Linking Land Use and Plant Functional Diversity Patterns in Sabah, Borneo, through Large-Scale Spatially Continuous Sentinel-2 Inference
Abstract
:1. Introduction
2. Methods
2.1. Study Area
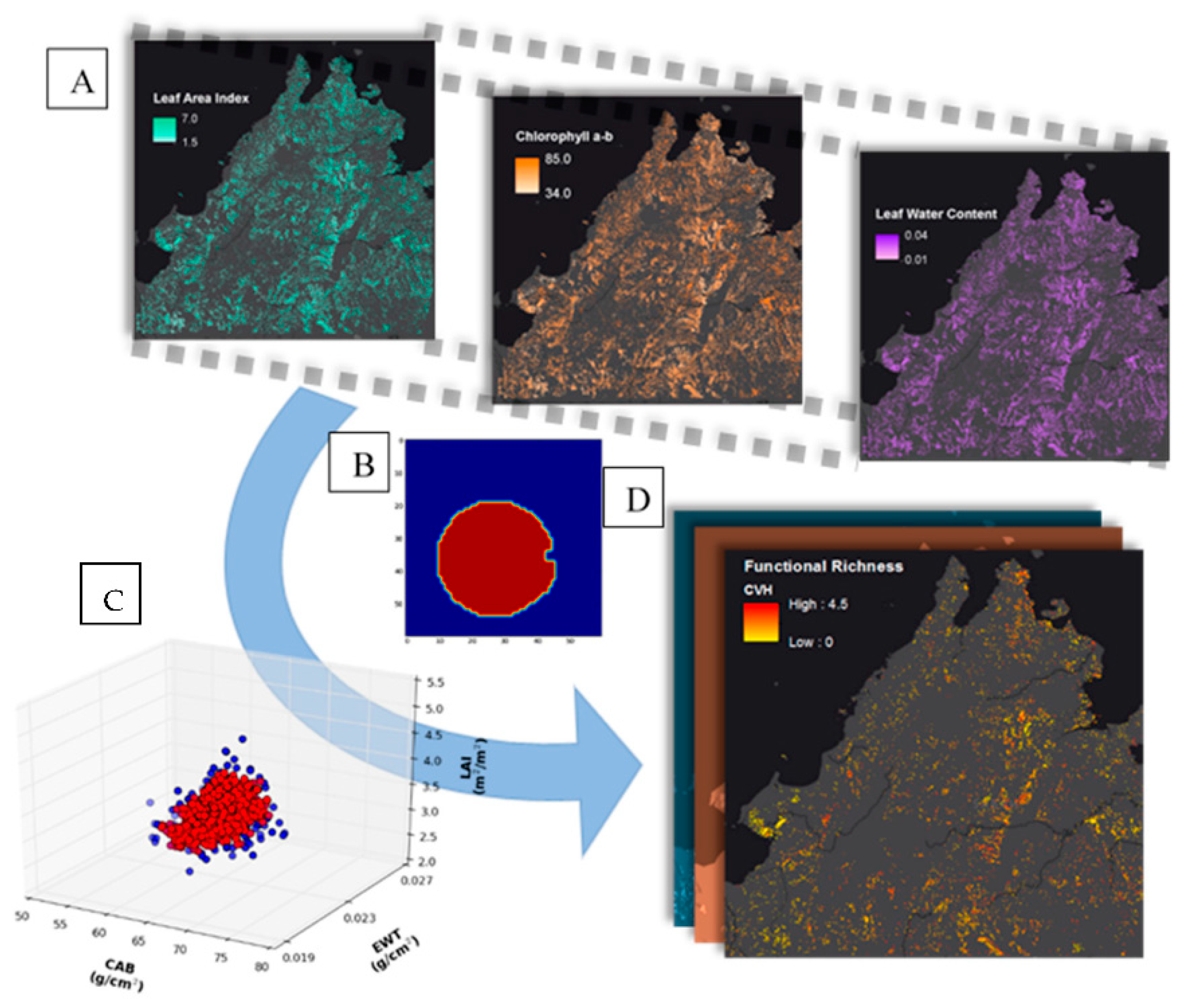
2.2. Retrieval of Functional Traits
2.3. Estimating Functional Diversity
2.4. Land-Use Data
2.5. Data Analysis
3. Results
3.1. From Mapping Spatially Explicit Spectral Trait Indicators to Functional Diversity Estimates
3.2. Land-Use Patterns of Plant Functional Diversity
4. Discussion
4.1. Trait Retrieval for Functional Diversity
4.2. Land-Use Gradient as Qualitative Assessment
4.3. Outlook
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A

| Land Use | N | Mean Elevation (m) | Mean Slope (% Change) | |
|---|---|---|---|---|
 | Intact Forest | 1192 | 439.90 | 1.88 |
 | Logged Forest | 3018 | 419.71 | 1.30 |
 | Oil Palm Plantations | 1416 | 63.84 | 0.74 |
Appendix A.1. Qualitative Assessment of the Validity of Spectral Trait Indicators
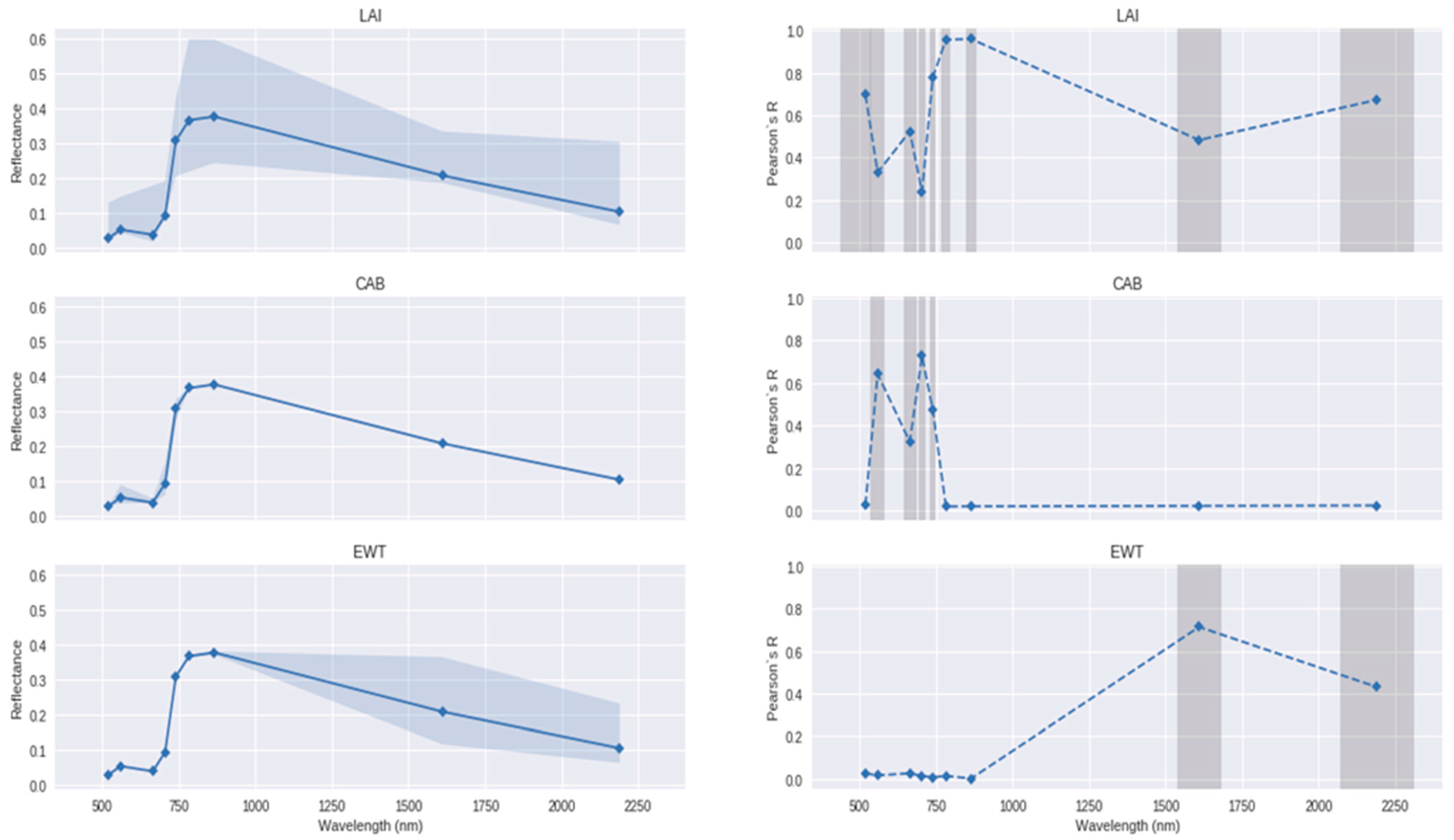
| RMSE | MAE | %nRMSE | Sampled Pixels (N) | |||
|---|---|---|---|---|---|---|
| Land Use | µ | σ | µ | σ | µ | |
| Intact Forest | 0.012 | 0.009 | 0.007 | 0.005 | 7.6 | 4774 |
| Logged Forest | 0.014 | 0.01 | 0.008 | 0.006 | 8.9 | 4274 |
| Oil Palm Plantations | 0.012 | 0.01 | 0.007 | 0.006 | 7.2 | 3952 |

Appendix A.2. Functional Diversity across Elevations

References
- IPBES. Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science—Policy Platform on Biodiversity and Ecosystem Services; IPBES Secretariat: Bonn, Germany, 2019. [Google Scholar]
- Newbold, T.; Hudson, L.N.; Arnell, A.P.; Contu, S.; De Palma, A.; Ferrier, S.; Hill, S.L.L.; Hoskins, A.J.; Lysenko, I.; Phillips, H.R.P.; et al. Has land use pushed terrestrial biodiversity beyond the planetary boundary? A global assessment. Science 2016, 353, 288–291. [Google Scholar] [CrossRef]
- UNDP. Biodiversity Conservation in Multiple-Use Forest Landscapes in Sabah, Malaysia; UNDP: Sabah, Malaysia, 2012. [Google Scholar]
- Wilcove, D.S.; Giam, X.; Edwards, D.P.; Fisher, B.; Koh, L.P. Navjot’s nightmare revisited: Logging, agriculture, and biodiversity in Southeast Asia. Trends Ecol. Evol. 2013, 28, 531–540. [Google Scholar] [CrossRef]
- Sodhi, N.S.; Koh, L.P.; Brook, B.W.; Ng, P.K.L. Southeast Asian biodiversity: An impending disaster. Trends Ecol. Evol. 2004, 19, 654–660. [Google Scholar] [CrossRef]
- Sodhi, N.S.; Koh, L.P.; Clements, R.; Wanger, T.C.; Hill, J.K.; Hamer, K.C.; Clough, Y.; Tscharntke, T.; Posa, M.R.C.; Lee, T.M. Conserving Southeast Asian forest biodiversity in human-modified landscapes. Biol. Conserv. 2010, 143, 2375–2384. [Google Scholar] [CrossRef]
- Gaveau, D.L.A.; Sloan, S.; Molidena, E.; Yaen, H.; Sheil, D.; Abram, N.K.; Ancrenaz, M.; Nasi, R.; Quinones, M.; Wielaard, N.; et al. Four decades of forest persistence, clearance and logging on Borneo. PLoS ONE 2014, 9, e101654. [Google Scholar] [CrossRef] [Green Version]
- Bryan, J.E.; Shearman, P.L.; Asner, G.P.; Knapp, D.E.; Aoro, G.; Lokes, B. Extreme Differences in Forest Degradation in Borneo: Comparing Practices in Sarawak, Sabah, and Brunei. PLoS ONE 2013, 8, e69679. [Google Scholar] [CrossRef] [Green Version]
- Gaveau, D.L.A.; Sheil, D.; Salim, M.A.; Arjasakusuma, S.; Ancrenaz, M.; Pacheco, P.; Meijaard, E. Rapid conversions and avoided deforestation: Examining four decades of industrial plantation expansion in Borneo. Sci. Rep. 2016, 6, 32017. [Google Scholar] [CrossRef]
- Maycock, C.R.; Kettle, C.J.; Khoo, E.; Pereira, J.T.; Sugau, J.B.; Nilus, R.; Ong, R.C.; Amaludin, N.A.; Newman, M.F.; Burslem, D.F.R.P. A Revised Conservation Assessment of Dipterocarps in Sabah. Biotropica 2012, 44, 649–657. [Google Scholar] [CrossRef] [Green Version]
- Fitzherbert, E.B.; Struebig, M.J.; Morel, A.; Danielsen, F.; Brühl, C.A.; Donald, P.F.; Phalan, B. How will oil palm expansion affect biodiversity? Trends Ecol. Evol. 2008, 23, 538–545. [Google Scholar] [CrossRef]
- Koh, L.P.; Wilcove, D.S. Is oil palm agriculture really destroying tropical biodiversity? Conserv. Lett. 2008, 1, 60–64. [Google Scholar] [CrossRef]
- Bernard, H.; Fjeldså, J.; Mohamed, M. A case study on the effects of disturbance and conversion of tropical lowland rain forest on the non-volant small mammals in north Borneo: Management implications. Mammal Study 2009, 34, 85–96. [Google Scholar] [CrossRef]
- Benedick, S.; Hill, J.K.; Mustaffa, N.; Chey, V.K.; Maryati, M.; Searle, J.B.; Schilthuizen, M.; Hamer, K.C. Impacts of rain forest fragmentation on butterflies in northern Borneo: Species richness, turnover and the value of small fragments. J. Appl. Ecol. 2006, 43, 967–977. [Google Scholar] [CrossRef]
- Barnes, A.D.; Jochum, M.; Mumme, S.; Haneda, N.F.; Farajallah, A.; Widarto, T.H.; Brose, U. Consequences of tropical land use for multitrophic biodiversity and ecosystem functioning. Nat. Commun. 2014, 5, 6351. [Google Scholar] [CrossRef]
- Edwards, F.a.; Edwards, D.P.; Larsen, T.H.; Hsu, W.W.; Benedick, S.; Chung, a.; Vun Khen, C.; Wilcove, D.S.; Hamer, K.C. Does logging and forest conversion to oil palm agriculture alter functional diversity in a biodiversity hotspot? Anim. Conserv. 2014, 17, 163–173. [Google Scholar] [CrossRef]
- Neo, L.; Tan, H.T.W.; Wong, K.M. Too little, too late? Conservation exigencies for Borneo inferred from biogeographic considerations of its endemic plant genera against intense landscape modifications. Biodivers. Conserv. 2021, 31, 59–76. [Google Scholar] [CrossRef]
- Jetz, W.; Cavender-Bares, J.; Pavlick, R.; Schimel, D.; Davis, F.W.; Asner, G.P.; Guralnick, R.; Kattge, J.; Latimer, A.M.; Moorcroft, P.; et al. Monitoring plant functional diversity from space. Nat. Plants 2016, 2, 16024. [Google Scholar] [CrossRef] [Green Version]
- Hortal, J.; De Bello, F.; Diniz-Filho, J.A.F.; Lewinsohn, T.M.; Lobo, J.M.; Ladle, R.J. Seven Shortfalls that Beset Large-Scale Knowledge of Biodiversity. Annu. Rev. Ecol. Evol. Syst. 2015, 46, 523–549. [Google Scholar] [CrossRef] [Green Version]
- Petchey, O.L.; Gaston, K.J. Functional diversity (FD), species richness and community composition. Ecol. Lett. 2002, 5, 402–411. [Google Scholar] [CrossRef]
- Flynn, D.F.B.; Gogol-Prokurat, M.; Nogeire, T.; Molinari, N.; Richers, B.T.; Lin, B.B.; Simpson, N.; Mayfield, M.M.; DeClerck, F. Loss of functional diversity under land use intensification across multiple taxa. Ecol. Lett. 2009, 12, 22–33. [Google Scholar] [CrossRef]
- de Souza, D.M.; Flynn, D.F.B.; DeClerck, F.; Rosenbaum, R.K.; de Melo Lisboa, H.; Koellner, T. Land use impacts on biodiversity in LCA: Proposal of characterization factors based on functional diversity. Int. J. Life Cycle Assess. 2013, 18, 1231–1242. [Google Scholar] [CrossRef]
- Diaz, S.; Cabido, M. Vive la difference: Plant functional diversity matters to ecosystem processes: Plant functional diversity matters to ecosystem processes. Trends Ecol. Evol. 2001, 16, 646–655. [Google Scholar] [CrossRef]
- Cadotte, M.W.; Carscadden, K.; Mirotchnick, N. Beyond species: Functional diversity and the maintenance of ecological processes and services. J. Appl. Ecol. 2011, 48, 1079–1087. [Google Scholar] [CrossRef]
- Fukami, T.; Bezemer, T.M.; Mortimer, S.R.; Van Der Putten, W.H. Species divergence and trait convergence in experimental plant community assembly. Ecol. Lett. 2005, 8, 1283–1290. [Google Scholar] [CrossRef]
- Violle, C.; Reich, P.B.; Pacala, S.W.; Enquist, B.J.; Kattge, J. The emergence and promise of functional biogeography. Proc. Natl. Acad. Sci. USA 2014, 111, 13690–13696. [Google Scholar] [CrossRef] [Green Version]
- Hooper, D.U. Species diversity, functional diversity and ecosystem functioning. In Biodiversity and Ecosystem Functioning: Synhesis and Perspectives; Oxford University Press on Demand: Oxford, UK, 2002. [Google Scholar]
- Asner, G.P.; Martin, R.E.; Anderson, C.B.; Knapp, D.E. Quantifying forest canopy traits: Imaging spectroscopy versus field survey. Remote Sens. Environ. 2015, 158, 15–27. [Google Scholar] [CrossRef]
- Götzenberger, L.; de Bello, F.; Bråthen, K.A.; Davison, J.; Dubuis, A.; Guisan, A.; Lepš, J.; Lindborg, R.; Moora, M.; Pärtel, M.; et al. Ecological assembly rules in plant communities-approaches, patterns and prospects. Biol. Rev. 2012, 87, 111–127. [Google Scholar] [CrossRef]
- Chiarucci, A.; Bacaro, G.; Scheiner, S.M. Old and new challenges in using species diversity for assessing biodiversity. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 2426–2437. [Google Scholar] [CrossRef] [Green Version]
- Granger, V.; Bez, N.; Fromentin, J.; Meynard, C.; Jadaud, A.; Mérigot, B. Mapping diversity indices: Not a trivial issue. Methods Ecol. Evol. 2015, 6, 688–696. [Google Scholar] [CrossRef] [Green Version]
- Takashina, N.; Economo, E.P. Developing generalized sampling schemes with known error properties: The case of a moving observer. Ecography 2021, 44, 293–306. [Google Scholar] [CrossRef]
- Schimel, D.; Pavlick, R.; Fisher, J.B.; Asner, G.P.; Saatchi, S.; Townsend, P.; Miller, C.; Frankenberg, C.; Hibbard, K.; Cox, P. Observing terrestrial ecosystems and the carbon cycle from space. Glob. Chang. Biol. 2015, 21, 1762–1776. [Google Scholar] [CrossRef]
- Jensen, J.R. Remote Sensing of the Environment: Pearson New International Edition: An Earth Resource Perspective; Pearson Education Limited: London, UK, 2013; ISBN 9781292034935. [Google Scholar]
- Butler, D. Earth observation enters next phase. Nature 2014, 508, 160–161. [Google Scholar] [CrossRef] [Green Version]
- Serbin, S.P.; Wu, J.; Ely, K.S.; Kruger, E.L.; Townsend, P.A.; Meng, R.; Wolfe, B.T.; Chlus, A.; Wang, Z.; Rogers, A. From the Arctic to the tropics: Multibiome prediction of leaf mass per area using leaf reflectance. New Phytol. 2019, 224, 1557–1568. [Google Scholar] [CrossRef]
- Ali, A.M.; Darvishzadeh, R.; Skidmore, A.; Gara, T.W.; O’Connor, B.; Roeoesli, C.; Heurich, M.; Paganini, M. Comparing methods for mapping canopy chlorophyll content in a mixed mountain forest using Sentinel-2 data. Int. J. Appl. Earth Obs. Geoinf. 2020, 87, 102037. [Google Scholar] [CrossRef]
- Ali, A.M.; Darvishzadeh, R.; Skidmore, A.; Heurich, M.; Paganini, M.; Heiden, U.; Mücher, S. Evaluating prediction models for mapping canopy chlorophyll content across biomes. Remote Sens. 2020, 12, 1788. [Google Scholar] [CrossRef]
- Brown, L.A.; Ogutu, B.O.; Dash, J. Estimating Forest Leaf Area Index and Canopy Chlorophyll Content with Sentinel-2: An Evaluation of Two Hybrid Retrieval Algorithms. Remote Sens. 2019, 11, 1752. [Google Scholar] [CrossRef] [Green Version]
- Verrelst, J.; Rivera-Caicedo, J.P.; Reyes-Muñoz, P.; Morata, M.; Amin, E.; Tagliabue, G.; Panigada, C.; Hank, T.; Berger, K. Mapping landscape canopy nitrogen content from space using PRISMA data. ISPRS J. Photogramm. Remote Sens. 2021, 178, 382–395. [Google Scholar] [CrossRef]
- Aguirre-gutiérrez, J.; Rifai, S.; Shenkin, A.; Oliveras, I.; Patrick, L.; Svátek, M.; Girardin, C.A.J.; Both, S.; Riutta, T.; Berenguer, E.; et al. Pantropical modelling of canopy functional traits using Sentinel-2 remote sensing data. Remote Sens. Environ. 2021, 252, 112122. [Google Scholar] [CrossRef]
- Hauser, L.T.; Féret, J.-B.; An Binh, N.; van der Windt, N.; Sil, Â.F.; Timmermans, J.; Soudzilovskaia, N.A.; van Bodegom, P.M. Towards scalable estimation of plant functional diversity from Sentinel-2: In-situ validation in a heterogeneous (semi-)natural landscape. Remote Sens. Environ. 2021, 262, 112505. [Google Scholar] [CrossRef]
- Rossi, C.; Kneubühler, M.; Schütz, M.; Schaepman, M.E.; Haller, R.M.; Risch, A.C. From local to regional: Functional diversity in differently managed alpine grasslands. Remote Sens. Environ. 2020, 236, 111415. [Google Scholar] [CrossRef]
- Ma, X.; Mahecha, M.D.; Migliavacca, M.; van der Plas, F.; Benavides, R.; Ratcliffe, S.; Kattge, J.; Richter, R.; Musavi, T.; Baeten, L.; et al. Inferring plant functional diversity from space: The potential of Sentinel-2. Remote Sens. Environ. 2019, 233, 111368. [Google Scholar] [CrossRef]
- Hauser, L.T.; Timmermans, J.; van der Windt, N.; Sil, Â.F.; César de Sá, N.; Soudzilovskaia, N.A.; van Bodegom, P.M. Explaining discrepancies between spectral and in-situ plant diversity in multispectral satellite earth observation. Remote Sens. Environ. 2021, 265, 112684. [Google Scholar] [CrossRef]
- Anderson, C.B. Biodiversity monitoring, earth observations and the ecology of scale. Ecol. Lett. 2018, 21, 1572–1585. [Google Scholar] [CrossRef] [PubMed]
- Abelleira Martínez, O.J.; Fremier, A.K.; Günter, S.; Ramos Bendaña, Z.; Vierling, L.; Galbraith, S.M.; Bosque-Pérez, N.a.; Ordoñez, J.C. Scaling up functional traits for ecosystem services with remote sensing: Concepts and methods. Ecol. Evol. 2016, 6, 4359–4371. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, A.N.; Dave, M.G.; Parmar, R.M.; Bhattacharya, B.; Marpu, P.R.; Singh, A.; Krishnayya, N.S.R. Inferring species diversity and variability over climatic gradient with spectral diversity metrics. Remote Sens. 2020, 12, 2130. [Google Scholar] [CrossRef]
- Durán, S.M.; Martin, R.E.; Díaz, S.; Maitner, B.S.; Malhi, Y.; Salinas, N.; Shenkin, A.; Silman, M.R.; Wieczynski, D.J.; Asner, G.P.; et al. Informing trait-based ecology by assessing remotely sensed functional diversity across a broad tropical temperature gradient. Sci. Adv. 2019, 5, aaw8114. [Google Scholar] [CrossRef] [Green Version]
- Villéger, S.; Mason, N.W.H.; Mouillot, D. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef] [Green Version]
- Kitayama, K. An altitudinal transect study of the vegetation on Mount Kinabalu, Borneo. Vegetatio 1992, 102, 149–171. [Google Scholar] [CrossRef]
- Aiba, S.; Kitayama, K. Structure, Composition and Species Diversity in an Altitude-Substrate Matrix of Rain Forest Tree Communities on Mount Kinabalu, Borneo. Plant Ecol. 2010, 140, 139–157. [Google Scholar] [CrossRef]
- Grytnes, J.A.; Beaman, J.H. Elevational species richness patterns for vascular plants on Mount Kinabalu, Borneo. J. Biogeogr. 2006, 33, 1838–1849. [Google Scholar] [CrossRef]
- Döbert, T.F.; Webber, B.L.; Sugau, J.B.; Dickinson, K.J.M.; Didham, R.K. Can leaf area index and biomass be estimated from Braun-Blanquet cover scores in tropical forests? J. Veg. Sci. 2015, 26, 1043–1053. [Google Scholar] [CrossRef]
- ESA. Sentinel-2 User Handbook; ESA Standard Document, Date 24/07/2015, Issue 1, Rev 2; ESA: Paris, France, 2015; p. 64. [Google Scholar]
- Gascon, F.; Cadau, E.; Colin, O.; Hoersch, B.; Isola, C.; Fernández, B.L.; Martimort, P. Copernicus Sentinel-2 Mission: Products, Algorithms and Cal/Val. In Proceedings of the Earth Observing Systems XIX; Butler, J.J., Xiong, X., Gu, X., Eds.; International Society for Optics and Photonics: Bellingham, WA, USA, 2014; Volume 9218, pp. 455–463. [Google Scholar]
- Louis, J.; Debaecker, V.; Pflug, B.; Main-Knorn, M.; Bieniarz, J.; Mueller-Wilm, U.; Cadau, E.; Gascon, F. Sentinel-2 Sen2Cor: L2A Processor for Users. In Proceedings of the Living Planet Symposium 2016, Prague, Czech Republic, 9–13 May 2016; pp. 1–8. [Google Scholar]
- Weiss, M.; Baret, F. Sentinel-2 ToolBox Level 2 Biophysical Product Algorithms; Version 1.1; ESA: Paris, France, 2016. [Google Scholar]
- Feret, J.B.; François, C.; Asner, G.P.; Gitelson, A.a.; Martin, R.E.; Bidel, L.P.R.; Ustin, S.L.; le Maire, G.; Jacquemoud, S. PROSPECT-4 and 5: Advances in the leaf optical properties model separating photosynthetic pigments. Remote Sens. Environ. 2008, 112, 3030–3043. [Google Scholar] [CrossRef]
- Fourty, T.; Baret, F. Amelioration de la Precision des Coefficients D’absorption Specifique de la Matiere Seche et des Pigments Photosynthetiques; INRA: Avignon, France, 1997. [Google Scholar]
- Verhoef, W. Light scattering by leaf layers with application to canopy reflectance modeling: The SAIL model. Remote Sens. Environ. 1984, 16, 125–141. [Google Scholar] [CrossRef] [Green Version]
- Jacquemoud, S.; Verhoef, W.; Baret, F.; Zarco-Tejada, P.J.; Asner, G.P.; François, C.; Ustin, S.L. PROSPECT + SAIL: 15 Years of Use for Land Surface Characterization. In Proceedings of the 2006 IEEE International Symposium on Geoscience and Remote Sensing, Denver, CO, USA, 31 July–4 August 2006; Volume 516, pp. 1992–1995. [Google Scholar] [CrossRef] [Green Version]
- Jacquemoud, S.; Verhoef, W.; Baret, F.; Bacour, C.; Zarco-Tejada, P.J.; Asner, G.P.; François, C.; Ustin, S.L. PROSPECT + SAIL models: A review of use for vegetation characterization. Remote Sens. Environ. 2009, 113, S56–S66. [Google Scholar] [CrossRef]
- Pfeifer, M.; Korhonen, L.; Wheeler, C.; Rautiainen, M. Remote Sensing of Environment Forest canopy structure and reflectance in humid tropical Borneo: A physically-based interpretation using spectral invariants. Remote Sens. Environ. 2017, 201, 314–330. [Google Scholar] [CrossRef] [Green Version]
- Asner, G.P.; Scurlock, J.M.O.; Hicke, J.A. Global synthesis of leaf area index observations: Implications for ecological and remote sensing studies. Glob. Ecol. Biogeogr. 2003, 12, 191–205. [Google Scholar] [CrossRef] [Green Version]
- Castillo, J.; Apan, A.A.; Maraseni, T.N.; Salmo, S.G. Estimation and mapping of above-ground biomass of mangrove forests and their replacement land uses in the Philippines using Sentinel imagery. ISPRS J. Photogramm. Remote Sens. 2017, 134, 70–85. [Google Scholar] [CrossRef]
- Zheng, G.; Moskal, L.M. Retrieving Leaf Area Index (LAI) Using Remote Sensing: Theories, Methods and Sensors. Sensors 2009, 9, 2719–2745. [Google Scholar] [CrossRef] [Green Version]
- Poorter, L.; Bongers, F. Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology 2006, 87, 1733–1743. [Google Scholar] [CrossRef]
- Croft, H.; Chen, J.M.; Luo, X.; Bartlett, P.; Chen, B.; Staebler, R.M. Leaf chlorophyll content as a proxy for leaf photosynthetic capacity. Glob. Chang. Biol. 2017, 23, 3513–3524. [Google Scholar] [CrossRef]
- Cao, K. Leaf anatomy and chlorophyll content of 12 woody species in contrasting light conditions in a Bornean heath forest. Can. J. Bot. 2000, 78, 1245–1253. [Google Scholar]
- Weiher, E.; van der Werf, A.; Thompson, K.; Roderick, M.; Garnier, E.; Eriksson, O. Challenging Theophrastus: A common core list of plant traits for functional ecology. J. Veg. Sci. 1999, 10, 609–620. [Google Scholar] [CrossRef]
- Saura-Mas, S.; Lloret, F. Leaf and Shoot Water Content and Leaf Dry Matter Content of Mediterranean Woody Species with Different Post-fire Regenerative Strategies. Ann. Bot. 2017, 99, 545–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Nieuwstadt, M.G.L.; Sheil, D. Drought, fire and tree survival in a Borneo rain forest, East Kalimantan, Indonesia. J. Ecol. 2005, 93, 191–201. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Cornic, G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 2002, 25, 275–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asbjornsen, H.; Goldsmith, G.R.; Alvarado-Barrientos, M.S.; Rebel, K.; Van Osch, F.P.; Rietkerk, M.; Chen, J.; Gotsch, S.; Tobón, C.; Geissert, D.R.; et al. Ecohydrological advances and applications in plant–water relations research: A review. J. Plant Ecol. 2011, 4, 3–22. [Google Scholar] [CrossRef]
- Damm, A.; Paul-Limoges, E.; Haghighi, E.; Simmer, C.; Morsdorf, F.; Schneider, F.D.; van der Tol, C.; Migliavacca, M.; Rascher, U. Remote sensing of plant-water relations: An overview and future perspectives. J. Plant Physiol. 2018, 227, 3–19. [Google Scholar] [CrossRef]
- Mason, N.W.H.; Mouillot, D.; Lee, W.G.; Wilson, J.B. Functional richness, functional evenness and functional divergence: The primary components of functional diversity. Oikos 2005, 111, 112–118. [Google Scholar] [CrossRef]
- Mouillot, D.; Mason, W.H.N.; Dumay, O.; Wilson, J.B. Functional regularity: A neglected aspect of functional diversity. Oecologia 2005, 142, 353–359. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Schwilk, D.W.; Ackerly, D.D. A Trait-Based Test for Habitat Filtering: Convex Hull Volume. Ecology 2006, 87, 1465–1471. [Google Scholar] [CrossRef]
- Mouchet, M.a.; Villéger, S.; Mason, N.W.H.; Mouillot, D. Functional diversity measures: An overview of their redundancy and their ability to discriminate community assembly rules. Funct. Ecol. 2010, 24, 867–876. [Google Scholar] [CrossRef]
- Aiba, M.; Katabuchi, M.; Takafumi, H.; Matsuzaki, S.I.S.; Sasaki, T.; Hiura, T. Robustness of trait distribution metrics for community assembly studies under the uncertainties of assembly processes. Ecology 2013, 94, 2873–2885. [Google Scholar] [CrossRef] [PubMed]
- Kraft, N.J.B.; Valencia, R.; Ackerly, D.D. Functional Traits and Niche-Based Tree Community Assembly in an Amazonian Forest. Science 2008, 322, 580–582. [Google Scholar] [CrossRef] [Green Version]
- Schneider, F.D.; Morsdorf, F.; Schmid, B.; Petchey, O.L.; Hueni, A.; Schimel, D.S.; Schaepman, M.E. Mapping functional diversity from remotely sensed morphological and physiological forest traits. Nat. Commun. 2017, 8, s41467–s42017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fornberg, B.; Flyer, N. A Primer on Radial Basis Functions with Applications to the Geosciences; SIAM, Society for Industrial and Applied Mathematics: Philadelphia, PA, USA, 2015. [Google Scholar]
- Sist, P.; Saridan, A. Stand structure and floristic composition of a primary lowland dipterocarp forest in East Kalimantan. J. Trop. For. Sci. 1999, 11, 704–722. [Google Scholar]
- Martin, R.; Chadwick, K.; Brodrick, P.; Carranza-Jimenez, L.; Vaughn, N.; Asner, G. An Approach for Foliar Trait Retrieval from Airborne Imaging Spectroscopy of Tropical Forests. Remote Sens. 2018, 10, 199. [Google Scholar] [CrossRef]
- Kattge, J.; Bönisch, G.; Díaz, S.; Lavorel, S.; Prentice, I.C.; Leadley, P.; Tautenhahn, S.; Werner, G.D.A.; Aakala, T.; Abedi, M.; et al. TRY plant trait database—Enhanced coverage and open access. Glob. Chang. Biol. 2020, 26, 119–188. [Google Scholar] [CrossRef] [Green Version]
- Apichatmeta, K.; Sudsiri, C.J.; Ritchie, R.J. Photosynthesis of oil palm (Elaeis guineensis). Sci. Hortic. 2017, 214, 34–40. [Google Scholar] [CrossRef]
- Kurokawa, H.; Nakashizuka, T. Leaf herbivory and decomposability in a Malaysian tropical rain forest. Ecology 2008, 89, 2645–2656. [Google Scholar] [CrossRef]
- Kattge, J.; Díaz, S.; Lavorel, S.; Prentice, I.C.; Leadley, P.; Bönisch, G.; Garnier, E.; Westoby, M.; Reich, P.B.; Wright, I.J.; et al. TRY—A global database of plant traits. Glob. Chang. Biol. 2011, 17, 2905–2935. [Google Scholar] [CrossRef]
- Wu, J.; Chavana-Bryant, C.; Prohaska, N.; Serbin, S.P.; Guan, K.; Albert, L.P.; Yang, X.; van Leeuwen, W.J.D.; Garnello, A.J.; Martins, G.; et al. Convergence in relationships between leaf traits, spectra and age across diverse canopy environments and two contrasting tropical forests. New Phytol. 2017, 214, 1033–1048. [Google Scholar] [CrossRef] [Green Version]
- de Bello, F.; Lavorel, S.; Díaz, S.; Harrington, R.; Cornelissen, J.H.C.; Bardgett, R.D.; Berg, M.P.; Cipriotti, P.; Feld, C.K.; Hering, D.; et al. Towards an assessment of multiple ecosystem processes and services via functional traits. Biodivers. Conserv. 2010, 19, 2873–2893. [Google Scholar] [CrossRef]
- Combal, B.; Baret, F.; Weiss, M. Improving canopy variables estimation from remote sensing data by exploiting ancillary information. Case study on sugar beet canopies. Agronomie 2002, 22, 205–215. [Google Scholar] [CrossRef]
- Verrelst, J.; Malenovský, Z.; Van der Tol, C.; Camps-Valls, G.; Gastellu-Etchegorry, J.P.; Lewis, P.; North, P.; Moreno, J. Quantifying Vegetation Biophysical Variables from Imaging Spectroscopy Data: A Review on Retrieval Methods. Surv. Geophys. 2019, 40, 589–629. [Google Scholar] [CrossRef] [Green Version]
- Dorigo, W.; Richter, R.; Baret, F.; Bamler, R.; Wagner, W. Enhanced automated canopy characterization from hyperspectral data by a novel two step radiative transfer model inversion approach. Remote Sens. 2009, 1, 1139–1170. [Google Scholar] [CrossRef] [Green Version]
- Berger, K.; Pablo, J.; Caicedo, R.; Martino, L.; Wocher, M.; Hank, T. A Survey of Active Learning for Quantifying Vegetation Traits from Terrestrial Earth Observation Data. Remote Sens. 2021, 13, 287. [Google Scholar] [CrossRef]
- Nguyen, B.A.; Hauser, L.T.; Hoa, P.V.; Thi, G.; Thao, P. Quantifying mangrove leaf area index from Sentinel-2 imagery using hybrid models and active learning. Int. J. Remote Sens. 2022, 1–22. [Google Scholar] [CrossRef]
- Berger, K.; Atzberger, C.; Danner, M.; D’Urso, G.; Mauser, W.; Vuolo, F.; Hank, T. Evaluation of the PROSAIL Model Capabilities for Future Hyperspectral Model Environments: A Review Study. Remote Sens. 2018, 10, 85. [Google Scholar] [CrossRef] [Green Version]
- Schlerf, M.; Atzberger, C. Inversion of a forest reflectance model to estimate structural canopy variables from hyperspectral remote sensing data. Remote Sens. Environ. 2006, 100, 281–294. [Google Scholar] [CrossRef]
- Kuusk, A.; Nilson, T. A directional multispectral forest reflectance model. Remote Sens. Environ. 2000, 72, 244–252. [Google Scholar] [CrossRef]
- Huang, J.; Zeng, Y.; Kuusk, A.; Wu, B.; Dong, L.; Mao, K.; Chen, J. Inverting a forest canopy reflectance model to retrieve the overstorey and understorey leaf area index for forest stands. Int. J. Remote Sens. 2011, 32, 7591–7611. [Google Scholar] [CrossRef]
- Pasqualotto, N.; D’Urso, G.; Bolognesi, S.F.; Belfiore, O.R.; Van Wittenberghe, S.; Delegido, J.; Pezzola, A.; Winschel, C.; Moreno, J. Retrieval of evapotranspiration from sentinel-2: Comparison of vegetation indices, semi-empirical models and SNAP biophysical processor approach. Agronomy 2019, 9, 663. [Google Scholar] [CrossRef] [Green Version]
- Vinué, D.; Camacho, F.; Fuster, B. Validation of Sentinel-2 LAI and FAPAR products derived from SNAP toolbox over a cropland site in Barrax and over an agroforested site in Liria. In Proceedings of the Fifth Recent Advances in Quantitative Remote Sensing, Torrent, Spain, 18–22 September 2018; p. 248. [Google Scholar]
- Xie, Q.; Dash, J.; Huete, A.; Jiang, A.; Yin, G.; Ding, Y.; Peng, D.; Hall, C.C.; Brown, L.; Shi, Y.; et al. Retrieval of crop biophysical parameters from Sentinel-2 remote sensing imagery. Int. J. Appl. Earth Obs. Geoinf. 2019, 80, 187–195. [Google Scholar] [CrossRef]
- Danner, M.; Berger, K.; Wocher, M.; Mauser, W.; Hank, T. Efficient RTM-based training of machine learning regression algorithms to quantify biophysical & biochemical traits of agricultural crops. ISPRS J. Photogramm. Remote Sens. 2021, 173, 278–296. [Google Scholar] [CrossRef]
- Vanino, S.; Nino, P.; De Michele, C.; Falanga Bolognesi, S.; D’Urso, G.; Di Bene, C.; Pennelli, B.; Vuolo, F.; Farina, R.; Pulighe, G.; et al. Capability of Sentinel-2 data for estimating maximum evapotranspiration and irrigation requirements for tomato crop in Central Italy. Remote Sens. Environ. 2018, 215, 452–470. [Google Scholar] [CrossRef]
- Noormets, A. Phenology of Ecosystem Processes; Springer Science & Business Media: New York, NY, USA, 2013; Volume 53, ISBN 978-1-107-05799-1. [Google Scholar]
- Gara, T.W.; Darvishzadeh, R.; Skidmore, A.K.; Wang, T.; Heurich, M. Accurate modelling of canopy traits from seasonal Sentinel-2 imagery based on the vertical distribution of leaf traits. ISPRS J. Photogramm. Remote Sens. 2019, 157, 108–123. [Google Scholar] [CrossRef]
- Asner, G.P.; Martin, R.E.; Anderson, C.B.; Kryston, K.; Vaughn, N.; Knapp, D.E.; Bentley, L.P.; Shenkin, A.; Salinas, N.; Sinca, F.; et al. Scale dependence of canopy trait distributions along a tropical forest elevation gradient. New Phytol. 2017, 214, 973–988. [Google Scholar] [CrossRef]
- Duraiappah, A.K.; Naeem, S.; Agardy, T.; Ash, N.J.; Cooper, H.D.; Diaz, S.; Faith, D.P.; Mace, G.; McNeely, J.A.; Mooney, H.A.; et al. Ecosystems and Human Well-Being: Biodiversity Synthesis: A Report of the Millennium Ecosystem Assessment; IPBES Secretariat: Bonn, Germany, 2005. [Google Scholar]
- Lin, B.B.; Flynn, D.F.B.; Bunker, D.E.; Uriarte, M.; Naeem, S. The effect of agricultural diversity and crop choice on functional capacity change in grassland conversions. J. Appl. Ecol. 2011, 48, 609–618. [Google Scholar] [CrossRef]
- Maeshiro, R.; Kusumoto, B.; Fujii, S.; Shiono, T.; Kubota, Y. Using tree functional diversity to evaluate management impacts in a subtropical forest. Ecosphere 2013, 4, 6. [Google Scholar] [CrossRef]
- Wang, Z.; Rahbek, C.; Fang, J. Effects of geographical extent on the determinants of woody plant diversity. Ecography 2012, 35, 1160–1167. [Google Scholar] [CrossRef]
- Zarnetske, P.L.; Read, Q.D.; Record, S.; Gaddis, K.; Pau, S.; Hobi, M.; Malone, S.; Costanza, J.; Dahlin, K.M.; Latimer, A.M.; et al. Towards connecting biodiversity and geodiversity across scales with satellite remote sensing. Glob. Ecol. Biogeogr. 2019, 28, 548–556. [Google Scholar] [CrossRef] [Green Version]
- Blonder, B.; Lamanna, C.; Violle, C.; Enquist, B.J. The n-dimensional hypervolume. Glob. Ecol. Biogeogr. 2014, 23, 595–609. [Google Scholar] [CrossRef]
- Bongers, F.; Poorter, L.; Hawthorne, W.D.; Sheil, D. The intermediate disturbance hypothesis applies to tropical forests, but disturbance contributes little to tree diversity. Ecol. Lett. 2009, 12, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.R.; Mallik, A.U. Disturbance effects on species diversity and functional diversity in riparian and upland plant communities. Ecology 2010, 91, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Castro, A.F.; Raymundo, M.; Bimler, M.; Mayfield, M.M. Using multi-scale spatially explicit frameworks to understand the relationship between functional diversity and species richness. Ecography 2022, 1–18. [Google Scholar] [CrossRef]
- Shipley, B. Community assembly, natural selection and maximum entropy models. Oikos 2010, 119, 604–609. [Google Scholar] [CrossRef]
- Huston, M.A.; Huston, M.A. Biological Diversity: The Coexistence of Species; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Jong, H.N. Proposal could redefine palm oil-driven deforestation as reforestation in Indonesia. Mongabay, 7 January 2022. [Google Scholar]
- Hinkes, C. Adding (bio)fuel to the fire: Discourses on palm oil sustainability in the context of European policy development. Environ. Dev. Sustain. 2020, 22, 7661–7682. [Google Scholar] [CrossRef]
- Pakeman, R.J. Functional diversity indices reveal the impacts of land use intensification on plant community assembly. J. Ecol. 2011, 99, 1143–1151. [Google Scholar] [CrossRef]
- Hauser, L.T.; Binh, N.A.; Hoa, P.V.; Quan, N.H.; Timmermans, J. Gap-free monitoring of annual mangrove forest dynamics in ca mau province, vietnamese mekong delta, using the landsat-7-8 archives and post-classification temporal optimization. Remote Sens. 2020, 12, 3729. [Google Scholar] [CrossRef]
- Lahoz, W.A.; Schneider, P. Data assimilation: Making sense of Earth Observation. Front. Environ. Sci. 2014, 2, 16. [Google Scholar] [CrossRef] [Green Version]
- Lewis, P.; Gomez-Dans, J.; Kaminski, T.; Settle, J.; Quaife, T.; Gobron, N.; Styles, J.; Berger, M. An Earth Observation Land Data Assimilation System (EO-LDAS). Remote Sens. Environ. 2012, 120, 219–235. [Google Scholar] [CrossRef] [Green Version]
- Belda, S.; Pipia, L.; Morcillo-Pallarés, P.; Rivera-Caicedo, J.P.; Amin, E.; De Grave, C.; Verrelst, J. DATimeS: A machine learning time series GUI toolbox for gap-filling and vegetation phenology trends detection. Environ. Model. Softw. 2020, 127, 104666. [Google Scholar] [CrossRef]
- Gu, C.; Du, H.; Mao, F.; Han, N.; Zhou, G.; Xu, X.; Sun, S.; Gao, G. Global sensitivity analysis of PROSAIL model parameters when simulating Moso bamboo forest canopy reflectance. Int. J. Remote Sens. 2016, 37, 5270–5286. [Google Scholar] [CrossRef]
- Verrelst, J.; Vicent, J.; Rivera-Caicedo, J.P.; Lumbierres, M.; Morcillo-Pallarés, P.; Moreno, J. Global sensitivity analysis of leaf-canopy-atmosphere RTMs: Implications for biophysical variables retrieval from top-of-atmosphere radiance data. Remote Sens. 2019, 11, 1923. [Google Scholar] [CrossRef] [Green Version]
- de Sá, N.C.; Baratchi, M.; Hauser, L.T.; van Bodegom, P.M. Exploring the Impact of Noise on Hybrid Inversion of PROSAIL RTM on Sentinel-2 Data. Remote Sens. 2021, 13, 648. [Google Scholar] [CrossRef]
- Azzeme, A.M.; Abdullah, S.N.A.; Aziz, M.A.; Wahab, P.E.M. Oil palm leaves and roots differ in physiological response, antioxidant enzyme activities and expression of stress-responsive genes upon exposure to drought stress. Acta Physiol. Plant. 2016, 38, 52. [Google Scholar] [CrossRef]

| N | Functional Richness | Functional Divergence | Functional Evenness | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | St. Dev. | Mean | St. Dev. | Mean | St. Dev. | |||
 | Intact Forest | 1192 | 5.474 | 3.354 | 0.737 | 0.020 | 0.833 | 0.010 |
| Logged Forest | 3018 | 4.971 | 2.331 | 0.732 | 0.015 | 0.833 | 0.008 | |
| Oil Palm Plantations | 1416 | 3.974 | 2.876 | 0.692 | 0.018 | 0.820 | 0.018 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hauser, L.T.; Timmermans, J.; Soudzilovskaia, N.A.; van Bodegom, P.M. Linking Land Use and Plant Functional Diversity Patterns in Sabah, Borneo, through Large-Scale Spatially Continuous Sentinel-2 Inference. Land 2022, 11, 572. https://doi.org/10.3390/land11040572
Hauser LT, Timmermans J, Soudzilovskaia NA, van Bodegom PM. Linking Land Use and Plant Functional Diversity Patterns in Sabah, Borneo, through Large-Scale Spatially Continuous Sentinel-2 Inference. Land. 2022; 11(4):572. https://doi.org/10.3390/land11040572
Chicago/Turabian StyleHauser, Leon T., Joris Timmermans, Nadejda A. Soudzilovskaia, and Peter M. van Bodegom. 2022. "Linking Land Use and Plant Functional Diversity Patterns in Sabah, Borneo, through Large-Scale Spatially Continuous Sentinel-2 Inference" Land 11, no. 4: 572. https://doi.org/10.3390/land11040572
APA StyleHauser, L. T., Timmermans, J., Soudzilovskaia, N. A., & van Bodegom, P. M. (2022). Linking Land Use and Plant Functional Diversity Patterns in Sabah, Borneo, through Large-Scale Spatially Continuous Sentinel-2 Inference. Land, 11(4), 572. https://doi.org/10.3390/land11040572






