshes with annual succulent halophytes, were completely extirpated from the Czech Republic, e.g., Salicornion prostratae [15]. After 1989 salt marshes, as well as other types of wetland habitats, are now actively restored at a great financial cost [5,16,17,18,19]. Post-socialist rural and agricultural landscapes are now undergoing programs aimed at supporting biodiversity enhancement [20,21], changes towards multifunctional agricultural practices [22], organic farming [23], and an appreciation of the non-productive functions of agricultural lands [24].
Saltmarsh biotopes form a specific niche harboring not only rare plant species but also are home to many specialized fungi [25,26], invertebrates [27], and bird species [28,29]. However, they have faced many long term threats, such as drying out, fertilization and subsequent eutrophication, weedy and ruderal plant species expansion, absence of halophytic species typical for these biotopes, and the succession of grasses with a greater competitive ability [10]. The high degree of fragmentation and overall degradation of Czech salt marsh biotypes make restoration and preservation problematic [6]. However, restoration is still possible where degradation is not too severe; it can be accomplished through hydrological regime change [30,31] influenced by micro-relief [32] and utilizing extant seed banks in remnant patches or remaining degraded wetlands [33,34]. One documented restoration method that is often employed over large areas uses the extant seed bank by peeling away the top layer of turf that has developed [34,35,36]. By removing the upper layer of soil [37], the restoration of halophytic plant species is enhanced and further augmented by the grazing of livestock or seed sowing of desired species [38].
Many of the restoration practices involve changes to the water level [39]. While physical and chemical properties of the water and soil are important, it is the water level below and above ground as well as its fluctuation during the year, the most important factor for vegetation formation in wetlands [8]. Differing inundation levels produce different vegetation types that will inherently affect which species establish and flourish [1,2]; the establishment of certain desirable species and associated vegetation affects the successful restoration of the wetland to provide an important ecological function on the landscape [18,19,34,37,40].
Water level differences are one of the most important factors influencing wetland restoration from the soil seed bank. To examine this more closely, an ex-situ experiment with an inland salt marsh soil seed bank was conducted where the water level was manipulated to investigate first, the impact of water level on the number of species grown from a soil seed bank; second, the impact of water level on species composition grown from a soil seed bank; and third, to assess the impact of water levels on halophytes growth. The combination of these three aims will assist in Central European inland salt marsh restoration, where substantial loss and biotype degradation has occurred.
2. Materials and Methods
2.1. Factors Influencing the Experimental Design
The majority of halophytic plant species descend from families that produce seeds with a physiological type of dormancy. To sprout, the seeds must be within a specific temperature range to interrupt the physiological dormancy. Besides, the seeds need to be able to germinate at a higher level of water saturation in the soil. Once prompted towards growth, the seeds are more receptive towards various positive factors such as light and hormones [33]. Halophytic plants are often tolerant of a saline environment during the germination stage while also not sensitive to salination during the reproductive stage (excluding the early flowering). However, they are most sensitive to salination during the formation and growth of young seedlings; for those seedlings to reproduce, seed germination must be initiated at a favorable time with favorable water conditions [41]—some are germinating in autumn, some during winter, and some in spring [42].
The seeds that germinate in autumn require a period of high summer temperatures so that their dormancy is terminated, and growth commences. Alternatively, the seeds that germinate in the spring require a period of low temperatures to terminate their dormancy and commence germination. For both seasonal germinations, the seedlings can better tolerate an increased salinity level, which is greatly affected by the amount of rainfall as salinity rises when water levels either increase, causing dilution or decrease, resulting in elution [33]. This ex-situ experiment aims to mimic those conditions and therefore sets and follows these rules and guidelines:
- to keep the time of germination consistent, it is better to sample the soil seed bank in autumn;
- the sampling time and outdoor experimental conditions ensure that the seeds experience “natural” temperatures needed for germination;
- water in a sufficient amount should be present at all times;
- seeds require and should experience similar light conditions as they would in their habitat outdoors.
2.2. Seed Bank
The experimental seed bank was obtained from a degraded salt marsh area (agriculturally managed wet meadow, 48.7752050 N, 16.7007611 E) in proximity to a still existing salt marsh near the salt fishpond Nesyt in the cadastre of the Sedlec municipality, South Moravia, Czech Republic (Figure 1).
Figure 1.
Location of seed bank origin and the place of the experiment.
Salt marshes in Sedlec are formed on wet areas near the largest Moravian fishpond, Nesyt, with mild-salty water. The well-preserved part of salty soils constitutes a Nature reserve of national importance because many threatened invertebrates [27], birds [29], and plants species [1] are present there. Many halophytes are reported from this area, such as Triglochin maritima, Tripolium pannonicum, Crypsis aculeata, Spergularia marina, Taraxacum bessarabicum, and Juncus gerardii; formerly also Salicornia prostrata and Suaeda prostrata [43].
We obtained our soil sample on 17 October 2017 to make the autumn or winter germination of certain species possible. Before we obtained the sample, the grass was removed; 15 dm3 of soil was taken from the depth down to approximately 15 cm. The shallow hole created by sampling was filled with molehill soils, and plants were replaced in their location. The soil sample was put into a plastic bag and transferred the same day to the Institute of Botany of the CAS (Třeboň, Czech Republic).
2.3. Experimental Design
An experimental tank with a size of 1100 × 1900 mm and a depth of 400 mm was prepared before the soil sampling in the Botanic Garden Třeboň (in outdoor conditions, located in The Institute of Botany of the CAS, 49.0060847 N, 14.7721886 E, Figure 1). A mixture of 100 L of peat, 200 L of sand, and 100 L of a profi-garden substrate with 8 L of clay was prepared as the substrate. This substrate was then homogenized and placed in the tank. To test the impact of the water level on the number and diversity of growing species, the surface of the substrate was sloped so that opposite ends of the tank would either be above or below the water level (Figure 2). There was a 150 mm substrate height difference between the two opposing ends, and the substrate thickness was a minimum of 100 mm to ensure a sufficient amount of nutrients for the seedlings (Figure 2).
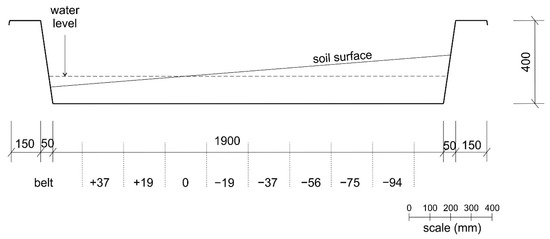
Figure 2.
Experimental design—the water level in the tank and position of belts with the different water levels, longitudinal side view, length units are in mm.
A drainage hole and stopper were provided with the tank. This allowed water to be retained in the tank but also released during heavier rainfall events. Throughout the experiment, the water level was kept in such a way that a quarter of the substrate was flooded with water throughout the experimental period (Figure 2); different inundation levels were +37 mm, +19 mm, 0 mm, −19 mm, −37 mm, −56 mm, −75 mm, and −94 mm, and further referred to as water level belts.
To prevent contamination by local seeds, the tank was covered by a white non-woven fabric before the beginning of the experiment and for the duration of the experiment, except for when the species were being recorded. To prevent the risk of substrate contamination when manipulating the fabric, a 200 mm inwards buffer area was used in the experiment (Figure 2).
2.4. Data Collection
Soil from the locality was homogenized and evenly distributed on the soil surface of the prepared substrate the next day after the sampling. Plant growth in the experimental tank was monitored until October 2019 at 7- to 21-day intervals, where the range of the interval depended on the vegetative stages of individual species. Unambiguously identifiable plants were recorded and regularly removed to provide space for germination and growth of other plants. All large unidentifiable plants were replanted to individual pots and left to grow until their identification was possible. The number of specimens of each species was monitored in the water level belts, each 200 mm in width, the positioning of which was such that it was possible to test the impact of the water level (Figure 2).
2.5. Data Analyses
To accomplish the aims of our study, three sets of analyses were performed. To resolve our first aim, a linear regression was utilized, where the number of species in a belt was set as the dependent variable, and the independent variable was calculated as the mean difference between the water level and surface in each belt (Figure 2).
The second aim was to examine the relationship between species composition of the different water level belts and their mean water level, which was tested in two steps. As species composition consists of many species (i.e., variables), a multivariate technique was utilized in the first step. Belts were used as replicates, and a non-metric multidimensional analysis (nMDS) was used to obtain an ordination of the assemblage. A square root transformation of the input data and the Bray–Curtis distances were calculated. The value of stress and Shepard diagram with non-metric fit were used for identification of how well the configuration in the two new dimensions fits the structure of input data. The permutation test was used to test the relationship between two nMDS axes and water level. All of these calculations were performed in R using the vegan package [44]. The second step was to use a test whether there would be a relationship between species composition and water level, the scores of each water level belt in the first nMDS axis were used as a dependent variable in a linear regression with the water level being as a predictor variable.
Our last aim was to consider the impact of water levels on halophyte growth. Species growing mostly on soils with at minimum a moderate salt content (i.e., species with Ellenberg’s indicator values 4 and more) are of special interest. Other species were divided into two groups–species that prefer salt-free soils or can grow on slightly salty soils (i.e., species with Ellenberg’s indicator values 0 and 1) and species that prefer low salt content (i.e., species with Ellenberg’s indicator values 2 and 3). A Fisher’s exact test was used to test potential differences in those three species groups across the different water level belts. Then, a linear regression was used to test whether or not there is a linear relationship between the abundance of individual species growing on soils of at least moderate salt content. Finally, Fisher’s exact test for count data with a simulated p-value based on 2000 replicates was used to test the differences in the number of individual plants among halophytic species and belts. Fisher’s exact tests were performed in R [45], while the linear regression was carried out in TIBCO Statistica [46].
3. Results
Three higher taxa and 40 species of vascular plants were determined from the seeds that grew from the soil seed bank sample in the experimental tank. Many members of the Poaceae family were found. However, several specimens could not be conclusively determined, as shown in Appendix A. A total of 1233 individual plants were recorded. Appendix A shows the frequency of species by water level belt location. Many of the species that germinated are rare; approximately 70.1% of species were recorded were less than or equal to seven in quantity, and more than one-third of all species (36.4% exactly) were represented by only one individual. Conversely, only six species were abundant—Mentha aquatica, Juncus articulates, Atriplex prostata, Lotus tenuis, Juncus compressus, and Plantago uliginosa (Appendix A) and represented by 30 individuals or more.
3.1. The Relationship between the Number of Species and Water Level
The number of species varied among the water level belts between 13 and 21 species. The potential relationship between the number of species and the level of the water in the belts was tested using linear regression. The results show a strong negative relationship between the average water level in a belt and the number of species found in a belt. This result shows that when the water level decreases, the number of species increases (Figure 3). The relationship is statistically significant and tight as adjusted R2 reached the value of 0.726.
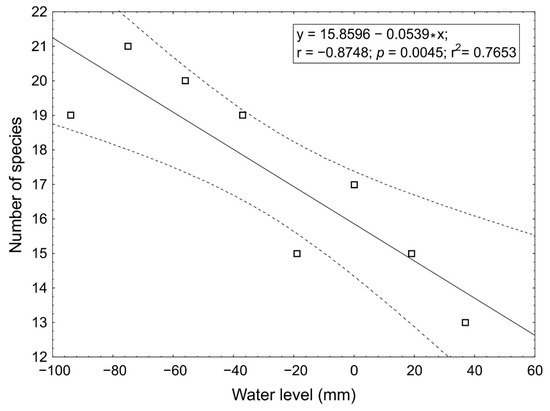
Figure 3.
Linear regression results comparing the number of species to the average water level in belts, regression line with 95% confidence intervals shown.
3.2. Species Composition and Water Level
The nMDS analysis using the square root transformed data and the Bray–Curtis distances produced a new two-dimensional space that fits the input data very well, as the stress value is very small at 0.09. A relatively small scatter around the line in the Shepard diagram suggests that the original dissimilarities are quite well preserved in the two new dimensions; the fit indices indicate and appropriateness of the transform (Figure 4).
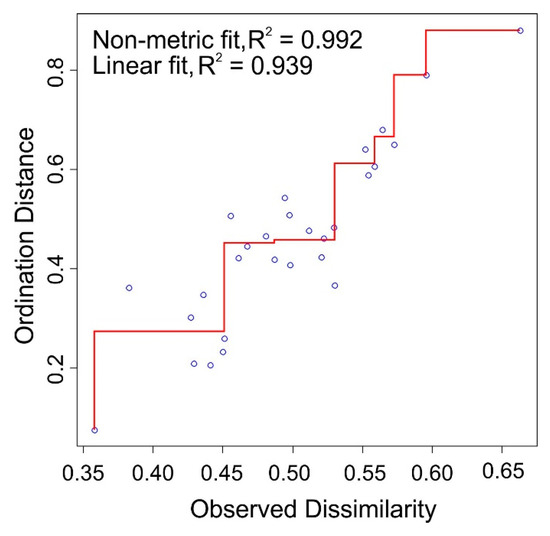
Figure 4.
Shepard diagram. Relationship between a non-metric multidimensional analysis (nMDS) ordination distance and originally observed distance.
The position of the water level belts corresponds to a somewhat diagonal line from the top left to the lower right-hand corner in the new two-dimensional space, as there are belts with low water level near the left-hand side and belts with a high water level on the right-hand side (Figure 5). The cosine of the angle of the water level to the first axis is 0.86458, and R2 is 0.8283 with a permutation-based p-value of 0.014.
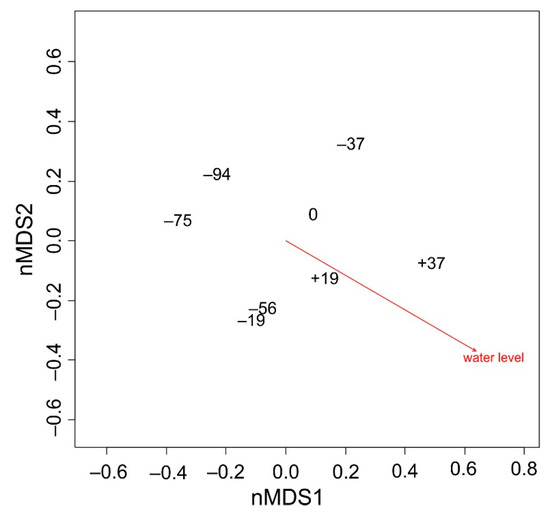
Figure 5.
Ordination diagram based on scores of two-dimensional nMDS. Positions of belts and water level are shown.
The scores of each water level belt from the first MDS axis were used as a dependent variable in a linear regression with the water level as the independent variable (Figure 6). The regression model is significant, with an adjusted R2 equal to 0.636.
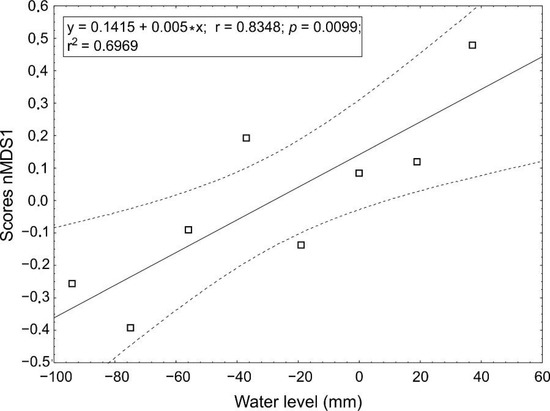
Figure 6.
Results for linear regression of scores on the first axis of nMDS on the average water level in belts, regression line with 95% confidence intervals shown.
3.3. Growth of Halophytes in Water Level Belts
There were 10 species with an Ellenberg’s salinity indicator value equal to or greater than 4. Ten of the forty species were considered to be halophytes—Pulicaria dysenterica with Ellenberg’s indicator value for salinity 4, Melilotus dentatus and Bolboschoenus maritimus with 5, Bupleurum tenuissimum, Lotus tenuis and Trifolium fragiferum with 6, Plantago maritima with 7, Spergularia maritima and Tripolium pannonicum with 8, and Crypsis schoenoides with 9.
Except for Lotus tenuis, the occurrence of all other species was rare or even unique. In many cases, the number of growing individuals was very low—Crypsis schoenoides, Plantago maritima, Pulicaria dysenterica only in one individual, Trifolium fragiferum in two individuals, and Bupleurum tenuissimum in three individuals.
The distribution of the number of species in three groups is completely independent of water level belts (X-squared = 3.8849, df = 14, p-value = 0.9961). No linear dependence was found between the number of species of halophytes and the water level (based on linear regression results). However, a significant (Fisher’s Exact Test p = 0.0285) difference among the number of halophytic species was found across water level belts (Figure 7).
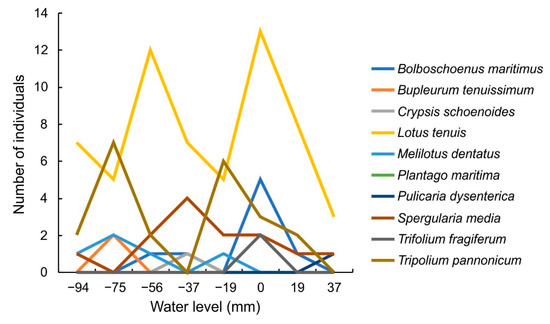
Figure 7.
Number of individuals for halophyte species grown in this experiment ordered by their water inundation levels.
There are differences in the number of individuals of halophyte species growing in different water level belts. Many of these individuals grew in belts with deeper water levels (Figure 7). Almost exclusively, Bupleurum tenuissimum, Crypsis schoenoides, Melilotus dentatus, and Plantago maritima grew in belts with water levels below the substrate. A large majority of individuals of Tripolium pannonicum and Spergularia maritima also grew in belts with water levels below the substrate. Individuals of two species were predominantly found in a particular belt with the water level just at the soil surface-Trifolium fragiferum and Bolboschoenus maritimus. Only Pulicaria dysenterica grew in an inundated belt, and Lotus tenuis grew in all types of belts.
4. Discussion
4.1. The Impact of Water Level on the Number of Species and Their Distribution
A sufficient amount of soil water is necessary for the seeds of halophytic plant species to germinate [33], and at the same time, a higher number of seeds will germinate in water with no or low levels of salt [33,47]. Simultaneously an increase in water salinity reduces the seed germination of the majority of halophytic species [33,47,48,49], and also delays the beginning of germination and reduces the germination speed of those seeds [48]. In our experiment, the salt content was lower in all belts as the soil seed bank was spread on the prepared substrate and watered. Thus seed from all belts had a chance to germinate due to reduced salinity. Results from the statistical analysis of the water level belts support that there is a relationship between the species variety and water levels. This is also confirmed by Wang et al. [50] in their experiments in wetland restoration using soil seed banks in northeastern China. More plant species will germinate when the water level is just below the level of the soil rather than if the soil is permanently inundated. Our results correspond with the results of Wang et al. [46].
Magee and Kentula [51] assume that to maintain the species richness in plant communities of seasonal wetland ecosystems, it is essential to understand what type of hydrological regime is required by plant species. They showed that plant communities rich in indigenous plant species were found in an environment with higher than the natural water level fluctuations. Even small changes in the natural hydrological regime might have an impact on species diversity, as more common and non-indigenous species characterized by growing in a wide range of hydrological conditions would have been favored over the less common indigenous taxon growing under a narrower range of conditions. According to Wang et al. [52], to restore the vegetation by utilizing the extant soil seed bank, it is essential first to determine what hydrological regime is necessary to aid in the germination of individual plant species from that seed bank.
4.2. Halophytes in Experiment
The number of species with a higher Ellenberg’s salinity indicator value is one-quarter of all germinated species. This is a high proportion of species on the type of locality under study, and it is much greater than the proportion of halophytes on the native flora of the Czech Republic [53], thus demonstrating clear evidence that the seed bank was taken from saline habitat. It is important to note that the possibility of species with a high Ellenberg’s salinity indicator value, as these species are tolerant of a high or extremely high soil salt content and specialize in tolerating no or low soil salt contents [54] and therefore are more endangered by changes to their habitats. Even though almost all the ten halophyte species that germinated in this experiment (see Appendix B) are extremely rare in the Czech Republic, all of them are quite abundant on the nearby still existing salt marshes and at margins of fishponds with mild-salty water. A detailed description of those species is available in Appendix B.
The water level influences the growth of halophytic species, and a higher number of these plant species are found on medium and low water content soils rather than on permanently flooded soils in salt marsh biotopes [55]. It is also known that different concentrations of soil salts directly impacts germination and prosperity species, such as Bupleurum tenuissimum [42] or Trifolium fragiferum [56]. This corresponds well with the results of this experiment, indicating that most halophytic plant species grown on the non-inundated belts in the experimental area.
5. Conclusions
A soil seed bank obtained from a degraded inland salt marsh was used to test the impact of the water level on the potential restoration of that biotype. Eight different water levels were tested, and 1233 individuals of 44 taxa of vascular plants were recorded, of which 10 are considered to be halophytic. Only 5 species were abundant, of which only Lotus tenuis is the lone abundant halophytic species, and in total, only five halophyte species were represented by more than three individuals. The water level has a significant impact on the number of species as well as species distribution. No effect was found on the composition of halophyte species, but differences in halophyte abundance were found.
Our experiment has important implications for salt marsh restoration in the Czech Republic. Where the turf peeling restoration method is utilized, the formation of these re-emergent salt marsh biotopes is fundamentally affected by the water level, as the amount of water in the soil and the salinity level both have an impact on the germination of individual species. The water regime is an important environmental factor that affects inland salt marsh vegetation; it is possible that inundation can cause extirpation, or with the correct conditions, it can bring about the restoration of these ecologically critical salt marsh vegetation biotypes. This work can inform on ecological restoration of some salt marshes; however, it remains unclear if the turf removal and water regime control method is applicable where salt marshes have experienced long-term degradation; further experimentation is required to determine optimal methods and conditions for prospering of halophyte plant species.
Author Contributions
Conceptualization, J.N. (Jana Navrátilová) and E.Č.; methodology, E.Č. and J.N. (Jana Navrátilová); formal analysis, S.M., E.Č. and J.N. (Josef Navrátil); investigation, E.Č.; resources, E.Č. and J.N. (Josef Navrátil); data curation, J.N. (Josef Navrátil), E.Č. and R.J.F.; writing—original draft preparation, E.Č., J.N. (Jana Navrátilová), S.M. and R.J.F.; writing—review and editing, J.N. (Josef Navrátil) and R.J.F.; visualization, J.N. (Josef Navrátil); supervision, J.N. (Jana Navrátilová); project administration, J.N. (Jana Navrátilová); funding acquisition, J.N. (Jana Navrátilová) and S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by institutional support from the Institute of Botany, Czech Academy of Sciences (J.N. (Jana Navrátilová); long-term research development project no. RVO 67985939), from institutional support of Palacký University Olomouc (S.M.), University of South Bohemia (J.N. (Josef Navrátil)), and Weber State University (R.J.F.).
Acknowledgments
Authors thank the Department of Experimental Garden and Collection of Aquatic and Wetland Plants of the Institute of Botany, Czech Academy of Sciences (Hortus Botanicus Třeboň, HBT) for technical support, Pálava Protected Landscape Area Administration for cooperation, and two anonymous reviewers for their substantial help with improving previous versions of our manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
The frequency of individual species in total and the frequency in the belts.
Table A1.
The frequency of individual species in total and the frequency in the belts.
| Species/Belt (mm) | −96 | −75 | −56 | −37 | −19 | 0 | +19 | +37 | Total Frequency | EIV Salinity |
|---|---|---|---|---|---|---|---|---|---|---|
| Alopecurus aequalis | 1 | 1 | 2 | 2 | ||||||
| Atriplex prostrata | 7 | 11 | 9 | 4 | 8 | 2 | 6 | 1 | 48 | 3 |
| Bolboschoenus maritimus | 1 | 1 | 5 | 1 | 8 | 5 | ||||
| Bupleurum tenuissimum | 2 | 1 | 3 | 6 | ||||||
| Cirsium vulgare | 1 | 1 | 2 | 1 | ||||||
| Centaurium pulchellum | 4 | 1 | 1 | 2 | 8 | 3 | ||||
| Cerastium brachypetalum | 1 | 1 | 2 | 0 | ||||||
| Cerastium dubium | 2 | 2 | 3 | |||||||
| Conyza canadensis | 1 | 1 | 1 | |||||||
| Epilobium hirsutum | 1 | 1 | 2 | 1 | ||||||
| Epilobium parviflorum | 1 | 1 | 1 | |||||||
| Geranium pusillum | 1 | 1 | 0 | |||||||
| Crypsis schoenoides | 1 | 1 | 9 | |||||||
| Hypericum sp. | 1 | 1 | - | |||||||
| Chenopodium album agg. | 1 | 1 | 1 | |||||||
| Inula britannica | 3 | 8 | 2 | 2 | 2 | 17 | 3 | |||
| Juncus articulatus | 3 | 6 | 9 | 3 | 5 | 8 | 6 | 4 | 44 | 1 |
| Juncus bufonius agg. | 1 | 7 | 1 | 2 | 5 | 1 | 3 | 1 | 21 | 1 |
| Juncus compressus | 41 | 45 | 20 | 35 | 36 | 27 | 38 | 20 | 262 | 3 |
| Juncus inflexus | 1 | 2 | 1 | 4 | 2 | |||||
| Lamium purpureum | 2 | 2 | 0 | |||||||
| Lotus tenuis | 7 | 5 | 12 | 7 | 5 | 13 | 8 | 3 | 60 | 6 |
| Lycopus europaeus | 2 | 2 | 1 | |||||||
| Medicago lupulina | 1 | 1 | 1 | |||||||
| Melilotus altissimus | 1 | 1 | 2 | |||||||
| Melilotus dentatus | 1 | 2 | 1 | 1 | 5 | 5 | ||||
| Mentha aquatica | 3 | 5 | 7 | 2 | 6 | 4 | 3 | 2 | 32 | 1 |
| Plantago lanceolata | 1 | 1 | 1 | |||||||
| Plantago major | 1 | 1 | 1 | |||||||
| Plantago maritima | 1 | 1 | 7 | |||||||
| Plantago uliginosa | 82 | 92 | 108 | 92 | 73 | 62 | 57 | 32 | 598 | 2 |
| Poa annua | 2 | 1 | 1 | 1 | 2 | 7 | 1 | |||
| Poaceae | 8 | 5 | 4 | 6 | 8 | 4 | 2 | 37 | - | |
| Potentilla supina | 2 | 2 | 4 | 1 | ||||||
| Pulicaria dysenterica | 1 | 1 | 4 | |||||||
| Ranunculus sceleratus | 1 | 1 | 1 | |||||||
| Typha sp. | 1 | 1 | 2 | - | ||||||
| Spergularia media | 1 | 2 | 4 | 2 | 2 | 1 | 1 | 13 | 8 | |
| Taraxacum sp. | 1 | 1 | - | |||||||
| Trifolium fragiferum | 2 | 2 | 6 | |||||||
| Tripolium pannonicum subsp. pannonicum | 2 | 7 | 2 | 6 | 3 | 2 | 22 | 8 | ||
| Veronica anagallis-aquatica | 1 | 2 | 2 | 1 | 6 | 2 | ||||
| Veronica anagalloides | 1 | 1 | 3 | |||||||
| Veronica scutellata | 1 | 1 | 0 |
Note: EIV = Ellenberg’s salinity indicator value, according to Chytry et al. [53].
Appendix B
Halophyte species grown from the soil seed bank:
- Pulicaria dysenterica is to be found usually on wet and mildly saline soils [57] and is known for chemicals contained in its oils [58,59]. In the Czech Republic, it is scattered only across the valleys in southern Moravia and can be rarely seen in the White Carpathians, in Haná region, or the Moravian Gate and Ostrava regions [60].
- Melilotus dentatus is species the prefers wet saline grasslands, often ruderalized [61]. In the Czech Republic, it can be found only in dry, warm areas of southern Moravia and central and north-western Bohemia. It is a species with declined occurrence due to the loss of suitable habitats in the Czech Republic [62,63].
- Bolboschoenus maritimus dominates reed vegetation of saline waters both along the seashore and inland saltwater bodies in Central Europe [64] as well as in other parts of the world [65]. In the Czech Republic, it is a rare species with declining occurrence [62] concentrated to the remains of saline wetlands in dry and warm areas of southern Moravia and central and north-western Bohemia [66].
- Bupleurum tenuissimum is an obligate halophyte that is competitively very weak and thus only grows in areas with open or disturbed types of vegetation. In the Czech Republic, it can be found very rarely [62] in the lowlands of the north-western Bohemia and southern Moravia [67].
- Lotus tenuis is European species of wet salt-rich soils and quite rare in the Czech Republic [62]. It is naturalized in other parts of the world, where it is known as an important forage crop of otherwise useless saline soils [68]. It is flood tolerant [69] and well established on restored saline wetlands [39].
- Trifolium fragiferum has many commonalities with the previously mentioned species. It is flood-tolerant species of alkali and salty wet meadows [62,68,69]. It is also an important forage crop [70].
- Plantago maritima is another obligate halophyte that tolerates being trodden down. However, it is often found on gradually disrupted saline soils (due to restoration) and in localities overgrown with other vegetation. Much of this species preferred habitat has disappeared from the Czech Republic, and thus its occurrence is rare and only in the north-western Bohemia and southern Moravia [71].
- Spergularia media is a halophyte plant that grows on wet and heavily saline soils and is rarely seen in the Czech Republic in the north-western Bohemia, southern Moravia, and Ostrava region [62,72,73].
- Tripolium pannonicum subsp. pannonicum grows on wet and saline soils that dry out in summer [74]. In the Czech Republic, it is to be found only on saline soils in southern Moravia [57,62,75].
- Crypsis schoenoides is also an obligate halophyte, but it grows on heavily saline soils that accumulate an extreme amount of salt during the dry season. At the same time, this species is to be found in full-sun localities with wet soils rich in nutrients. In the Czech Republic, they can be found very rarely [62], only in southern Moravia [76].
References
- Šumberová, K.; Novák, J.; Sádlo, J. Slaniskové trávníky. In Vegetace České Republiky. 2, Travinná a Keříčková Vegetace, 2nd ed.; Chytrý, M., Ed.; Academia: Praha, Czech Republic, 2010; pp. 150–164. [Google Scholar]
- Sádlo, J. T7 Slaniska. In Katalog Biotopů České Republiky, 2nd ed.; Chytrý, M., Kučera, T., Kočí, M., Grulich, V., Lustyk, P., Eds.; Agentura Ochrany Přírody a Krajiny ČR: Praha, Czech Republic, 2010; pp. 240–243. [Google Scholar]
- Piernik, A. Ecological Pattern of Inland Salt Marsh Vegetation in Central Europe; Wydawnictwo Naukowe Uniwersytetu Mikołaja Kopernika: Toruń, Czech Republic, 2012; p. 219. [Google Scholar]
- Šumberová, K.; Chytrý, M.; Sádlo, J. Rákosiny a vegetace vysokých ostřic. In Katalog Biotopů České Republiky, 2nd ed.; Chytrý, M., Kučera, T., Kočí, M., Grulich, V., Lustyk, P., Eds.; Agentura Ochrany Přírody a Krajiny České Republiky: Praha, Czech Republic, 2010; pp. 34–52. [Google Scholar]
- Galvanek, D.; Dite, D.; Elias, P.; Dite, Z. Regeneration of threatened alkali steppe vegetation after a heavy disturbance by disk tillage. Plant Ecol. 2020, 221, 1177–1186. [Google Scholar] [CrossRef]
- Elias, P.; Sopotlieva, D.; Dite, D.; Hajkova, P.; Apostolova, I.; Senko, D.; Meleckova, Z.; Hajek, M. Vegetation diversity of salt-rich grasslands in Southeast Europe. Appl. Veg. Sci. 2013, 16, 521–537. [Google Scholar] [CrossRef]
- Eliáš, P.; Dítě, D.; Dítě, Z. Halophytic Vegetation in the Pannonian Basin: Origin, Syntaxonomy, Threat, and Conservation. In Handbook of Halophytes: From Molecules to Ecosystems towards Biosaline Agriculture; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 1–38. [Google Scholar]
- Chytry, M. Vegetation of the Czech Republic: Diversity, ecology, history and dynamics. Preslia 2012, 84, 427–504. [Google Scholar]
- Hroudová, Z.; Hrivnák, R.; Šumberová, K. MCB01. Astero Pannonici-Bolboschoenetum Compacti. In Vegetace České Republiky. 3, Vodní a Mokřadní Vegetace, 1st ed.; Chytrý, M., Ed.; Academia: Praha, Czech Republic, 2011; pp. 434–437. [Google Scholar]
- Šumberová, K. Mokřady a pobřežní vegetace. In Červený Seznam Biotopů České Republiky; Agentura Ochrany Přírody a Krajiny ČR: Praha, Czech Republic, 2020; Volume 41, pp. 47–52. [Google Scholar]
- Chytrý, M. Sekundární trávníky a vřesoviště. In Červený Seznam Biotopů České Republiky; Agentura Ochrany Přírody a Krajiny ČR: Praha, Czech Republic, 2020; Volume 41, pp. 63–69. [Google Scholar]
- Navratilova, J.; Havlicek, M.; Navratil, J.; Frazier, R.J. Land cover changes on temperate organic substrates over last 150years: Evidence from the Czech Republic. Biologia 2019, 74, 361–373. [Google Scholar] [CrossRef]
- Hroudová, Z. Svaz, M.C.B. Meliloto Dentati-Bolboschoenion Maritimi. In Vegetace České republiky. 3, Vodní a Mokřadní Vegetace, 1st ed.; Chytrý, M., Ed.; Academia: Praha, Czech Republic, 2011; pp. 428–434. [Google Scholar]
- Ditetova, Z.; Dite, D.; Elias, P.; Galvanek, D. The impact of grazing absence in inland saline vegetation—A case study from Slovakia. Biologia 2016, 71, 980–988. [Google Scholar] [CrossRef]
- Šumberová, K. Vegetace jednoletých sukulentních halofytů. In Vegetace České Republiky. 2, Travinná a Keříčková Vegetace, 2nd ed.; Chytrý, M., Ed.; Academia: Praha, Czech Republic, 2010; pp. 143–149. [Google Scholar]
- Boon, P.J.; Baxter, J.M. Aquatic conservation: Reflections on the first 25 years. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 809–816. [Google Scholar] [CrossRef]
- Kaplan, Z.; Sumberova, K.; Formanova, I.; Duchacek, M. Re-establishment of an extinct population of the endangered aquatic plant Potamogeton coloratus. Aquat. Bot. 2014, 119, 91–99. [Google Scholar] [CrossRef]
- Schaich, H.; Barthelmes, B. Management of grasslands in rewetted floodplains: Effects of grazing and cutting on vegetation development. Tuexenia 2012, 32, 207–231. [Google Scholar]
- Klotzli, F.; Grootjans, A.P. Restoration of natural and semi-natural wetland systems in Central Europe: Progress and predictability of developments. Restor. Ecol. 2001, 9, 209–219. [Google Scholar] [CrossRef]
- Williams, P.; Biggs, J.; Stoate, C.; Szczur, J.; Brown, C.; Bonney, S. Nature based measures increase freshwater biodiversity in agricultural catchments. Biol. Conserv. 2020, 244. [Google Scholar] [CrossRef]
- Jellinek, S.; Harrison, P.A.; Tuck, J.; Te, T. Replanting agricultural landscapes: How well do plants survive after habitat restoration? Restor. Ecol. 2020, 28, 1454–1463. [Google Scholar] [CrossRef]
- Van der Horst, D.; Martinat, S.; Navratil, J.; Dvorak, P.; Chmielova, P. What can the location of biogas plants tell us about agricultural change? A Case Study from the Czech Republic. Deturope Cent. Eur. J. Reg. Dev. Tour. 2018, 10, 33–52. [Google Scholar]
- Alonso, N.M.; Muniz, I.O.; Aja, A.H.; Garcia, F.F. Challenges for the revitalisation of peri-urban agriculture in Spain: Territorial analysis of the Madrid and Oviedo metropolitan areas. Morav. Geogr. Rep. 2017, 25, 192–207. [Google Scholar] [CrossRef]
- Chodkowska-Miszczuk, J.; Kulla, M.; Novotny, L. Biogas Energy—A Chance for Agriculture and Rural Development? Insight from the Post-Communist Central Europe. Deturope Cent. Eur. J. Reg. Dev. Tour. 2019, 11, 30–53. [Google Scholar]
- Hildebrandt, U.; Janetta, K.; Ouziad, F.; Renne, B.; Nawrath, K.; Bothe, H. Arbuscular mycorrhizal colonization of halophytes in Central European salt marshes. Mycorrhiza 2001, 10, 175–183. [Google Scholar] [CrossRef]
- Muhsin, T.M.; Booth, T. Fungi Associated with Halophytes of an Inland Salt-Marsh, Manitoba, Canada. Can. J. Bot. 1987, 65, 1137–1151. [Google Scholar] [CrossRef]
- Sychra, J.; Adamek, Z.; Petrivalska, K. Distribution and diversity of littoral macroinvertebrates within extensive reed beds of a lowland pond. Ann. Limnol. Int. J. Limnol. 2010, 46, 281–289. [Google Scholar] [CrossRef]
- Beyen, W.; Meire, P. Ecohydrology of saline grasslands: Consequences for their restoration. Appl. Veg. Sci. 2003, 6, 153–160. [Google Scholar] [CrossRef]
- Miklin, J.; Machacek, P. Birds of Lednicke rybniky Fishponds (Czech Republic). J. Maps 2016, 12, 239–248. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Wu, X.; Nairn, R.W.; Weihe, P.E.; Wang, N.; Deal, R.; Boucher, C.E. Creating and restoring wetlands. BioScience 1998, 48, 1019–1030. [Google Scholar] [CrossRef]
- Zedler, J.B. Progress in wetland restoration ecology. Trends Ecol. Evol. 2000, 15, 402–407. [Google Scholar] [CrossRef]
- Wang, G.M.; Lv, J.Z.; Han, G.X.; Zhu, S.Y.; Liu, X.L.; Wang, A.D.; Guan, B.; Zhao, Y.J. Ecological Restoration of Degraded Supratidal Wetland Based on Microtopography Modification: A Case Study in the Yellow River Delta. Wetlands 2020. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press: Cambridge, MA, USA, 1998. [Google Scholar]
- Wolters, M.; de Vries, S.; Ozinga, W.A.; Bakker, J.P. Restoration of inland brackish vegetation by large-scale transfer of coastal driftline material. Appl. Veg. Sci. 2017, 20, 641–650. [Google Scholar] [CrossRef]
- Gilhaus, K.; Vogt, V.; Holzel, N. Restoration of sand grasslands by topsoil removal and self-greening. Appl. Veg. Sci. 2015, 18, 661–673. [Google Scholar] [CrossRef]
- Meleckova, Z.; Galvanek, D.; Dite, D.; Elias, P. Effect of experimental top soil removal on vegetation of Pannonian salt steppes. Cent. Eur. J. Biol. 2013, 8, 1204–1215. [Google Scholar] [CrossRef]
- Bakker, J.P.; Esselink, P.; Dijkema, K.S.; Van Duin, W.E.; De Jong, D.J. Restoration of salt marshes in the Netherlands. Hydrobiologia 2002, 478, 29–51. [Google Scholar] [CrossRef]
- Šumberová, K.; Chytrý, M. Vegetace jednoletých vlhkomilných bylin. In Katalog Biotopů České Republiky, 2nd ed.; Chytrý, M., Kučera, T., Kočí, M., Grulich, V., Lustyk, P., Eds.; Agentura Ochrany Přírody a Krajiny České Republiky: Praha, Czech Republic, 2010; pp. 54–62. [Google Scholar]
- Meleckova, Z.; Dite, D.; Elias, P.; Pis, V.; Galvanek, D. Succession of saline vegetation in Slovakia after a large-scale disturbance. Ann. Bot. Fenn. 2014, 51, 285–296. [Google Scholar] [CrossRef]
- Bakker, J.P.; Berendse, F. Constraints in the restoration of ecological diversity in grassland and heathland communities. Trends Ecol. Evol. 1999, 14, 63–68. [Google Scholar] [CrossRef]
- Shannon, M.C.; Grieve, C.M.; Francois, L.E. Whole—Plant Response to Salinity. In Plant—Environment Interactions; Wilkinson, R.E., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1994; pp. 199–244. [Google Scholar]
- Al-Hawija, B.N.; Partzsch, M.; Hensen, I. Effects of temperature, salinity and cold stratification on seed germination in halophytes. Nord. J. Bot. 2012, 30, 627–634. [Google Scholar] [CrossRef]
- Grulich, V.; Danihelka, J. NPR Slanisko u Nesytu. In Chráněná Území Přírody ČR, Svazek IX. Brněnsko; Mackovcin, P., Ed.; AOPK ČR, EkoCentrum Brno: Praha, Czech Republic, 2007; pp. 766–767. [Google Scholar]
- Oksanen, J.; Guillaume Blanchet, F.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Package ‘Vegan’. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 12 November 2020).
- The-R-Foundation. The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 12 November 2020).
- TIBCO. TIBCO Statistica™ Quick Reference. Available online: https://docs.tibco.com/pub/stat/13.3.0/doc/pdf/TIB_stat_13.3_quick_ref.pdf (accessed on 12 November 2020).
- Keiffer, C.H.; Ungar, I.A. The effect of extended exposure to hypersaline conditions on the germination of five inland halophyte species. Am. J. Bot. 1997, 84, 104–111. [Google Scholar] [CrossRef]
- Ungar, I.A. Germination ecology of halophytes. In Contributions to the Ecology of Halophytes; Sen, D.N., Rajpurohit, K.S., Eds.; Springer: Dordrecht, The Netherlands, 1982; Volumes 143–154, p. 272. [Google Scholar]
- Elsey-Quirk, T.; Middleton, B.A.; Proffitt, C.E. Seed flotation and germination of salt marsh plants: The effects of stratification, salinity, and/or inundation regime. Aquat. Bot. 2009, 91, 402009. [Google Scholar] [CrossRef]
- Wang, M.; Wang, G.D.; Lu, X.G.; Jiang, M.; Wang, S.Z. Soil seed banks and their implications for wetland restoration along the Nongjiang River, Northeastern China. Ecol. Eng. 2016, 96, 26–33. [Google Scholar] [CrossRef]
- Magee, T.K.; Kentula, M.E. Response of wetland plant species to hydrologic conditions. Wetl. Ecol. Manag. 2005, 13, 163–181. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, H.; Yin, L.; Li, J.; Zhang, Z.; Li, Y. Effects of water treatments on the activation of soil seed banks-A case study on the lower reaches of the Tarim River. Prog. Nat. Sci. 2009, 19, 733–740. [Google Scholar] [CrossRef]
- Chytry, M.; Tichy, L.; Drevojan, P.; Sadlo, J.; Zeleny, D. Ellenberg-type indicator values for the Czech flora. Preslia 2018, 90, 83–103. [Google Scholar] [CrossRef]
- Zeleny, D.; Chytry, M. Ecological Specialization Indices for species of the Czech flora. Preslia 2019, 91, 93–116. [Google Scholar] [CrossRef]
- Deák, B.; Valkó, O.; Török, P.; Tóthmérész, B. Solonetz meadow vegetation (Beckmannion eruciformis) in East-Hungary—An alliance driven by moisture and salinity. Tuexenia 2014, 34, 187–203. [Google Scholar] [CrossRef]
- Can, E.; Arslan, M.; Sener, O.; Daghan, H. Response of strawberry clover (Trifolium fragiferum L.) to salinity stress. Res. Crops 2013, 14, 576–584. [Google Scholar]
- Kaplan, Z.; Danihelka, J.; Sumberova, K.; Chrtek, J.; Rotreklova, O.; Ekrt, L.; Stepankova, J.; Taraska, V.; Travnicek, B.; Prancl, J.; et al. Distributions of vascular plants in the Czech Republic. Part 5. Preslia 2017, 89, 333–439. [Google Scholar] [CrossRef]
- Basta, A.; Tzakou, O.; Couladis, M.; Pavlovic, M. Chemical composition of Pulicaria dysenterica (L.) Bernh. from Greece. J. Essent. Oil Res. 2007, 19, 333–335. [Google Scholar] [CrossRef]
- Cdiz-Gurrea, M.D.; Zengin, G.; Kayacik, O.; Lobine, D.; Mahomoodally, M.F.; Leyva-Jimnez, F.J.; Segura-Carretero, A. Innovative perspectives on Pulicaria dysenterica extracts: Phyto-pharmaceutical properties, chemical characterization and multivariate analysis. J. Sci. Food Agric. 2019, 99, 6001–6010. [Google Scholar] [CrossRef] [PubMed]
- Hrouda, L. Pulicaria Gaertner. In Květena České Republiky 7; Slavík, B., Štěpánková, J., Eds.; Academia: Praha, Czech Republic, 2004. [Google Scholar]
- Tishchenko, M.P.; Korolyuk, A.Y. The syntaxonomy of the meadow vegetation of Kulunda and Kasmala Pine Forest Strips (Altai Territory). Rastit. Ross. 2018, 2018, 101–119. [Google Scholar] [CrossRef]
- Danihelka, J.; Chrtek, J.; Kaplan, Z. Checklist of vascular plants of the Czech Republic. Preslia 2012, 84, 647–811. [Google Scholar]
- Chytry, M.; Hajek, M.; Koci, M.; Pesout, P.; Rolecek, J.; Sadlo, J.; Sumberova, K.; Sychra, J.; Boublik, K.; Douda, J.; et al. Red List of Habitats of the Czech Republic. Ecol. Indic. 2019, 106. [Google Scholar] [CrossRef]
- Ljevnaic-Masic, B.; Dzigurski, D.; Nikolic, L.; Brdar-Jokanovic, M.; Cabilovski, R.; Ciric, V.; Petrovic, A. Assessment of the habitat conditions of a rare and endangered inland saline wetland community with Bolboschoenus maritimus (L.) Palla dominance in Southeastern Europe: The effects of physical-chemical water and soil properties. Wetl. Ecol. Manag. 2020, 28, 421–438. [Google Scholar] [CrossRef]
- Kettenring, K.M. Viability, dormancy, germination, and intraspecific variation of Bolboschoenus maritimus (alkali bulrush) seeds. Aquat. Bot. 2016, 134, 26–30. [Google Scholar] [CrossRef]
- Hroudova, Z.; Zakravsky, P.; Duchacek, M.; Marhold, K. Taxonomy, distribution and ecology of Bolboschoenus in Europe. Ann. Bot. Fenn. 2007, 44, 81–102. [Google Scholar]
- Šourková, M.; Hrouda, L.; Bupleurum, L. Květena České Republiky 5; Slavík, B., Ed.; Academia: Praha, Czech Republic, 1997. [Google Scholar]
- Espasandin, F.D.; Brugnoli, E.A.; Ayala, P.G.; Ayala, L.P.; Ruiz, O.A.; Sansberro, P.A. Long-term preservation of Lotus tenuis adventitious buds. Plant Cell Tissue Organ Cult. 2019, 136, 373–382. [Google Scholar] [CrossRef]
- Striker, G.G.; Colmer, T.D. Flooding tolerance of forage legumes. J. Exp. Bot. 2017, 68, 1851–1872. [Google Scholar] [CrossRef]
- Haerinasab, M.; Ali-Farsangi, F.; Bordbar, F.; Farouji, A.E. Genetic Diversity and Infraspecific Relationships of Trifolium fragiferum L. in Iran. Iran. J. Sci. Technol. Trans. A Sci. 2020, 44, 345–354. [Google Scholar] [CrossRef]
- Chrtek, J.S.; Plantago, L. Květena České Republiky 6; Slavík, B., Ed.; Academia: Praha, Czech Republic, 2000. [Google Scholar]
- Kaplan, Z.; Danihelka, J. Květena České Republiky 2; Hejný, S., Slavík, B., Eds.; Academia: Praha, Czech Republic, 1990. [Google Scholar]
- Kaplan, Z.; Danihelka, J.; Stepankova, J.; Ekrt, L.; Chrtek, J.; Zazvorka, J.; Grulich, V.; Repka, R.; Prancl, J.; Duchacek, M.; et al. Distributions of vascular plants in the Czech Republic. Part 2. Preslia 2016, 88, 229–322. [Google Scholar]
- Eliáš, P.; Dítě, D.; Dítě, Z.; Eliášová, M. Distribution and habitat preferences of Tripolium pannonicum subsp. pannonicum (Asteraceae) in Slovakia. Thaiszia J. Bot. 2018, 28, 111–123. [Google Scholar]
- Kovanda, K.; Kubát, K.; Aster, L. Květena České Republiky 7; Slavík, B., Štěpánková, J., Eds.; Academia: Praha, Czech Republic, 2004. [Google Scholar]
- Kaplan, Z.; Danihelka, J.; Ekrt, L.; Stech, M.; Repka, R.; Chrtek, J.; Grulich, V.; Rotreklova, O.; Drevojan, P.; Sumberova, K.; et al. Distributions of vascular plants in the Czech Republic. Part 9. Preslia 2020, 92, 255–340. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).