Soil Characteristics and Fertility of the Unique Jarrah Forest of Southwestern Australia, with Particular Consideration of Plant Nutrition and Land Rehabilitation
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling of Soil Materials
2.2. Chemical Properties of Soils
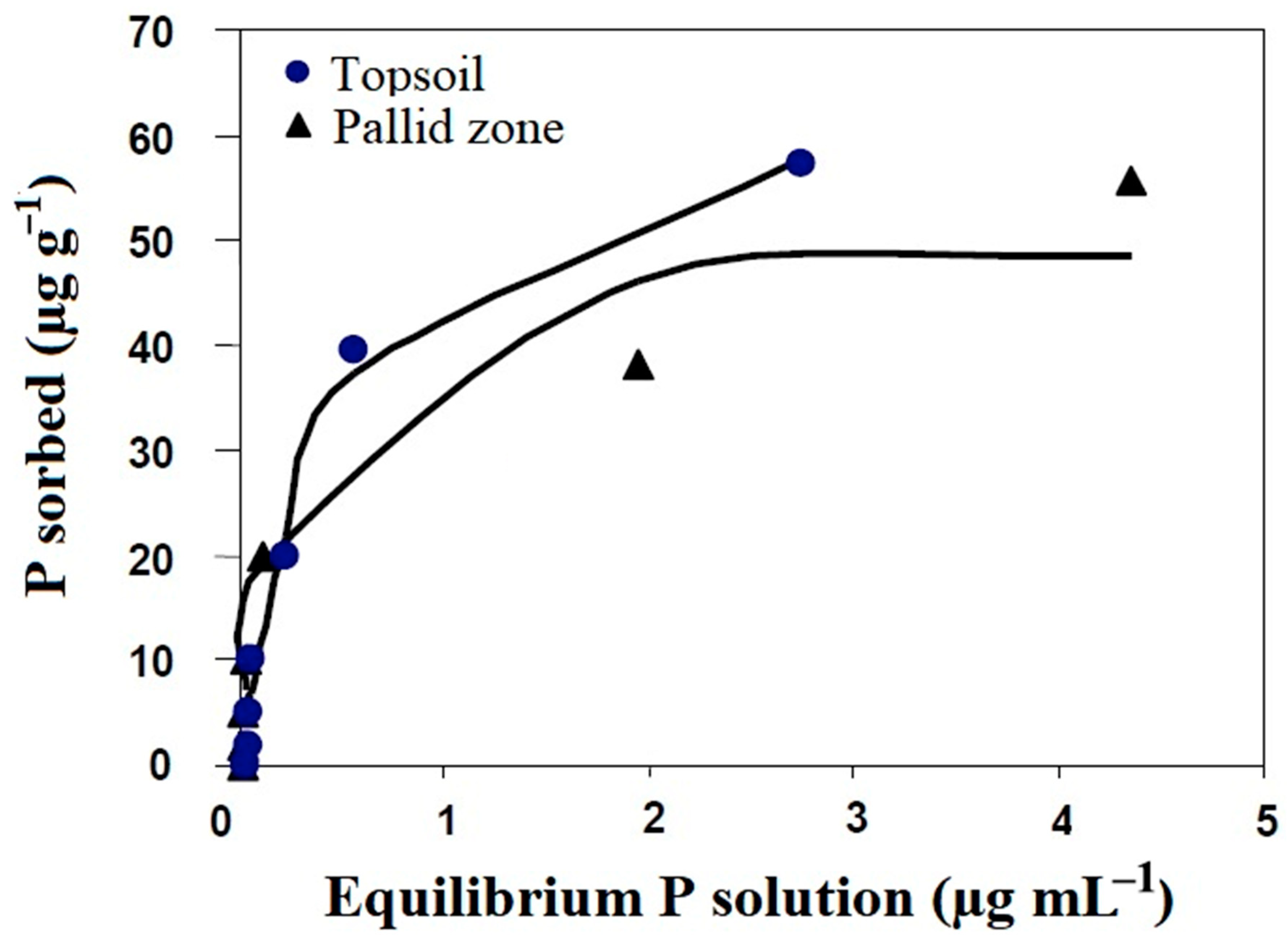
2.3. Phosphorus Sorption Isotherm
2.4. 31P Nuclear Magnetic Resonance (31P-NMR) Spectroscopy
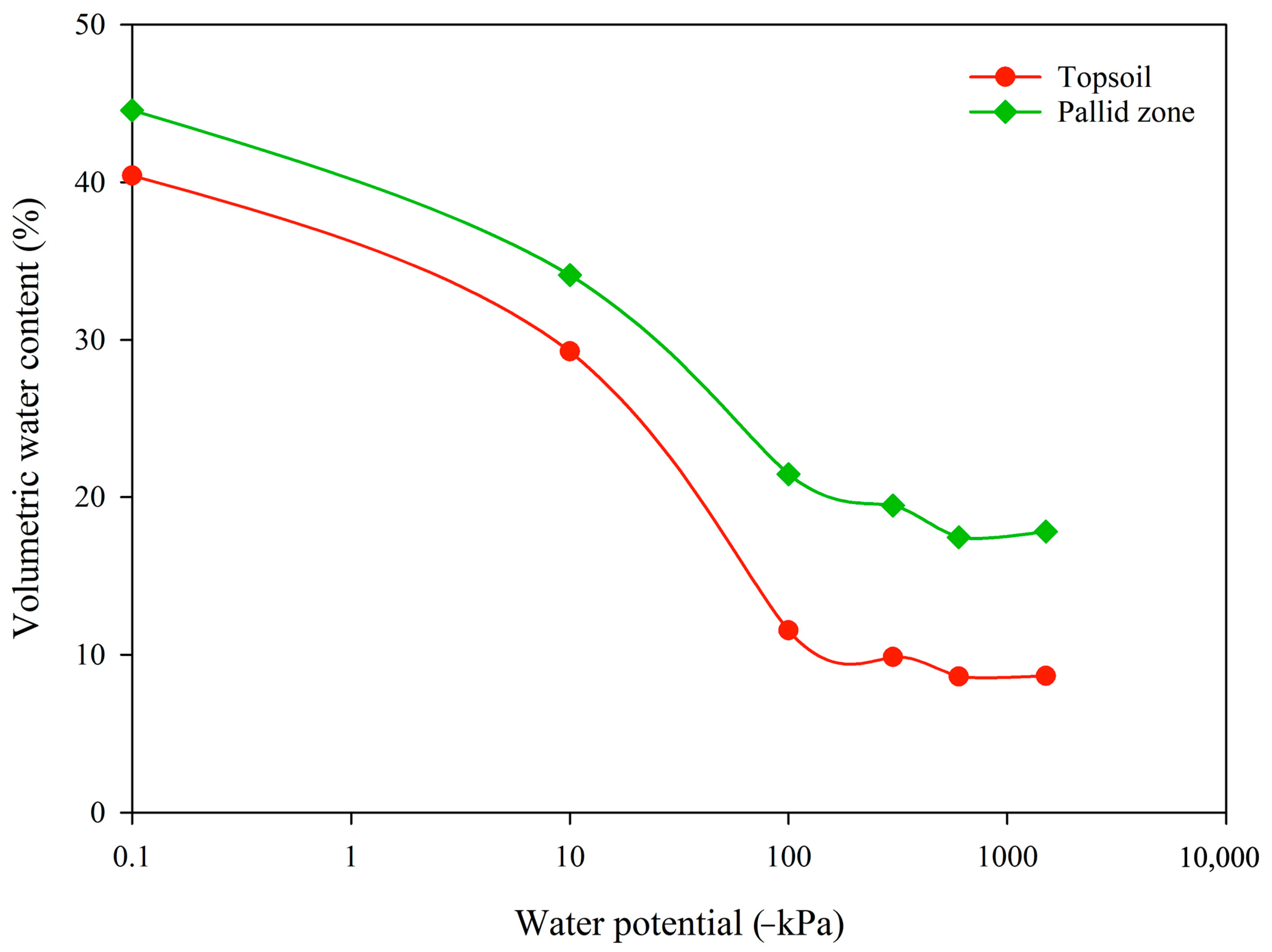
2.5. Physical Properties of Soils
3. Results
4. Discussion
4.1. Basic Properties of Soils
4.2. Chemical Fertility
4.3. Phosphorus Fertility
4.4. Key Physical Properties
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Connell, A.M.; Grove, T.S.; Dimmock, G.M. Nutrients in the litter on jarrah forest soils. Aust. J. Ecol. 1978, 3, 253–260. [Google Scholar] [CrossRef]
- Hopper, S.D.; Gioia, P. The southwest Australian floristic region: Evolution and conservation of a global hot spot of biodiversity. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 623–650. [Google Scholar] [CrossRef]
- Hopper, S.D. OCBIL theory: Towards an integrated understanding of the evolution, ecology and conservation of biodiversity on old, climatically buffered, infertile landscapes. Plant Soil. 2009, 322, 49–86. [Google Scholar] [CrossRef]
- Daws, M.I.; Walters, S.J.; Harris, R.J.; Tibbett, M.; Grigg, A.H.; Morald, T.K.; Hobbs, R.J.; Standish, R.J. Nutrient enrichment diminishes plant diversity and density, and alters long-term ecological trajectories, in a biodiverse forest restoration. Ecol. Eng. 2021, 165, 106222. [Google Scholar] [CrossRef]
- Mulcahy, M.J.; Churchward, H.M.; Dimmock, G.M. Landforms and soils on an uplifted peneplain in the darling range, western australia. Aust. J. Soil Res. 1972, 10, 1–14. [Google Scholar] [CrossRef]
- Butt, C.R.M. The nature and origin of the lateritic weathering mantle, with particular reference to Western Australia. Geophys. Prospect. Deep. Weather. Terrains 1981, 6, 11–29. [Google Scholar]
- Bettenay, E.; Smith, R.E.; Butt, C.R.M. Physical features of the Yilgarn Block. In Proceedings of the 25th International Geological Congress, Sydney, Australia, 16–25 August 1976; pp. 5–10. [Google Scholar]
- Lambers, H.; Brundrett, M.C.; Raven, J.A.; Hopper, S.D. Plant mineral nutrition in ancient landscapes: High plant species diversity on infertile soils is linked to functional diversity for nutritional strategies. Plant Soil. 2011, 348, 7–27. [Google Scholar] [CrossRef]
- Nemchin, A.A.; Pidgeon, R.T. Evolution of the Darling range batholith, Yilgarn craton, western Australia: A SHRIMP zircon study. J. Petrol. 1997, 38, 625–649. [Google Scholar] [CrossRef]
- Kirke, E.A. Alumina from Darling Range bauxite. Proc. Aust. Inst. Min. Metall. Conf. Perth 1983, 288, 553–564. [Google Scholar]
- Walters, S.J.; Harris, R.J.; Daws, M.I.; Gillett, M.J.; Richardson, C.G.; Tibbett, M.; Grigg, A.H. The benefits of fertiliser application on tree growth are transient in restored jarrah forest. Trees For. People 2021, 5, 100112. [Google Scholar] [CrossRef]
- Daws, M.I.; Grigg, A.H.; Blackburn, C.; Barker, J.M.; Standish, R.J.; Tibbett, M. Initial conditions can have long-term effects on plant species diversity in jarrah forest restored after bauxite mining. In Proceedings of the Mine Closure 2022: 15th International Conference on Mine Closure, Australian Centre for Geomechanics, Perth, Australia, 4–6 October 2022; Fourie, A.B., Tibbett, M., Boggs, G., Eds.; pp. 857–868. [Google Scholar]
- Koch, J.M.; Hobbs, R.J. Synthesis: Is Alcoa Successfully Restoring a Jarrah Forest Ecosystem after Bauxite Mining in Western Australia? Restor. Ecol. 2007, 15, S137–S144. [Google Scholar] [CrossRef]
- Koch, J.M. Mining and Ecological Restoration in the Jarrah Forest of Western Australia. In Mining in Ecologically Sensitive Landscapes; Routledge: London, UK, 2015; pp. 111–140. [Google Scholar]
- Spain, A.V.; Tibbett, M.; Ridd, M.; McLaren, T.I. Phosphorus dynamics in a tropical forest soil restored after strip mining. Plant Soil. 2018, 427, 105–123. [Google Scholar] [CrossRef]
- Tibbett, M. Large-scale mine site restoration of Australian eucalypt forests after bauxite mining: Soil management and ecosystem development. Ecol. Ind. Pollut. 2010, 309–326. [Google Scholar]
- Banning, N.C.; Lalor, B.M.; Grigg, A.H.; Phillips, I.R.; Colquhoun, I.J.; Jones, D.L.; Murphy, D.V. Rehabilitated Mine-Site Management, Soil Health and Climate Change. In Soil Health and Climate Change; Springer: Berlin/Heidelberg, Germany, 2011; pp. 287–314. [Google Scholar]
- Koch, J.M. Restoring a jarrah forest understorey vegetation after bauxite mining in Western Australia. Restor. Ecol. 2007, 5, 137–144. [Google Scholar] [CrossRef]
- Standish, R.J.; Morald, T.K.; Koch, J.M.; Hobbs, R.J.; Tibbett, M. Restoring jarrah forest after bauxite mining in Western Australia–the effect of fertiliser on floristic diversity and composition. In Proceedings of the Third International Seminar on Mine Closure, Johannesburg, South Africa, 14–17 October 2008; pp. 14–17. [Google Scholar]
- Tibbett, M.; Daws, M.I.; George, S.J.; Ryan, M.H. The where, when and what of phosphorus fertilisation for seedling establishment in a biodiverse jarrah forest restoration after bauxite mining in Western Australia. Ecol. Eng. 2020, 153, 105907. [Google Scholar] [CrossRef]
- Daws, M.I.; Standish, R.J.; Koch, J.M.; Morald, T.K.; Tibbett, M.; Hobbs, R.J. Phosphorus fertilisation and large legume species affect jarrah forest restoration after bauxite mining. For. Ecol. Manag. 2015, 354, 10–17. [Google Scholar] [CrossRef]
- Tibbett, M.; O’Connor, R.; Daws, M. Too much of a good thing: Phosphorus over-fertilisation in rehabilitated landscapes of high biodiversity value. In Proceedings of the 13th International Conference on Mine Closure, Crawley, Australia, 5 September 2019; pp. 651–666. [Google Scholar] [CrossRef]
- DeJong, J.; Tibbett, M.; Fourie, A. Geotechnical systems that evolve with ecological processes. Environ. Earth Sci. 2015, 73, 1067–1082. [Google Scholar] [CrossRef]
- di Carlo, E.; Chen, C.R.; Haynes, R.J.; Phillips, I.R.; Courtney, R. Soil quality and vegetation performance indicators for sustainable rehabilitation of bauxite residue disposal areas: A review. Soil Res. 2019, 57, 419–446. [Google Scholar] [CrossRef]
- Worthington, T.; Braimbridge, M.F.; Vlahos, S.; Amoah, N.; Tibbett, M. Geotechnical Materials and Wood Wastes for Embankment Stabilisation of Tailing Storage Facilities. In Geotechnical Materials and Wood Wastes for Embankment Stabilisation of Tailing Storage Facilities; Australian Centre for Geomechanics: Crawley, Australia, 2007; pp. 655–664. [Google Scholar]
- Rayment, G.E.; Higginson, F.R. Australian Laboratory Handbook of Soil and Water Chemical Methods; Inkata Press Pty Ltd.: Melbourne, Australia, 1992. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Keeney, D.R.; Nelson, D.W. Inorganic Forms of Nitrogen. Methods of Soil Analysis, Part 2; Wiley: New York, NY, USA, 1982; pp. 643–698. [Google Scholar]
- Colwell, J.D. The estimation of the phosphorus fertilizer requirements of wheat in southern New South Wales by soil analysis. Aust. J. Exp. Agric. 1963, 3, 190–197. [Google Scholar] [CrossRef]
- Blair, G.J.; Chinoim, N.; Lefroy, R.D.B.; Anderson, G.C.; Crocker, G.J. A soil sulfur test for pastures and crops. Soil Res. 1991, 29, 619–626. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Fox, R.L.; Kamprath, E.J. Phosphate sorption isotherms for evaluating the phosphate requirements of soils. Soil Sci. Soc. Am. J. 1970, 34, 902–907. [Google Scholar] [CrossRef]
- Olsen, S.R.; Watanabe, F.S. A method to determine a phosphorus adsorption maximum of soils as measured by the Langmuir isotherm. Soil Sci. Soc. Am. J. 1957, 21, 144–149. [Google Scholar] [CrossRef]
- Sanyal, S.K.; de Datta, S.K. Chemistry of Phosphorus Transformations in Soil. In Advances in Soil Science; Springer: Berlin/Heidelberg, Germany, 1991; pp. 1–120. [Google Scholar]
- Barrow, N.J. The description of phosphate adsorption curves. J. Soil Sci. 1978, 29, 447–462. [Google Scholar] [CrossRef]
- Bolster, C.H.; Hornberger, G.M. On the use of linearized Langmuir equations. Soil Sci. Soc. Am. J. 2007, 71, 1796–1806. [Google Scholar] [CrossRef]
- Ratkowsky, D.A. A statistical study of seven curves for describing the sorption of phosphate by soil. J. Soil Sci. 1986, 37, 183–189. [Google Scholar] [CrossRef]
- Ozanne, P.G.; Shaw, T.C. Phosphate sorption by soils as a measure of the phosphate requirement for pasture growth. Aust. J. Agric. Res. 1967, 18, 601–612. [Google Scholar] [CrossRef]
- Cade-Menun, B.J.; Preston, C.M. A comparison of soil extraction procedures for 31P NMR spectroscopy. Soil Sci. 1996, 161, 770–785. [Google Scholar] [CrossRef]
- Newman, R.H.; Tate, K.R. Soil phosphorus characterisation by 31P nuclear magnetic resonance. Commun. Soil Sci. Plant Anal. 1980, 11, 835–842. [Google Scholar] [CrossRef]
- Zhang, T.Q.; Mackenzie, A.F.; Sauriol, F. Nature of soil organic phosphorus as affected by long-term fertilization under continuous corn (Zea Mays L.): A 31P NMR study. Soil Sci. 1999, 164, 662–670. [Google Scholar] [CrossRef]
- Klute, A.; Page, A.L. Methods of Soil Analysis. Part 1. Physical and Mineralogical Methods. Part 2. In Chemical and Microbiological Properties; American Society of Agronomy, Inc.: Madison, WI, USA, 1986. [Google Scholar]
- McDonald, R.C.; Isbell, R.F.; Speight, J.G.; Walker, J.; Hopkins, M.S. Australian Soil and Land Survey: Field Handbook; CSIRO Publishing: Victoria, Australia, 1998. [Google Scholar]
- Cresswell, H.P.; Green, T.W.; McKenzie, N.J. The adequacy of pressure plate apparatus for determining soil water retention. Soil Sci. Soc. Am. J. 2008, 72, 41–49. [Google Scholar] [CrossRef]
- Slattery, W.J.; Conyers, M.K.; Aitken, R.L. Soil pH, Aluminium, Manganese and Lime Requirement. In Soil Analysis: An Interpretation Manual; CSIRO Publishing: Victoria, Australia, 1999; pp. 103–128. [Google Scholar]
- Shaw, R.J. Soil Salinity-Electrical Conductivity and Chloride. In Soil Analysis: An Interpretation Manual; CSIRO Publishing: Victoria, Australia, 1999; pp. 129–145. [Google Scholar]
- Purdie, B.R.; Tille, P.J.; Schoknecht, N.R. Soil-Landscape Mapping in South-Western Australia: An Overview of Methodology and Outputs; Department of Agriculture and Food: Perth, Australia, 2004.
- Havel, J.J.; Dell, B.; Malajczuk, N. The Jarrah Forest: A Complex Mediterranean Ecosystem; Kluwer Academic Publishers: Alphen aan den Rijn, The Netherlands, 1989. [Google Scholar]
- McArthur, W.M. Reference Soils of South-Western Australia; ASSSI: Perth, WA, Australia, 1991. [Google Scholar]
- Ward, S.C. Soil development on rehabilitated bauxite mines in south-west Australia. Soil Res. 2000, 38, 453–464. [Google Scholar] [CrossRef]
- Hazelton, P.; Murphy, B. Interpreting Soil Test Results: What Do All the Numbers Mean? CSIRO Publishing: Victoria, Australia, 2016. [Google Scholar]
- Schofield, N.J.; Stoneman, G.L.; Loh, I.C. Hydrology of the Jarrah Forest. In The Jarrah Forest; Springer: Berlin/Heidelberg, Germany, 1989; pp. 179–201. [Google Scholar]
- Ferdowsian, R.; George, R.; Lewis, F.; McFarlane, D.; Short, R.; Speed, R. The extent of dryland salinity in Western Australia. In Proceedings of the Productive Use of Saline Lands Conference, Albany, Australia, 25–30 March 1996; pp. 89–97. [Google Scholar]
- Baldock, J.; Skjemstad, J.O. Soil Organic Carbon/Soil Organic Matter; CSIRO Publishing: Victoria, Australia, 1999. [Google Scholar]
- Moore, T.R.; Turunen, J. Carbon accumulation and storage in mineral subsoil beneath peat. Soil Sci. Soc. Am. J. 2004, 68, 690–696. [Google Scholar] [CrossRef]
- Hingston, F.J.; O’Connell, A.M.; Grove, T.S. Nutrient Cycling in Jarrah Forest. In The Jarrah Forest; Springer: Berlin/Heidelberg, Germany, 1989; pp. 155–177. [Google Scholar]
- George, S.J.; Kelly, R.N.; Greenwood, P.F.; Tibbett, M. Soil carbon and litter development along a reconstructed biodiverse forest chronosequence of South-Western Australia. Biogeochemistry 2010, 101, 197–209. [Google Scholar] [CrossRef]
- Gibbons, F.R. Soil Mapping in Australia. In Soils: An Australian Viewpoint; Academic Press: Cambridge, MA, USA, 1983. [Google Scholar]
- Schwertmann, U.; Taylor, R.M. Iron oxides. Minerals in soil environments. Miner. Soil Environ. 1989, 1, 379–438. [Google Scholar]
- Kome, G.K.; Enang, R.K.; Tabi, F.O.; Yerima, B.P.K. Influence of Clay Minerals on Some Soil Fertility Attributes: A Review. Open J. Soil Sci. 2019, 09, 155–188. [Google Scholar] [CrossRef]
- Bohn, H.; McNeal, B.; O’Connor, G. Soil Chemistry, 3rd ed.; Wiley Inter-Science: New York, NY, USA, 2001. [Google Scholar]
- Lambers, H.; Finnegan, P.M.; Laliberté, E.; Pearse, S.J.; Ryan, M.H.; Shane, M.W.; Veneklaas, E.J. Phosphorus nutrition of Proteaceae in severely phosphorus-impoverished soils: Are there lessons to be learned for future crops? Plant Physiol. 2011, 156, 1058–1066. [Google Scholar] [CrossRef]
- Rengasamy, P.; Churchman, G.J. Cation Exchange Capacity, Exchangeable Cations and Sodicity; CSIRO Publishing: Victoria, Australia, 1999. [Google Scholar]
- Tucker, B.M. Basic Exchangeable Cations; American Society of Agronomy, Inc.: Madison, WI, USA, 1983. [Google Scholar]
- Sumner, M.E.; Miller, W.P. Chapter 40 Cation Exchange Capacity and Exchange Coefficients; Wiley: New York, NY, USA, 1996. [Google Scholar]
- O’Connell, A.M.; Grove, T.S. Biomass Production, Nutrient Uptake and Nutrient Cycling in the Jarrah (Eucalyptus Marginata) and Karri (Eucalyptus Diversicolor) Forests of South-Western Australia; CSIRO Publishing: Victoria, Australia, 1996. [Google Scholar]
- Strong, W.M.; Mason, M.G. Nitrogen. In Soil Analysis: An Interpretation Manual; CSIRO Publishing: Victoria, Australia, 1999. [Google Scholar]
- Adams, M.A.; Attiwill, P.M. Role of Acacia spp. in nutrient balance and cycling in regenerating Eucalyptus regnans F. Muell. forests. II. Field studies of acetylene reduction. Aust. J. Bot. 1984, 32, 217–223. [Google Scholar] [CrossRef]
- Grove, T.S.; Malajczuk, N. Nitrogen Fixation by Legume Understorey in Karri (Eucalyptus Diversicolor) Forest. Current Perspectives in Nitrogen Fixation; FAO: Roma, Italy, 1981. [Google Scholar]
- Chapin, F.S.; Moilanen, L.; Kielland, K. Preferential use of organic nitrogen for growth by a non-mycorrhizal arctic sedge. Nature 1993, 361, 150–153. [Google Scholar] [CrossRef]
- Bell, R.W. Boron. In Soil Analysis: An Interpretation Manual; Peverill, K., Sparrow, L., Reuter, D.J., Eds.; CSIRO Publishing: Victoria, Australia, 1999; pp. 309–317. [Google Scholar]
- Brennan, R.F. Zinc. In Soil Guide: A Handbook for Understanding and Managing Agricultural Soil; Department of Primary Industries and Regional Development: Perth, Australia, 2004. [Google Scholar]
- Brennan, R.F.; Best, E. Copper. In Soil Analysis: An Interpretation Manual; CSIRO Publishing: Victoria, Australia, 1999. [Google Scholar]
- Uren, N.C. Manganese. In Soil Analysis: An Interpretation Manual; CSIRO Publishing: Victoria, Australia, 1999. [Google Scholar]
- Armour, J.D.; Brennan, R.F. Zinc. In Soil Analysis: An Interpretation Manual; CSIRO Publishing: Victoria, Australia, 1999. [Google Scholar]
- Bruce, R.C.; Rayment, G.E. Analytical Methods and Interpretations Used by the Agricultural Chemistry Branch for Soil and Land Use Surveys; Queensland Department of Primary Industries: Brisbane, QLD, Australia, 1982.
- Grierson, P.F.; Adams, M.A. Nutrient Cycling and Growth in Forest Ecosystems of South Western Australia: Relevance to Agricultural Landscapes; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Singh, B.; Cilkes, R.J. Phosphorus sorption in relation to soil properties for the major soil types of South-Western Australia. Aust. J. Soil Res. 1991, 29, 603–618. [Google Scholar] [CrossRef]
- Crane, W.J.B. Phosphorus stability in eucalypt forests. Aust. For. 1978, 41, 118–127. [Google Scholar] [CrossRef]
- Cade-Menun, B.J. Characterizing phosphorus in environmental and agricultural samples by 31P nuclear magnetic resonance spectroscopy. Talanta 2005, 66, 359–371. [Google Scholar] [CrossRef] [PubMed]
- McDowell, R.W.; Cade-Menun, B.; Stewart, I. Organic phosphorus speciation and pedogenesis: Analysis by solution 31P nuclear magnetic resonance spectroscopy. Eur. J. Soil Sci. 2007, 58, 1348–1357. [Google Scholar] [CrossRef]
- Peverill, K.I.; Sparrow, L.A.; Reuter, D.J. Soil Analysis: An Interpretation Manual; CSIRO Publishing: Victoria, Australia, 1999. [Google Scholar]
- Fourie, A.B.; Tibbett, M.; Worthington, T.; King, A.E. Quantifying the Effect of Substrate Compaction on Root Development in Cover Systems. In Mine Closure 2008, Proceedings of the Third International Conference on Mine Closure, Johannesburg, South Africa, 10–12 October 2008; Fourie, A.B., Tibbett, M., Weiersbye, I.M., Dye, P., Eds.; Australian Centre for Geomechanics: Perth, Australia, 2008; pp. 27–34. [Google Scholar]
- Thompson, I.; Mackey, B.; McNulty, S.; Mosseler, A. Forest resilience, biodiversity, and climate change. In Secretariat of the Convention on Biological Diversity, Montreal. Tech. Ser. 2009, 43, 1–67. [Google Scholar]
- Lambers, H.; Ahmedi, I.; Berkowitz, O.; Dunne, C.; Finnegan, P.M.; Hardy, G.E.S.J.; Jost, R.; Laliberte, E.; Pearse, S.J.; Teste, F.P. Phosphorus nutrition of phosphorus-sensitive Australian native plants: Threats to plant communities in a global biodiversity hotspot. Conserv. Physiol. 2013, 1, cot010. [Google Scholar] [CrossRef] [PubMed]


| Topsoil | Pallid Zone | |
|---|---|---|
| EC (µS/cm) 1 EC classification 2 | 49.3 ± 1.0 Low | 27.1 ± 0.7 Low |
| pH (H2O) 1 | 5.56 ± 0.04 | 4.62 ± 0.02 |
| pH standard classification 3 | Moderately acidic | Strongly acidic |
| pH revised classification | Normal | Moderately acidic |
| pH (CaCl2) | 4.60 ± 0.02 | 3.92 ± 0.01 |
| Soil organic C (%) 4 | 2.45 | 0.22 |
| Total N (%) | 0.08 ± 0.00 | 0.01 ± 0.00 |
| Total Al (%) 5 | 3.82 | 14.5 |
| Total Si (%) 5 | 40.5 | 31.0 |
| Total Ti (%) 5 | 0.27 | 0.31 |
| Total Fe (%) 5 | 0.54 | 0.71 |
| Total Mn (mg kg−1) 5 | 31 | nd |
| Total Ca (mg kg−1) 5 | 357 | 71 |
| Total K (mg kg−1) 5 | 332 | 581 |
| Total Mg (mg kg−1) 5 | nd | 422 |
| Total P (mg kg−1) 5 | 20 | 96 |
| Total V (mg kg−1) 5 | nd | 66 |
| Total Cr (mg kg−1) 5 | nd | 49 |
| Total Ni (mg kg−1) 5 | 18 | 38 |
| Total Rb (mg kg−1) 5 | 22 | 29 |
| Total Zr (mg kg−1) 5 | 345 | 261 |
| Total Hf (mg kg−1) 5 | 9 | 9 |
| Total Th (mg kg−1) 5 | 21 | 23 |
| Quartz (%) 6 | 98 | 67 |
| Gibbsite (%) 6 | 1 | 3 |
| Kaolinite (%) 6 | 1 | 30 |
| Topsoil | Pallid Zone | |
|---|---|---|
| NO3−-N (mg kg−1) 1 | 6.33 ± 0.33 | 1.00 ± 0.00 |
| NH4+-N (mg kg−1) 1 | 9.67 ± 0.33 | 1.00 ± 0.00 |
| Exc. Ca (meq 100 g−1) 2 | 2.23 ± 0.70 | 0.92 ± 0.12 |
| Exc. Mg (meq 100 g−1) 2 | 0.84 ± 0.23 | 0.52 ± 0.03 |
| Exc. Na (meq 100 g−1) 2 | 0.17 ± 0.05 | 0.09 ± 0.01 |
| Exc. K (meq 100 g−1) 2 | 0.15 ± 0.05 | 0.00 ± 0.00 |
| Exc. Al (meq 100 g−1) 2 | 0.19 ± 0.04 | 0.78 ± 0.14 |
| Al_KCl (meq 100 g−1) 3 | 0.32 ± 0.02 | 1.03 ± 0.18 |
| H_KCl (meq 100 g−1) 4 | 0.06 ± 0.02 | 0.13 ± 0.02 |
| Exc. Acidity (meq 100 g−1) 5 | 0.39 ± 0.03 | 1.16 ± 0.19 |
| ECEC (meq 100 g−1) | 3.75 ± 1.03 | 2.67 ± 0.16 |
| ESP (%) 6 | 4.7 | 3.9 |
| Extractable P (mg kg−1) 7 | 3.0 ± 0.0 | 1.0 ± 0.0 |
| Extractable K (mg kg−1) 7 | 45.00 ± 0.015 | 21.33 ± 3.28 |
| Extractable S (mg kg−1) 7 | 11.90 ± 0.50 | 75.33 ± 2.32 |
| Extractable Fe (mg kg−1) 7 | 32.72 ± 1.23 | 10.86 ± 0.55 |
| Extractable B (mg kg−1) 7 | 0.25 ± 0.05 | 0.25 ± 0.05 |
| Extractable Cu (mg kg−1) 7 | 0.16 ± 0.01 | 0.02 ± 0.00 |
| Extractable Mn (mg kg−1) 7 | 4.79 ± 0.25 | 0.04 ± 0.00 |
| Extractable Zn (mg kg−1) 7 | 0.23 ± 0.09 | 0.02 ± 0.01 |
| Pallid Zone | Topsoil | |||||||
|---|---|---|---|---|---|---|---|---|
| Langmuir Parameters | Freundlich Parameters | Langmuir Parameters | Freundlich Parameters | |||||
| Parameters | b (mL μg−1) | Xm (μg g−1) | k (μg g−1) | n (mL μg−1) | b (mL μg−1) | Xm (μg g−1) | k (μg g−1) | n (mL μg−1) |
| Initial estimates | 7.12 | 40.83 | 35.46 | 0.38 | 1.50 | 72.20 | 41.91 | 0.38 |
| Fitted values | 8.72 | 40.21 | 30.98 | 0.34 | 2.18 | 67.55 | 38.44 | 0.45 |
| Standard errors | 1.51 | 2.08 | 3.01 | 0.06 | 0.39 | 4.27 | 3.64 | 0.08 |
| Approx 95% confidence limits | ||||||||
| Lower bound | 3.92 | 33.60 | 21.40 | 0.14 | 1.09 | 55.78 | 28.33 | 0.23 |
| Upper bound | 13.52 | 46.82 | 40.57 | 0.55 | 3.27 | 79.33 | 48.55 | 0.66 |
| P-buffering capacity (μg g−1) | 2.34 | 3.37 | ||||||
| Goodness of fit statistics | ||||||||
| Sum of standard errors (SEE) | 130.52 | 59.12 | 28.32 | 171.59 | ||||
| Model efficiency | 0.94 | 0.97 | 0.99 | 0.93 | ||||
| Akaike’s information criterion (AIC) | 36.48 | 31.73 | 27.31 | 38.12 | ||||
| Topsoil | Pallid Zone | |
|---|---|---|
| Material < 2 mm (%) | 64.8 ± 12.8 | 80.6 ± 2.6 |
| Clay (%) | 9.6 | 25.8 |
| Silt (%) | 4.2 | 23.1 |
| Sand (%) | 86.2 | 51.1 |
| Soil texture 1 | Sandy loam | Clay loam |
| Average k-sat (mm day−1) 2 | 2539 ± 686 | 266 ± 114 |
| Drainage class 2 | Moderately rapid | Moderate |
| Bulk density (g cm−3) | 1.9 | 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soltangheisi, A.; George, S.; Tibbett, M. Soil Characteristics and Fertility of the Unique Jarrah Forest of Southwestern Australia, with Particular Consideration of Plant Nutrition and Land Rehabilitation. Land 2023, 12, 1236. https://doi.org/10.3390/land12061236
Soltangheisi A, George S, Tibbett M. Soil Characteristics and Fertility of the Unique Jarrah Forest of Southwestern Australia, with Particular Consideration of Plant Nutrition and Land Rehabilitation. Land. 2023; 12(6):1236. https://doi.org/10.3390/land12061236
Chicago/Turabian StyleSoltangheisi, Amin, Suman George, and Mark Tibbett. 2023. "Soil Characteristics and Fertility of the Unique Jarrah Forest of Southwestern Australia, with Particular Consideration of Plant Nutrition and Land Rehabilitation" Land 12, no. 6: 1236. https://doi.org/10.3390/land12061236
APA StyleSoltangheisi, A., George, S., & Tibbett, M. (2023). Soil Characteristics and Fertility of the Unique Jarrah Forest of Southwestern Australia, with Particular Consideration of Plant Nutrition and Land Rehabilitation. Land, 12(6), 1236. https://doi.org/10.3390/land12061236








