Does Pastoral Land-Use Legacy Influence Topsoil Carbon and Nitrogen Accrual Rates in Tallgrass Prairie Restorations?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. GIS Topographical Analysis
2.3. Soil Sampling and Processing
2.4. Soil Bulk Density and Nutrient Content Calculations
2.5. Statistical Analysis
3. Results
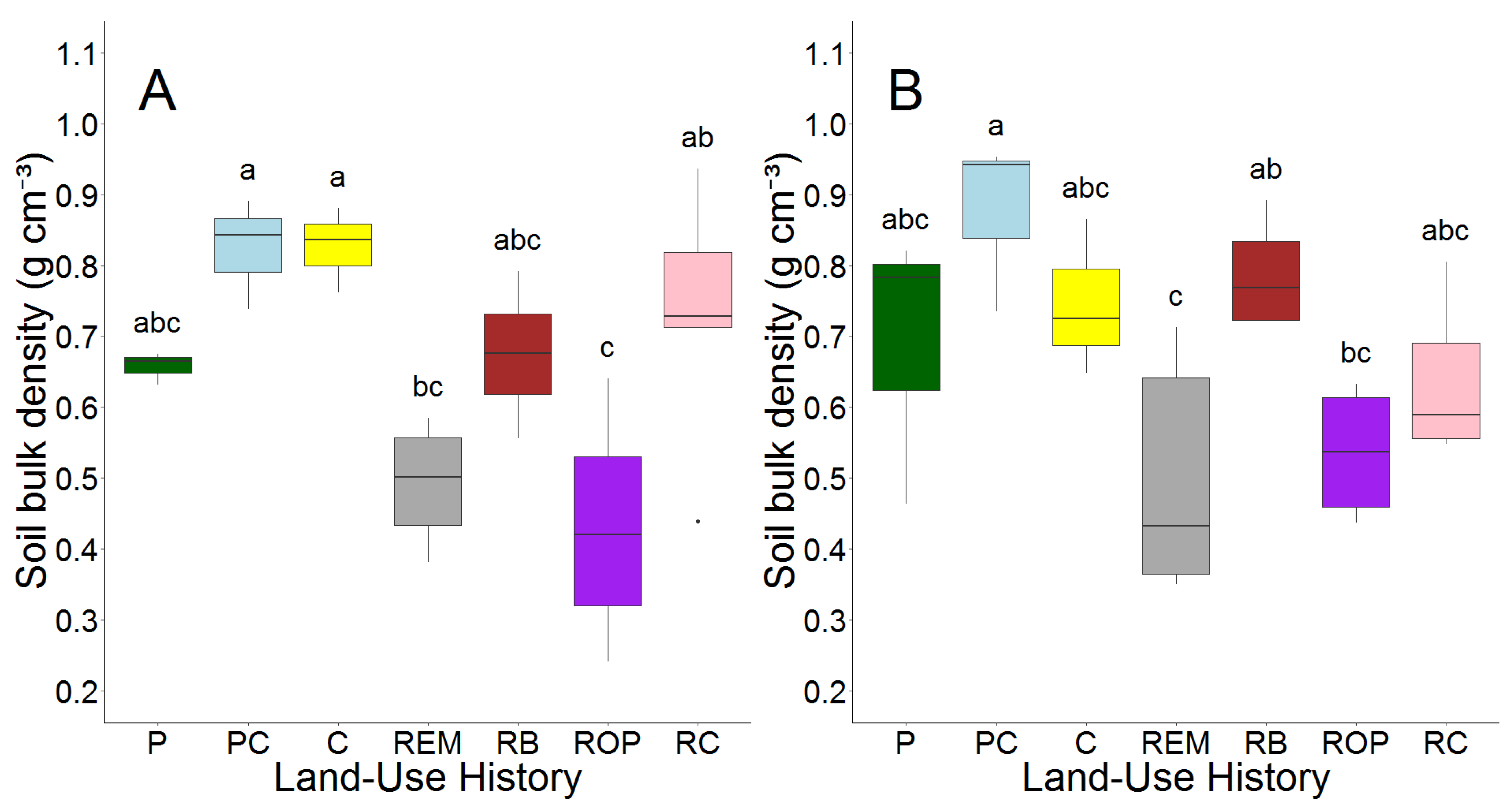
3.1. Topographical Effects and Soil Physical Properties
3.2. Belowground Plant and Soil Chemistry
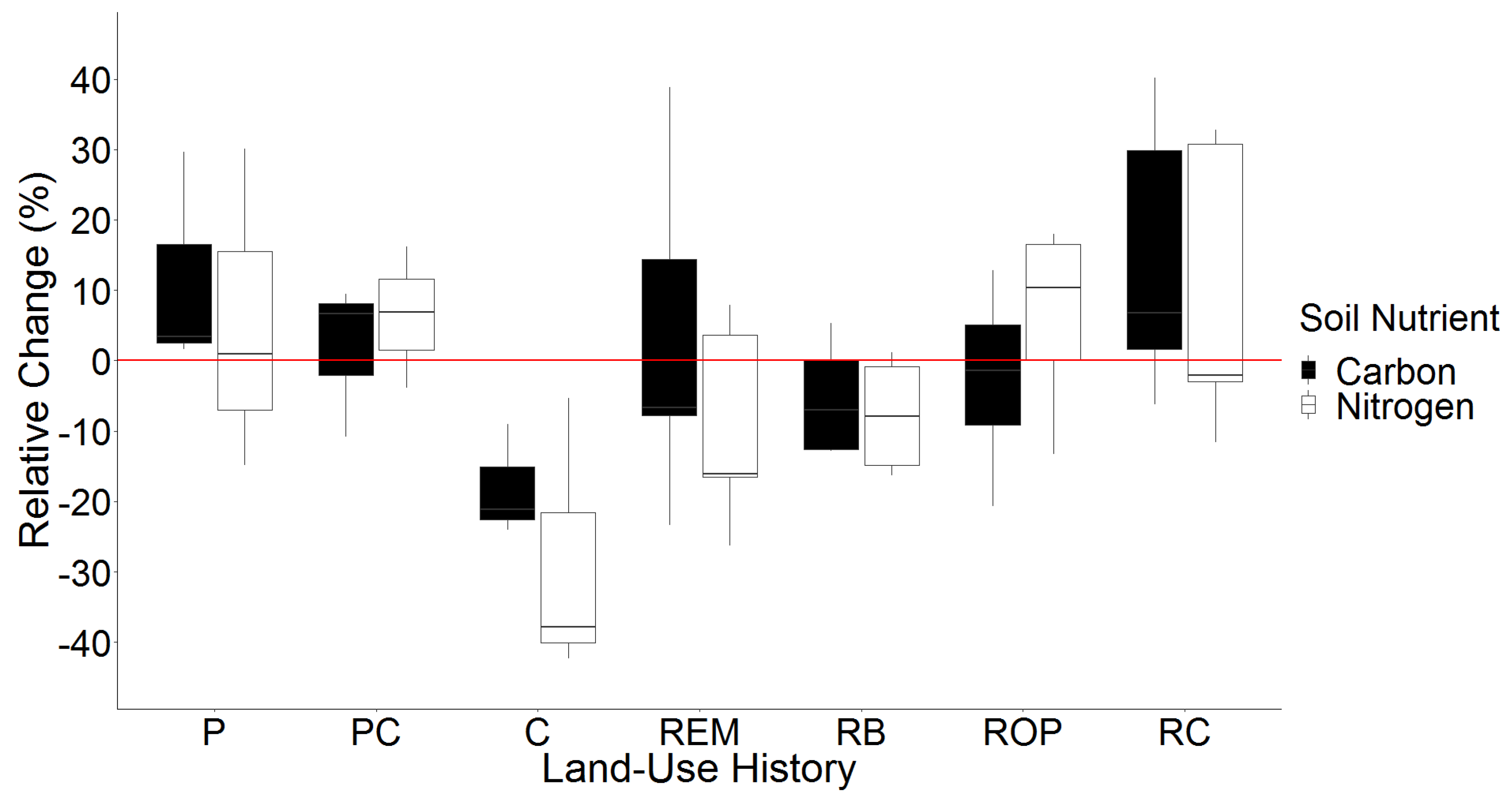
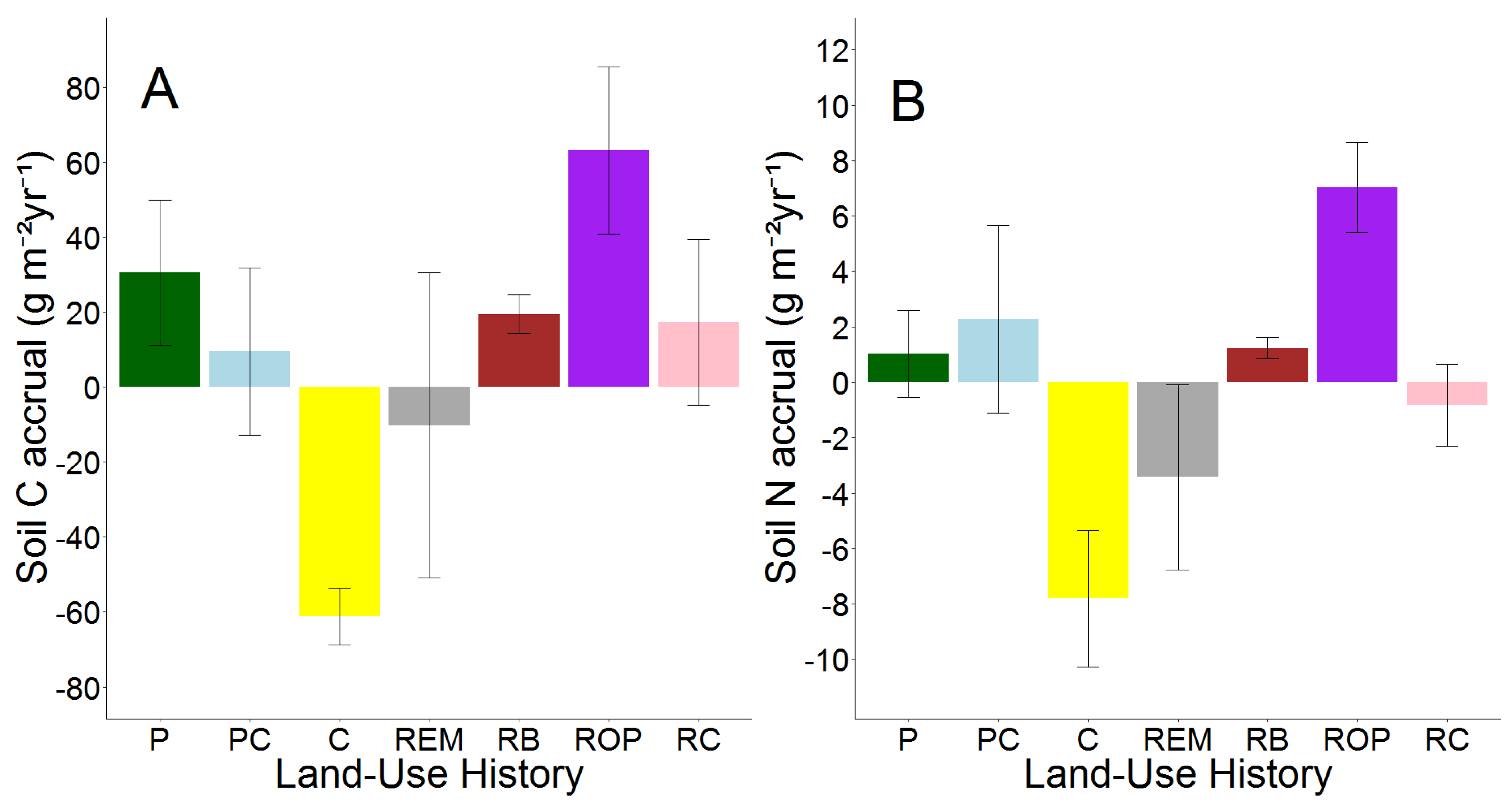
3.3. Temporal Changes in Soil C and N
4. Discussion
4.1. Environmental and Land Management Interactions
4.2. Soil and Root Nutrient Content Dynamics in Remnants
4.3. Land-Use Legacy Effects in Tallgrass Prairie Restorations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samson, F.; Knopf, F. Prairie Conservation in North America. BioScience 1994, 44, 418–421. [Google Scholar] [CrossRef]
- Kucharik, C.J.; Fayram, N.J.; Cahill, K.N. A paired study of prairie carbon stocks, fluxes and phenology: Comparing the world’s oldest prairie restoration with an adjacent remnant. Glob. Chang. Biol. 2006, 12, 122–139. [Google Scholar] [CrossRef]
- Schilling, K.; Drobney, P. Restoration of Prairie Hydrology at the Watershed Scale: Two Decades of Progress at Neal Smith National Wildlife Refuge, Iowa. Land 2014, 3, 206–238. [Google Scholar] [CrossRef]
- O’Brien, S.L.; Jastrow, J.D.; Grimley, D.A.; Gonzalez-Meler, M.A. Moisture and vegetation controls on decadal-scale accrual of soil organic carbon and total nitrogen in restored grasslands. Glob. Chang. Biol. 2010, 16, 2573–2588. [Google Scholar] [CrossRef]
- Bengtsson, J.; Bullock, J.M.; Egoh, B.; Everson, C.; O’Connor, T.; O’Farrell, P.J.; Smith, H.G.; Lindborg, R. Grasslands—More important for ecosystem services than you might think. Ecosphere 2019, 10, e02582. [Google Scholar] [CrossRef]
- Six, J.; Elliott, E.T.; Paustian, K. Soil macroaggregate turnover and microaggregate formation: A mechanism for C sequestration under no-tillage agriculture. Soil Biol. Biochem. 2000, 32, 2099–2103. [Google Scholar] [CrossRef]
- Qu, R.; Han, G. The Grain for Green Project May Enrich the Mercury Concentration in a Small Karst Catchment, Southwest China. Land 2020, 9, 354. [Google Scholar] [CrossRef]
- Tisdall, J.M. Possible role of soil microorganisms in aggregation in soils. Plant Soil 1994, 159, 115–121. [Google Scholar] [CrossRef]
- Jastrow, J.D. Soil aggregate formation and the accrual of particulate and mineral-associated organic matter. Soil Biol. Biochem. 1996, 28, 665–676. [Google Scholar] [CrossRef]
- Foster, B.L.; Murphy, C.A.; Keller, K.R.; Aschenbach, T.A.; Questad, E.J.; Kindscher, K. Restoration of prairie community structure and ecosystem function in an abandoned hayfield: A sowing experiment. Restor. Ecol. 2007, 15, 652–661. [Google Scholar] [CrossRef]
- Zylka, J.J.; Whelan, C.J.; Molano-Flores, B. Restoration Implications of Land Management Legacy on Aboveground and Seed Bank Composition of North American Grasslands. Am. Midl. Nat. 2016, 176, 36–59. [Google Scholar] [CrossRef]
- Lal, R. Soil erosion and the global carbon budget. Environ. Int. 2003, 29, 437–450. [Google Scholar] [CrossRef]
- West, T.O.; Six, J. Considering the influence of sequestration duration and carbon saturation on estimates of soil carbon capacity. Clim. Chang. 2007, 80, 25–41. [Google Scholar] [CrossRef]
- Todd, T.C.; Powers, T.O.; Mullin, P.G. Sentinel nematodes of land-use change and restoration in tallgrass prairie. J. Nematol. 2006, 38, 20–27. [Google Scholar]
- De Graaff, M.A.; Six, J.; Jastrow, J.D.; Schadt, C.W.; Wullschleger, S.D. Variation in root architecture among switchgrass cultivars impacts root decomposition rates. Soil Bio. Biochem. 2013, 58, 198–206. [Google Scholar] [CrossRef]
- Baer, S.G.; Kitchen, D.J.; Blair, J.M.; Rice, C.W. Changes in ecosystem structure and function along a chronosequence of restored grasslands. Ecol. Appl. 2002, 12, 1688–1701. [Google Scholar] [CrossRef]
- Sørenson, L.H. Stabilization of newly formed amino-acid metabolites in soil by clay minerals. Soil Sci. Soc. Am. J. 1972, 114, 5–11. [Google Scholar] [CrossRef]
- Tisdall, J.M.; Oades, J.M. Organic matter and water-stable aggregates in soils. J. Soil Sci. 1982, 33, 141–163. [Google Scholar] [CrossRef]
- Elliott, E.T. Aggregate structure and carbon, nitrogen, and phosphorus in native and cultivated soils. Soil Sci. Soc. Am. J. 1986, 50, 627–633. [Google Scholar] [CrossRef]
- Six, J.; Paustian, K.; Elliott, E.T.; Combrink, C. Soil structure and organic matter: I. Distribution of aggregate-size classes and aggregate-associated carbon. Soil Sci. Soc. Am. J. 2000, 64, 681–689. [Google Scholar] [CrossRef]
- Trueman, R.J.; Gonzalez-Meler, M.A. Accelerated belowground C cycling in a managed agriforest ecosystem exposed to elevated carbon dioxide concentrations. Glob. Chang. Biol. 2005, 11, 1258–1271. [Google Scholar] [CrossRef]
- Neff, J.C.; Townsend, A.R.; Gleixner, G.; Lehman, S.J.; Turnbull, J.; Bowman, W.D. Variable effects of nitrogen additions on the stability and turnover of soil carbon. Nature 2002, 419, 915–917. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Sources of CO2 efflux from soil and review of partitioning methods. Soil Bio. Biochem. 2006, 38, 425–428. [Google Scholar] [CrossRef]
- Schäfer, D.; Klaus, V.H.; Kleinebecker, T.; Boeddinghaus, R.S.; Hinderling, J.; Kandeler, E.; Marhan, S.; Nowak, S.; Sonnemann, I.; Wurst, S.; et al. Recovery of ecosystem functions after experimental disturbance in 73 grasslands differing in land-use intensity, plant species richness and community composition. J. Ecol. 2019, 107, 2635–2649. [Google Scholar] [CrossRef]
- John, K.; Abraham Isong, I.; Michael Kebonye, N.; Okon Ayito, E.; Chapman Agyeman, P.; Marcus Afu, S. Using Machine Learning Algorithms to Estimate Soil Organic Carbon Variability with Environmental Variables and Soil Nutrient Indicators in an Alluvial Soil. Land 2020, 9, 487. [Google Scholar] [CrossRef]
- Håkansson, I.; Reeder, R.C. Subsoil compaction by vehicles with high axle load extent, persistence and crop response. Soil Tillage Res. 1994, 29, 277–304. [Google Scholar] [CrossRef]
- Peng, X.; Horn, R. Time-dependent, anisotropic pore structure and soil strength in a 10-year period after intensive tractor wheeling under conservation and conventional tillage. J. Plant Nutr. Soil Sci. 2008, 171, 936–944. [Google Scholar] [CrossRef]
- Berisso, F.E.; Schjønning, P.; Keller, T.; Lamandé, M.; Etana, A.; de Jonge, L.; Iversen, B.V.; Arvidsson, J.; Forkman, J. Persistent effects of subsoil compaction on pore characteristics and functions in a loamy soil. Soil Tillage Res. 2012, 122, 42–51. [Google Scholar] [CrossRef]
- Keller, T.; Colombi, T.; Ruiz, S.; Manalili, M.P.; Rek, J.; Stadelmann, V.; Wunderli, H.; Breitenstein, D.; Reiser, R.; Oberholzer, H.; et al. Long-Term Soil Structure Observatory for Monitoring Post-Compaction Evolution of Soil Structure. Vadose Zone J. 2017, 16, 1–16. [Google Scholar] [CrossRef]
- Lal, R. Soils and world food security. Soil Tillage Res. 2009, 102, 1–4. [Google Scholar] [CrossRef]
- Hamza, M.A.; Anderson, W.K. Soil compaction in cropping systems. A review of the nature, causes and possible solutions. Soil Tillage Res. 2005, 82, 121–145. [Google Scholar] [CrossRef]
- Jabro, J.D.; Allen, B.L.; Rand, T.; Dangi, S.R.; Campbell, J.W. Effect of Previous Crop Roots on Soil Compaction in 2 Yr Rotations under a No-Tillage System. Land 2021, 10, 202. [Google Scholar] [CrossRef]
- Dick, R.P. A review: Long-term effects of agricultural systems on soil biochemical and microbial parameters. Agric. Ecosyst. Environ. 1992, 40, 25–36. [Google Scholar] [CrossRef]
- Nissen, T.M.; Wander, M.M. Management and Soil-Quality Effects on Fertilizer-Use Efficiency and Leaching. Soil Sci. Soc. Am. J. 2003, 67, 1524–1532. [Google Scholar] [CrossRef]
- Barak, P.; Jobe, B.O.; Krueger, A.R.; Peterson, L.A.; Laird, D.A. Effects of long-term soil acidification due to nitrogen fertilizer inputs in Wisconsin. Plant Soil 1997, 197, 61–69. [Google Scholar] [CrossRef]
- O’Brien, S.L.; Jastrow, J.D.; Grimley, D.A.; Gonzalez-Meler, M.A. Edaphic controls on soil organic carbon stocks in restored grasslands. Geoderma 2015, 251–252, 117–123. [Google Scholar] [CrossRef]
- Donkor, N.T.; Gedir, J.V.; Hudson, R.J.; Bork, E.W.; Chanasyk, D.S.; Naeth, M.A. Impacts of grazing systems on soil compaction and pasture production in Alberta. Can. J. Soil Sci. 2002, 82, 1–8. [Google Scholar] [CrossRef]
- Cherubin, M.R.; Carvalho, J.L.N.; Cerri, C.E.P.; Nogueira, L.A.H.; Souza, G.M.; Cantarella, H. Land Use and Management Effects on Sustainable Sugarcane-Derived Bioenergy. Land 2021, 10, 72. [Google Scholar] [CrossRef]
- Augustine, D.J.; Frank, D.A. Effects of migratory grazers on spatial heterogeneity of soil nitrogen properties in a grassland ecosystem. Ecology 2001, 82, 3149–3162. [Google Scholar]
- Hamilton, E.W.; Frank, D.A.; Hinchey, P.M.; Murray, T.R. Defoliation induces root exudation and triggers positive rhizospheric feedbacks in a temperate grassland. Soil Bio. Biochem. 2008, 40, 2865–2873. [Google Scholar] [CrossRef]
- Giese, M.; Gao, Y.Z.; Zhao, Y.; Pan, Q.; Lin, S.; Peth, S.; Brueck, H. Effects of grazing and rainfall variability on root and shoot decomposition in a semi-arid grassland. Appl. Soil Ecol. 2009, 41, 8–18. [Google Scholar] [CrossRef]
- Tracy, B.F.; Frank, D.A. erbivore influence on soil microbial biomass and nitrogen mineralization in a northern grassland ecosystem: Yellowstone National Park. Oecologia 1998, 114, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Stark, S.; Strömmer, R.; Tuomi, J. Reindeer grazing and soil microbial processes in two suboceanic and two subcontinental tundra heaths. Oikos 2002, 97, 69–78. [Google Scholar] [CrossRef]
- Sankaran, M.; Augustine, D.J. Large herbivores suppress decomposer abundance in a semiarid grazing ecosystem. Ecology 2004, 85, 1052–1061. [Google Scholar] [CrossRef]
- Leidinger, J.L.G.; Gossner, M.M.; Weisser, W.W.; Koch, C.; Rosadio Cayllahua, Z.L.; Podgaiski, L.R.; Duarte, M.M.; Araújo, A.S.F.; Overbeck, G.E.; Hermann, J.-M.; et al. Historical and recent land use affects ecosystem functions in subtropical grasslands in Brazil. Ecosphere 2017, 8, e02032. [Google Scholar] [CrossRef]
- Matamala, R.; Jastrow, J.D.; Miller, R.M.; Garten, C.T. Temporal changes in C and N stocks of restored prairie: Implications for C sequestration strategies. Ecol. Appl. 2008, 18, 1470–1488. [Google Scholar] [CrossRef]
- Walker, L.R.; Wardle, D.A.; Bardgett, R.D.; Clarkson, B.D. The use of chronosequences in studies of ecological succession and soil development. J. Ecol. 2010, 98, 725–736. [Google Scholar] [CrossRef]
- Baer, S.G.; Bach, E.M.; Meyer, C.K.; Du Preez, C.C.; Six, J. Belowground Ecosystem Recovery During Grassland Restoration: South African Highveld Compared to US Tallgrass Prairie. Ecosystems 2015, 18, 390–403. [Google Scholar] [CrossRef]
- Perring, M.P.; De Frenne, P.; Baeten, L.; Maes, S.L.; Depauw, L.; Blondeel, H.; Carón, M.M.; Verheyen, K. Global environmental change effects on ecosystems: The importance of land-use legacies. Glob. Chang. Biol. 2016, 22, 1361–1371. [Google Scholar] [CrossRef]
- Van Der Molen, M.K.; Dolman, A.J.; Ciais, P.; Eglin, T.; Gobron, N.; Law, B.E.; Meir, P.; Peters, W.; Phillips, O.L.; Reichstein, M.; et al. Drought and ecosystem carbon cycling. Agric. For. Meteorol. 2011, 151, 765–773. [Google Scholar] [CrossRef]
- Inglett, K.S.; Inglett, P.W.; Reddy, K.R.; Osborne, T.Z. Temperature sensitivity of greenhouse gas production in wetland soils of different vegetation. Biogeochemistry 2012, 108, 77–90. [Google Scholar] [CrossRef]
- Szafranek-Nakonieczna, A.; Stêpniewska, Z. Aerobic and anaerobic respiration in profiles of Polesie Lubelskie peatlands. Int. Agrophys. 2014, 28, 219–229. [Google Scholar] [CrossRef]
- Chen, H.; Zou, J.; Cui, J.; Nie, M.; Fang, C. Wetland drying increases the temperature sensitivity of soil respiration. Soil Bio. Biochem. 2018, 120, 24–27. [Google Scholar] [CrossRef]
- Rhoades, C.C.; Eckert, G.E.; Coleman, D.C. Soil Carbon Differences among Forest, Agriculture, and Secondary Vegetation in Lower Montane Ecuador. Ecol. Appl. 2000, 10, 497–505. [Google Scholar] [CrossRef]
- Chen, W.; Huang, D.; Liu, N.; Zhang, Y.; Badgery, W.B.; Wang, X.; Shen, Y. Improved grazing management may increase soil carbon sequestration in temperate steppe. Sci. Rep. 2015, 5, 10892. [Google Scholar] [CrossRef] [PubMed]
- Geraei, D.S.; Hojati, S.; Landi, A.; Cano, A.F. Total and labile forms of soil organic carbon as affected by land use change in southwestern Iran. Geoderma Reg. 2016, 7, 29–37. [Google Scholar] [CrossRef]
- Lucci, G.M. Pastures and drought: A review of processes and implications for nitrogen and phosphorus cycling in grassland systems. Soil Res. 2019, 57, 101–112. [Google Scholar] [CrossRef]
- Soil Survey Staff, Natural Resources Conservation Service, United States Department of Agriculture. Web Soil Survey. Available online: http://websoilsurvey.sc.egov.usda.gov/ (accessed on 25 October 2018).
- Midwestern Regional Climate Center, Illinois State Water Survey, Prairie Research Institute, University of Illinois at Urbana-Champaign. cli-MATE: MRCC Application Tools Environment. Available online: http://mrcc.illinois.edu/CLIMATE/welcome.jsp (accessed on 31 October 2018).
- Soil Survey Staff, Natural Resources Conservation Service, United States Department of Agriculture. Official Soil Series Descriptions. Available online: https://www.nrcs.usda.gov/wps/portal/nrcs/detail/soils/survey/geo/?cid=nrcs142p2_053587 (accessed on 25 October 2018).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: http://www.R-project.org/ (accessed on 24 December 2017).
- Tang, Y.; Horikoshi, M.; Li, W. Ggfortify: Unified Interface to Visualize Statistical Result of Popular R Packages. R J. 2016, 8, 478–489. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- National Integrated Drought Information System, U.S. National Integrated Drought Information System NIDIS—Drought.gov—U.S. Drought Portal. United States. 2008. Available online: https://www.drought.gov/about (accessed on 31 October 2018).
- Larson, D.L.; Hernández, D.L.; Larson, J.L.; Leone, J.B.; Pennarola, N. Management of remnant tallgrass prairie by grazing or fire: Effects on plant communities and soil properties. Ecosphere 2020, 11. [Google Scholar] [CrossRef]
- Munjonji, L.; Ayisi, K.K.; Mudongo, E.I.; Mafeo, T.P.; Behn, K.; Mokoka, M.V.; Linstädter, A. Disentangling Drought and Grazing Effects on Soil Carbon Stocks and CO2 Fluxes in a Semi-Arid African Savanna. Front. Environ. Sci. 2020, 8. [Google Scholar] [CrossRef]
- Barber, N.A.; Chantos-Davidson, K.M.; Amel Peralta, R.; Sherwood, J.P.; Swingley, W.D. Soil microbial community composition in tallgrass prairie restorations converge with remnants across a 27-year chronosequence. Environ. Microbiol. 2017, 19, 3118–3131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; He, N.; Liu, C.; Xu, L.; Chen, Z.; Li, Y.; Wang, R.; Yu, G.; Sun, W.; Xiao, C.; et al. Variation and evolution of C:N ratio among different organs enable plants to adapt to N-limited environments. Glob. Chang. Biol. 2020, 26, 2534–2543. [Google Scholar] [CrossRef]
- Silver, W.L.; Miya, R.K. Global patterns in root decomposition: Comparisons of climate and litter quality effects. Oecologia 2001, 129, 407–419. [Google Scholar] [CrossRef]
- Jelinski, N.A.; Kucharik, C.J. Land-use Effects on Soil Carbon and Nitrogen on a U.S. Midwestern Floodplain. Soil Sci. Soc. Am. J. 2009, 73, 217–225. [Google Scholar] [CrossRef]
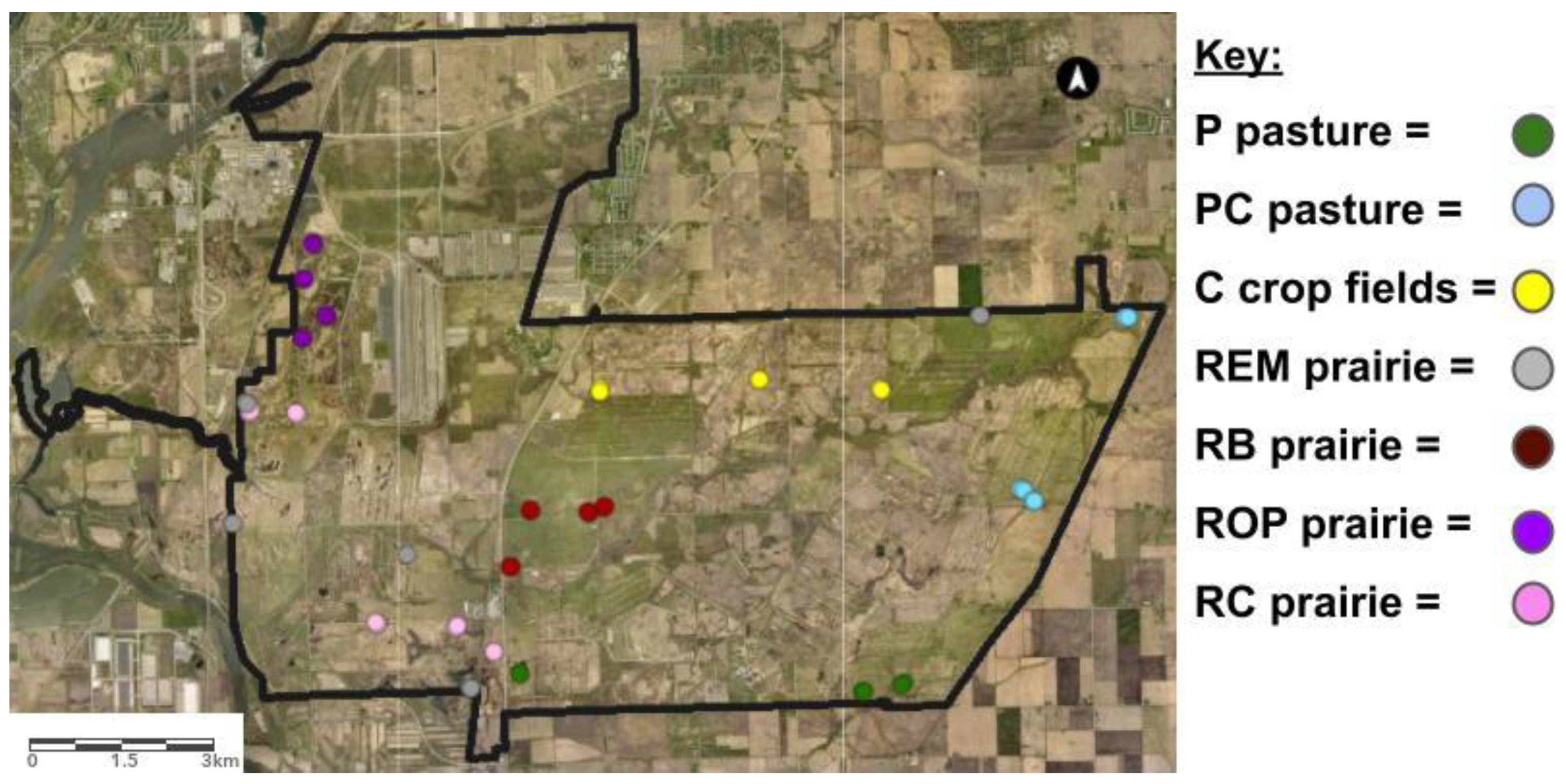
| Land-Use Histories | 1996 | 2000 | 2004 | 2008 | 2012 | 2018 |
|---|---|---|---|---|---|---|
| P | Pasture | Pasture | Pasture | Pasture | Pasture | Pasture |
| PC | Row crops | Pasture | Pasture | Pasture | Pasture | Pasture |
| C | Row crops | Row crops | Row crops | Row crops | Row crops | Row crops |
| REM | Remnant | Remnant | Remnant | Remnant | Remnant | Remnant |
| RB | Pasture/Row crops | Pasture | Pasture | Pasture | Pasture | Restoration (bison) |
| ROP | Pasture | Fallow | Fallow | Fallow | Fallow/Restoration | Restoration |
| RC | Pasture | Row crops | Row crops/Restoration | Restoration | Restoration | Restoration |
| Soil Bulk Density | Root C:N | Soil C:N | g C kg−1 Soil | g N kg−1 Soil | kg C m−2 | kg N m−2 | |
|---|---|---|---|---|---|---|---|
| T-statistic | −0.6 | 0.4 | 2.9 | −0.2 | −1.2 | 1.0 | −0.1 |
| p-value | 0.6 | 0.7 | 0.007 | 0.9 | 0.2 | 0.3 | 0.95 |
| 95% CI | −0.08–0.05 | −4.9–7.6 | −1.3–−0.2 | −3.6–3.0 | −0.4–0.1 | −0.1–0.3 | −0.02–0.02 |
| Year | 2008 | 2018 | ||
|---|---|---|---|---|
| Land-Use History | Root C:N | Soil C:N | Root C:N | Soil C:N |
| P | 42 ± 1 | 11.4 ± 0.2 | 49 ± 3 | 12.3 ± 0.6 |
| PC | 41 ± 4 | 12.2 ± 0.2 | 40 ± 3 | 12.1 ± 0.7 |
| C | 15 ± 3 | 10.6 ± 0.2 | 27 ± 2 | 13.5 ± 0.4 |
| REM | 54 ± 4 | 11.8 ± 0.2 | 38 ± 4 | 13.4 ± 0.5 |
| RB | 42 ± 4 | 12.0 ± 0.2 | 51 ± 8 | 12.3 ± 0.3 |
| ROP | 52 ± 7 | 12.3 ± 0.2 | 43 ± 5 | 11.3 ± 0.3 |
| RC | 45 ± 2 | 11.7 ± 0.2 | 55 ± 9 | 12.6 ± 0.5 |
| Land-Use History | δ1⁵N | δ13C | C3 Carbon Proportion |
|---|---|---|---|
| P | 5.31 ± 0.14 | −24.49 ± 0.34 | 0.78 ± 0.02 |
| PC | 5.72 ± 0.24 | −23.01 ± 0.38 | 0.69 ± 0.02 |
| C | 6.76 ± 0.08 | −21.11 ± 0.33 | 0.57 ± 0.02 |
| REM | 4.54 ± 0.88 | −22.78 ± 0.82 | 0.67 ± 0.05 |
| RB | 5.16 ± 0.27 | −24.28 ± 0.36 | 0.77 ± 0.02 |
| ROP | 4.97 ± 0.69 | −25.51 ± 0.65 | 0.84 ± 0.04 |
| RC | 5.84 ± 0.20 | −22.56 ± 0.60 | 0.66 ± 0.04 |
| Land-Use History | Ca | Mg | P | Cu | Mn | Na | NO3 | NH4 | K | Zn |
|---|---|---|---|---|---|---|---|---|---|---|
| P | 48 ± 18 | 84 ± 11 | 11 ± 3 | 12 ± 2 | 40 ± 4 | 41 ± 12 | 52 ± 18 | 30 ± 6 | 286 ± 57 | 6 ± 1 |
| PC | 52 ± 4 | 76 ± 12 | 11 ± 1 | 9 ± 1 | 51 ± 19 | 49 ± 6 | 32 ± 1 | 18 ± 2 | 200 ± 43 | 6 ± 1 |
| C | 47 ± 26 | 79 ± 17 | 7 ± 2 | 15 ± 5 | 15 ± 7 | 51 ± 23 | 33 ± 2 | 3 ± 2 | 180 ± 51 | 4 ± 1 |
| REM | 89 ± 18 | 103 ± 5 | 15 ± 3 | 8 ± 2 | 27 ± 4 | 73 ± 12 | 41 ± 6 | 20 ± 3 | 184 ± 26 | 10 ± 2 |
| RB | 50 ± 8 | 88 ± 10 | 19 ± 9 | 8 ± 1 | 37 ± 7 | 32 ± 8 | 41 ± 9 | 17 ± 1 | 247 ± 22 | 6 ± 2 |
| ROP | 124 ± 5 | 32 ± 7 | 20 ± 6 | 16 ± 6 | 37 ± 6 | 86 ± 9 | 45 ± 9 | 12 ± 2 | 231 ± 19 | 10 ± 1 |
| RC | 82 ± 19 | 67 ± 17 | 18 ± 4 | 14 ± 6 | 30 ± 5 | 68 ± 6 | 23 ± 7 | 13 ± 2 | 170 ± 19 | 6 ± 1 |
| Response Variable | Land-Use History | Estimate | 95% CI |
|---|---|---|---|
| Relative Change in g C kg−1 soil | ROP (Intercept) | −2.68 | −20.07–14.72 |
| P | −2.74 | −27.34–21.86 | |
| PC | 14.23 | −12.34–40.80 | |
| C | 4.46 | −22.11–31.03 | |
| REM | 17.11 | −6.23–40.44 | |
| RB | 5.75 | −17.59–29.09 | |
| RC | −15.39 | −41.96–11.19 | |
| Relative Change in g N kg−1 soil | ROP (Intercept) | 6.33 | −10.97–23.63 |
| P | −0.95 | −27.37–25.48 | |
| PC | 0.07 | −26.35–26.50 | |
| C | −34.83 | −61.25–−8.41 | |
| REM | −15.8 | −39.01–7.41 | |
| RB | −14.09 | −38.55–10.38 | |
| RC | 3.03 | −20.18–26.24 | |
| g C m−2 yr−1 | ROP (Intercept) | 63.11 | 9–118 |
| P | −32.61 | −116–51 | |
| PC | −53.58 | −137–30 | |
| C | −124.25 | −207–−41 | |
| REM | −73.34 | −146–−0.37 | |
| RB | −43.66 | −121–33 | |
| RC | −45.81 | −119–27 | |
| g N m−2 yr−1 | ROP (Intercept) | 7.02 | 2.24–11.79 |
| P | −5.99 | −13.29–1.31 | |
| PC | −4.74 | −12.04–2.56 | |
| C | −14.82 | −22.13–−7.52 | |
| REM | −10.44 | −16.85–−4.03 | |
| RB | −5.78 | −12.54–0.98 | |
| RC | −7.84 | −14.26–−1.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reed, N.; Molano-Flores, B.; Dias de Oliveira, E.; Meraz, E.; Umar, S.; Whelan, C.J.; Gonzalez-Meler, M.A. Does Pastoral Land-Use Legacy Influence Topsoil Carbon and Nitrogen Accrual Rates in Tallgrass Prairie Restorations? Land 2021, 10, 735. https://doi.org/10.3390/land10070735
Reed N, Molano-Flores B, Dias de Oliveira E, Meraz E, Umar S, Whelan CJ, Gonzalez-Meler MA. Does Pastoral Land-Use Legacy Influence Topsoil Carbon and Nitrogen Accrual Rates in Tallgrass Prairie Restorations? Land. 2021; 10(7):735. https://doi.org/10.3390/land10070735
Chicago/Turabian StyleReed, Nichole, Brenda Molano-Flores, Eduardo Dias de Oliveira, Erika Meraz, Samira Umar, Christopher J. Whelan, and Miquel A. Gonzalez-Meler. 2021. "Does Pastoral Land-Use Legacy Influence Topsoil Carbon and Nitrogen Accrual Rates in Tallgrass Prairie Restorations?" Land 10, no. 7: 735. https://doi.org/10.3390/land10070735
APA StyleReed, N., Molano-Flores, B., Dias de Oliveira, E., Meraz, E., Umar, S., Whelan, C. J., & Gonzalez-Meler, M. A. (2021). Does Pastoral Land-Use Legacy Influence Topsoil Carbon and Nitrogen Accrual Rates in Tallgrass Prairie Restorations? Land, 10(7), 735. https://doi.org/10.3390/land10070735






