Organoclay Microparticle-Enhanced Microfiltration for the Removal of Acid Red 27 in Aqueous Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Organoclay Synthesis
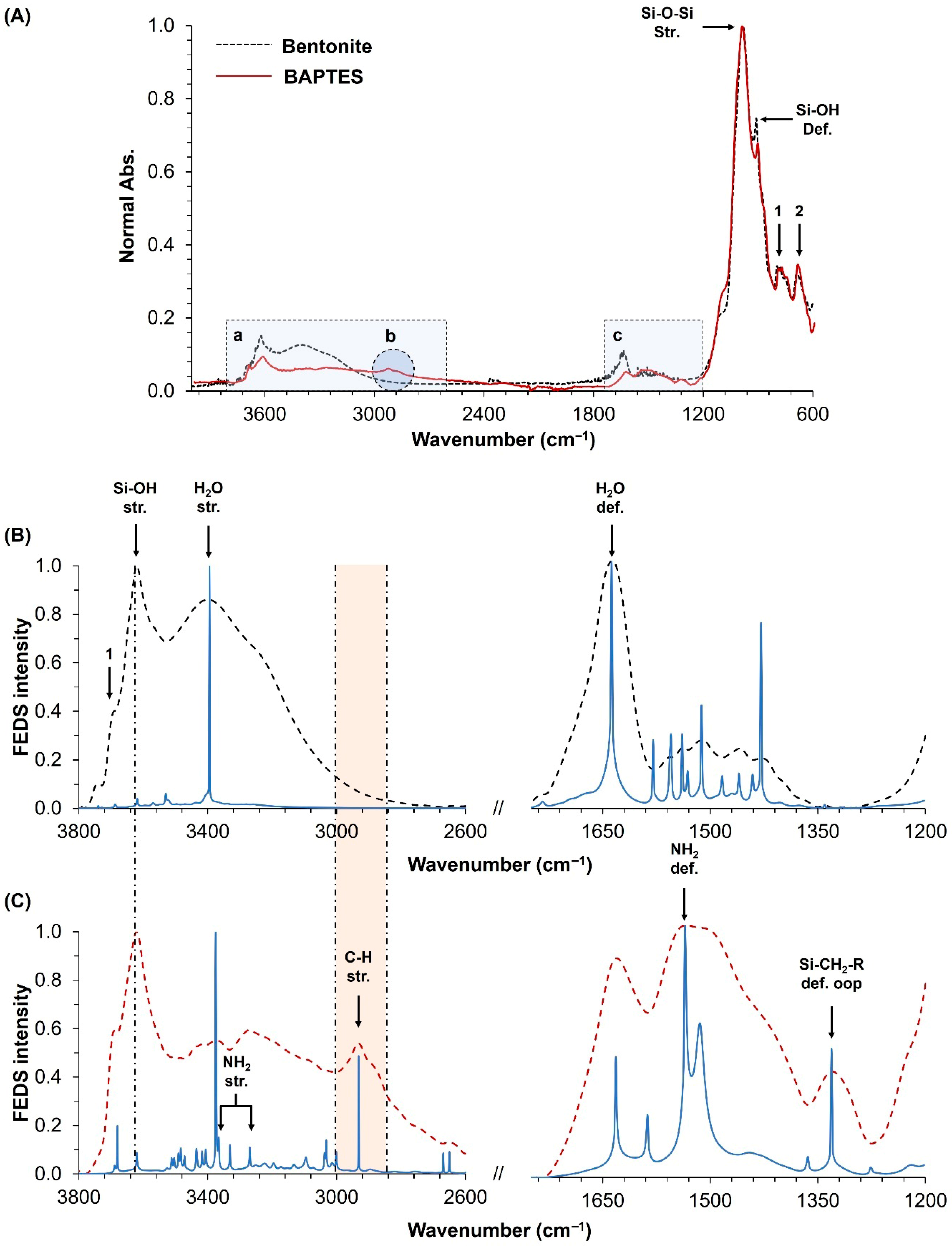
2.3. Structural Characterization of the Organoclay
2.4. Spectral Analysis Through Functionally Enhanced Derivative Spectroscopy (FEDS)
2.5. Study of the Adsorption Capacity of Dye
2.6. Study of Adsorption Kinetics
2.7. Theoretical Models of Adsorption Kinetics
2.8. Dye Adsorption Experiments in Equilibrium
2.9. Adsorption Isotherms
2.10. Determination of the Thermodynamic Parameters of the Dye Adsorption Process
2.11. Evaluation of BAPTES Microparticle-Enhanced Microfiltration for AR27 Removal
2.12. Study of the Desorption Capacity of the Dye
2.13. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of the BAPTES Organoclay
3.2. Study of the Adsorption Capacity of AR27
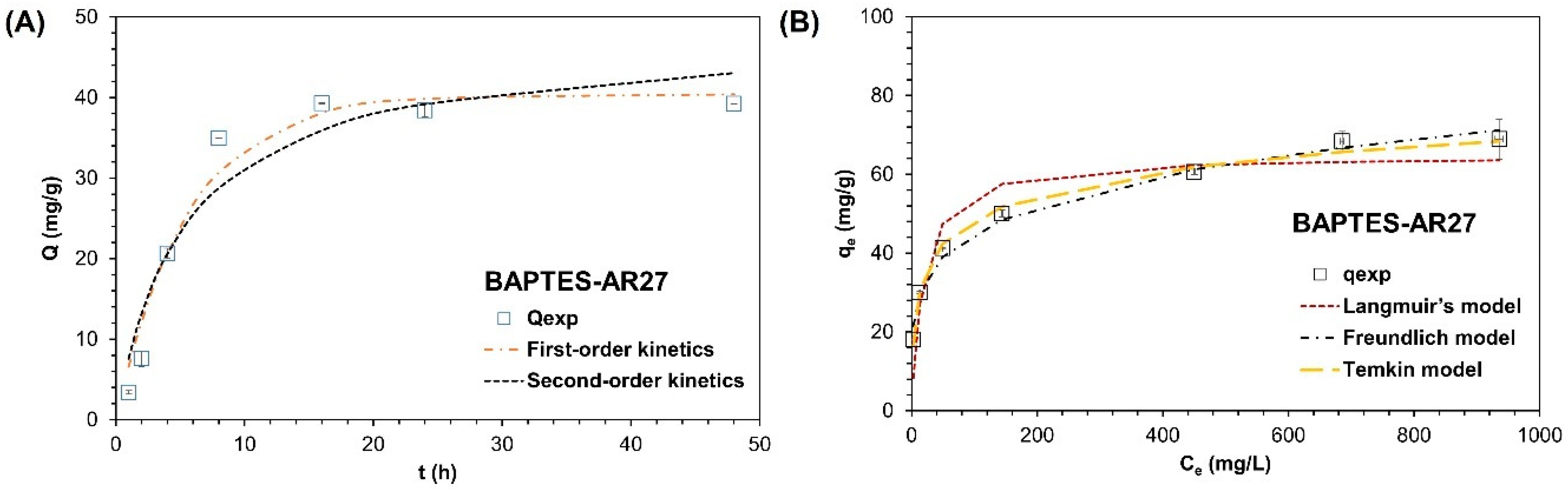
3.3. Study of Dye Adsorption Kinetics
3.4. Study of Dye Adsorption in Equilibrium
3.5. Study of the Thermodynamic Parameters of the Dye Adsorption Process
3.6. Evaluation of BAPTES Microparticle-Enhanced Microfiltration for AR27 Removal
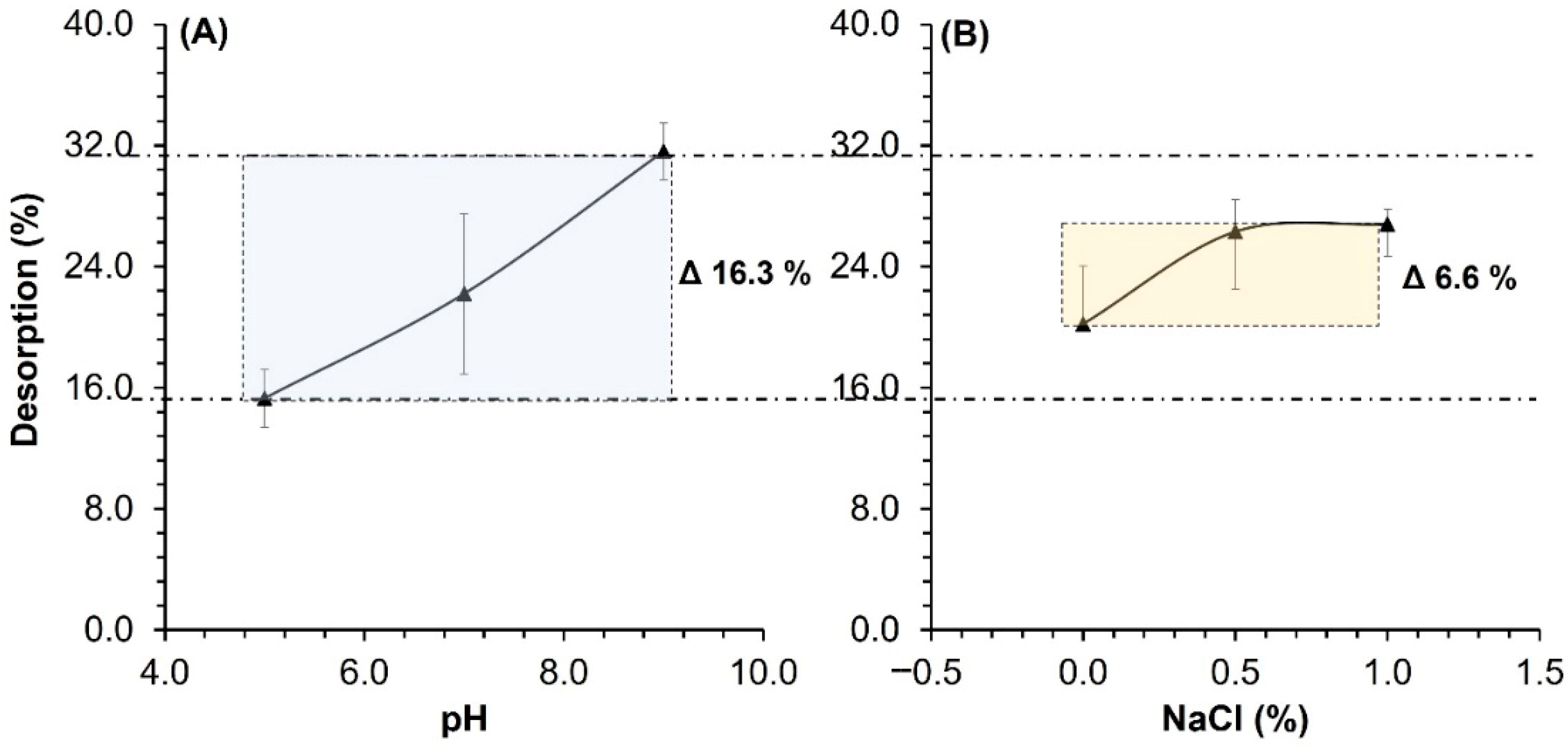
3.7. Study of the Desorption Capacity of Dye
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ngo, A.C.R.; Tischler, D. Microbial Degradation of Azo Dyes: Approaches and Prospects for a Hazard-Free Conversion by Microorganisms. Int. J. Environ. Res. Public Health 2022, 19, 4740. [Google Scholar] [CrossRef]
- Khan, M.; Lo, I.M.C. Removal of Ionizable Aromatic Pollutants from Contaminated Water Using Nano γ-Fe2O3 Based Magnetic Cationic Hydrogel: Sorptive Performance, Magnetic Separation and Reusability. J. Hazard. Mater. 2017, 322, 195–204. [Google Scholar] [CrossRef]
- Özcan, A.S.; Özcan, A. Adsorption of Acid Dyes from Aqueous Solutions onto Acid-Activated Bentonite. J. Colloid Interface Sci. 2004, 276, 39–46. [Google Scholar] [CrossRef]
- Shi, M.; Zhang, K.; Zhuang, Q.; Zhang, C.; Lin, X.; Xie, A.; Dong, W. Sulfonated Tetraphenylethylene Polymers with Negative Charges for High-Capacity Removal of Organic Dyes from Waste Water. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 128948. [Google Scholar] [CrossRef]
- Cesaratto, A.; Centeno, S.A.; Lombardi, J.R.; Shibayama, N.; Leona, M. A Complete Raman Study of Common Acid Red Dyes: Application to the Identification of Artistic Materials in Polychrome Prints. J. Raman Spectrosc. 2017, 48, 601–609. [Google Scholar] [CrossRef]
- Salem, A.N.M.; Ahmed, M.A.; El-Shahat, M.F. Selective Adsorption of Amaranth Dye on Fe3O4/MgO Nanoparticles. J. Mol. Liq. 2016, 219, 780–788. [Google Scholar] [CrossRef]
- Ahmad, R.; Kumar, R. Adsorption of Amaranth Dye onto Alumina Reinforced Polystyrene. CLEAN–Soil Air Water 2011, 39, 74–82. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, A.E.; Morales-Barrera, L.; Cristiani-Urbina, E. Continuous Biosorption of Acid Red 27 Azo Dye by Eichhornia Crassipes Leaves in a Packed-Bed Column. Sci. Rep. 2021, 11, 18413. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Aboamera, N.M.; Nasser, W.S.; Mahmoud, W.H.; Mohamed, G.G. Photodegradation of Organic Dyes by PAN/SiO2-TiO2-NH2 Nanofiber Membrane under Visible Light. Sep. Purif. Technol. 2019, 224, 509–514. [Google Scholar] [CrossRef]
- Adnan, L.A.; Hadibarata, T.; Sathishkumar, P.; Yusoff, A.R.M. Biodegradation Pathway of Acid Red 27 by White-Rot Fungus Armillaria Sp. F022 and Phytotoxicity Evaluation. CLEAN–Soil Air Water 2016, 44, 239–246. [Google Scholar] [CrossRef]
- Al-Zawahreh, K.; Barral, M.T.; Al-Degs, Y.; Paradelo, R. Competitive Removal of Textile Dyes from Solution by Pine Bark-Compost in Batch and Fixed Bed Column Experiments. Environ. Technol. Innov. 2022, 27, 102421. [Google Scholar] [CrossRef]
- Chamorro, A.F.; Lerma, T.A.; Palencia, M. CTAB Surfactant Promotes Rapid, Efficient, and Simultaneous Removal of Cationic and Anionic Dyes through Adsorption on Glycerol/Citrate Polyester. Water 2024, 16, 1860. [Google Scholar] [CrossRef]
- Palencia, M.; Martínez, J.M.; Arrieta, Á. Removal of Acid Blue 129 Dye by Polymer-Enhanced Ultrafiltration (PEUF). J. Sci. Technol. Appl. 2017, 2, 65–74. [Google Scholar] [CrossRef]
- Obiora-Okafo, I.A.; Onukwuli, O.D.; Igwegbe, C.A.; Onu, C.E.; Omotioma, M. Enhanced Performance of Natural Polymer Coagulants for Dye Removal from Wastewater: Coagulation Kinetics, and Mathematical Modelling Approach. Environ. Process. 2022, 9, 20. [Google Scholar] [CrossRef]
- Saeed, M.; Muneer, M.; Haq, A.U.; Akram, N. Photocatalysis: An Effective Tool for Photodegradation of Dyes—A Review. Environ. Sci. Pollut. Res. 2021, 29, 293–311. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of Various Recent Wastewater Dye Removal Methods: A Review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Naeem, H.T.; Hassan, A.A.; Al-Khateeb, R.; Naeem, H.T.; Al-Khateeb, R.T. Wastewater-(Direct Red Dye) Treatment-Using Solar Fenton Process. J. Pharm. Sci. Res. 2018, 10, 2309–2313. [Google Scholar]
- Benjelloun, M.; Miyah, Y.; Evrendilek, G.A.; Zerrouq, F.; Lairini, S. Recent Advances in Adsorption Kinetic Models: Their Application to Dye Types. Arab. J. Chem. 2021, 14, 103031. [Google Scholar] [CrossRef]
- Heybet, E.N.; Ugraskan, V.; Isik, B.; Yazici, O. Adsorption of Methylene Blue Dye on Sodium Alginate/Polypyrrole Nanotube Composites. Int. J. Biol. Macromol. 2021, 193, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Xia, D.; Kazlauciunas, A.; Thornton, P.; Lin, L.; Menzel, R. Dye-Mediated Interactions in Chitosan-Based Polyelectrolyte/Organoclay Hybrids for Enhanced Adsorption of Industrial Dyes. ACS Appl. Mater. Interfaces 2019, 11, 11961–11969. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Calderon, J.; Marpu, S.B.; Omary, M.A.; Shi, S.Q. Mesoporous Activated Carbon as a Green Adsorbent for the Removal of Heavy Metals and Congo Red: Characterization, Adsorption Kinetics, and Isotherm Studies. J. Contam. Hydrol. 2021, 243, 103869. [Google Scholar] [CrossRef]
- Paradelo, R.; Al-Zawahreh, K.; Barral, M.T. Utilization of Composts for Adsorption of Methylene Blue from Aqueous Solutions: Kinetics and Equilibrium Studies. Materials 2020, 13, 2179. [Google Scholar] [CrossRef] [PubMed]
- Kafle, S.R.; Adhikari, S.; Shrestha, R.; Ban, S.; Khatiwada, G.; Gaire, P.; Tuladhar, N.; Jiang, G.; Tiwari, A. Advancement of Membrane Separation Technology for Organic Pollutant Removal. Water Sci. Technol. 2024, 89, 2290–2310. [Google Scholar] [CrossRef]
- Loganathan, P.; Kandasamy, J.; Ratnaweera, H.; Vigneswaran, S. Submerged Membrane/Adsorption Hybrid Process in Water Reclamation and Concentrate Management—A Mini Review. Environ. Sci. Pollut. Res. 2023, 30, 42738–42752. [Google Scholar] [CrossRef]
- Palencia, M. Liquid-Phase Polymer-Based Retention: Theory, Modeling, and Application for the Removal of Pollutant Inorganic Ions. J. Chem. 2015, 2015, 965624. [Google Scholar] [CrossRef]
- López-Rodríguez, D.; Micó-Vicent, B.; Jordán-Núñez, J.; Bonet-Aracil, M.; Bou-Belda, E. Uses of Nanoclays and Adsorbents for Dye Recovery: A Textile Industry Review. Appl. Sci. 2021, 11, 11422. [Google Scholar] [CrossRef]
- Alexander, J.A.; Zaini, M.A.A.; Surajudeen, A.; Aliyu, E.N.U.; Omeiza, A.U. Surface Modification of Low-Cost Bentonite Adsorbents—A Review. Part. Sci. Technol. 2019, 37, 538–549. [Google Scholar] [CrossRef]
- Huang, Z.; Li, Y.; Chen, W.; Shi, J.; Zhang, N.; Wang, X.; Li, Z.; Gao, L.; Zhang, Y. Modified Bentonite Adsorption of Organic Pollutants of Dye Wastewater. Mater. Chem. Phys. 2017, 202, 266–276. [Google Scholar] [CrossRef]
- Song, R.; Li, Z.; Li, W.; An, Y.; Li, M.; Qin, H.; Liu, C. Improved Adsorption and Desorption Behavior of Cd on Thiol-Modified Bentonite Grafted with Cysteamine Hydrochloride. Res. Chem. Intermed. 2022, 48, 2721–2744. [Google Scholar] [CrossRef]
- Farghali, R.A.; Basiony, M.S.; Gaber, S.E.; Ibrahim, H.; Elshehy, E.A. Adsorption of Organochlorine Pesticides on Modified Porous Al30/Bentonite: Kinetic and Thermodynamic Studies. Arab. J. Chem. 2020, 13, 6730–6740. [Google Scholar] [CrossRef]
- Derakhshani, E.; Naghizadeh, A. Optimization of Humic Acid Removal by Adsorption onto Bentonite and Montmorillonite Nanoparticles. J. Mol. Liq. 2018, 259, 76–81. [Google Scholar] [CrossRef]
- Hank, D.; Azi, Z.; Hocine, S.A.; Chaalal, O.; Hellal, A. Optimization of Phenol Adsorption onto Bentonite by Factorial Design Methodology. J. Ind. Eng. Chem. 2014, 20, 2256–2263. [Google Scholar] [CrossRef]
- Haounati, R.; Ouachtak, H.; El Haouti, R.; Akhouairi, S.; Largo, F.; Akbal, F.; Benlhachemi, A.; Jada, A.; Addi, A.A. Elaboration and Properties of a New SDS/CTAB@Montmorillonite Organoclay Composite as a Superb Adsorbent for the Removal of Malachite Green from Aqueous Solutions. Sep. Purif. Technol. 2021, 255, 117335. [Google Scholar] [CrossRef]
- Belhadri, M.; Mokhtar, A.; Bengueddach, A.; Sassi, M. Efficient Adsorbent Based on Bentonite Functionalized with 3-Aminopropyltriethoxysilane for Dyes Removal from Aqueous Solutions. Eurasian Chem. Commun. 2021, 3, 881–892. [Google Scholar] [CrossRef]
- Lerma, T.A.; Chate-Galvis, N.; Palencia, M. Study of the Sorption Capacity of Dyes by Organo-Clays Based on Bentonite and Organosilanes. J. Sci. Technol. Appl. 2022, 12, 1–7. [Google Scholar] [CrossRef]
- Guo, W.; Umar, A.; Du, Y.; Wang, L.; Pei, M. Surface Modification of Bentonite with Polymer Brushes and Its Application as an Efficient Adsorbent for the Removal of Hazardous Dye Orange I. Nanomaterials 2020, 10, 1112. [Google Scholar] [CrossRef]
- Fernandes, J.V.; Rodrigues, A.M.; Menezes, R.R.; Neves, G.d.A. Adsorption of Anionic Dye on the Acid-Functionalized Bentonite. Materials 2020, 13, 3600. [Google Scholar] [CrossRef] [PubMed]
- Thue, P.S.; Sophia, A.C.; Lima, E.C.; Wamba, A.G.N.; de Alencar, W.S.; dos Reis, G.S.; Rodembusch, F.S.; Dias, S.L.P. Synthesis and Characterization of a Novel Organic-Inorganic Hybrid Clay Adsorbent for the Removal of Acid Red 1 and Acid Green 25 from Aqueous Solutions. J. Clean. Prod. 2018, 171, 30–44. [Google Scholar] [CrossRef]
- Wamba, A.G.N.; Lima, E.C.; Ndi, S.K.; Thue, P.S.; Kayem, J.G.; Rodembusch, F.S.; dos Reis, G.S.; de Alencar, W.S. Synthesis of Grafted Natural Pozzolan with 3-Aminopropyltriethoxysilane: Preparation, Characterization, and Application for Removal of Brilliant Green 1 and Reactive Black 5 from Aqueous Solutions. Environ. Sci. Pollut. Res. 2017, 24, 21807–21820. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Mansor, E.S.; Taha, G.M. Microfiltration and Adsorptive Membranes for Simultaneous Removal of Methyl Orange and Methylene Blue Using Hybrid Composites. Polym. Bull. 2022, 79, 7891–7908. [Google Scholar] [CrossRef]
- Alardhi, S.M.; Albayati, T.M.; Alrubaye, J.M. A Hybrid Adsorption Membrane Process for Removal of Dye from Synthetic and Actual Wastewater. Chem. Eng. Process. Process Intensif. 2020, 157, 108113. [Google Scholar] [CrossRef]
- Albayati, T.M. Application of Nanoporous Material MCM-41 in a Membrane Adsorption Reactor (MAR) as a Hybrid Process for Removal of Methyl Orange. Desalination Water Treat. 2019, 151, 138–144. [Google Scholar] [CrossRef]
- Januário, E.F.D.; Vidovix, T.B.; Bergamasco, R.; Vieira, A.M.S. Performance of a Hybrid Coagulation/Flocculation Process Followed by Modified Microfiltration Membranes for the Removal of Solophenyl Blue Dye. Chem. Eng. Process. Process Intensif. 2021, 168, 108577. [Google Scholar] [CrossRef]
- Lerma, T.A.; Paradelo, R.; Palencia, M. New Substrate for Plant Growth Based on Granite Powder and Biodegradable Geomimetic Composites Obtained from Bentonite-Poly(Glycerol Citrate). Mater. Today Commun. 2024, 41, 110226. [Google Scholar] [CrossRef]
- Palencia, M. Functional Transformation of Fourier-Transform Mid-Infrared Spectrum for Improving Spectral Specificity by Simple Algorithm Based on Wavelet-like Functions. J. Adv. Res. 2018, 14, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.H.; Al-Jubouri, S.M. Facile Preparation of Dual Functions Zeolite-Carbon Composite for Zinc Ion Removal from Aqueous Solutions. Asia-Pac. J. Chem. Eng. 2024, 19, e2967. [Google Scholar] [CrossRef]
- Khairuddin, K.; Ridhawansa, M.A.; Ruslan, R.; Sardi, B. Efficient Activation of Bentonite Clay for Cyanide Adsorption Using Sulfuric Acid and Sodium Ion Intercalation. Clean. Waste Syst. 2025, 10, 100225. [Google Scholar] [CrossRef]
- Ding, J.; Huang, D.; Wang, W.; Wang, Q.; Wang, A. Effect of Removing Coloring Metal Ions from the Natural Brick-Red Palygorskite on Properties of Alginate/Palygorskite Nanocomposite Film. Int. J. Biol. Macromol. 2019, 122, 684–694. [Google Scholar] [CrossRef]
- Qi, J.; Yu, J.; Shah, K.J.; Shah, D.D.; You, Z. Applicability of Clay/Organic Clay to Environmental Pollutants: Green Way—An Overview. Appl. Sci. 2023, 13, 9395. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Liu, Y.; Ren, J.; Rahman, M.M.; Zhang, H.; Hasan Johir, M.A.; Shon, H.K.; Naidu, R. Capability of Organically Modified Montmorillonite Nanoclay as a Carrier for Imidacloprid Delivery. ACS Agric. Sci. Technol. 2022, 2, 57–68. [Google Scholar] [CrossRef]
- Kgabi, D.P.; Ambushe, A.A. Characterization of South African Bentonite and Kaolin Clays. Sustainability 2023, 15, 12679. [Google Scholar] [CrossRef]
- Pisareva, A.S.; Tikhomirova, T.I. Sorption of Synthetic Anionic Amaranth Dye from an Aqueous Solution on Hydrophobized Silica and Alumina. Russ. J. Phys. Chem. A 2019, 93, 534–537. [Google Scholar] [CrossRef]
- Simonin, J.P. On the Comparison of Pseudo-First Order and Pseudo-Second Order Rate Laws in the Modeling of Adsorption Kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption Kinetic Models: Physical Meanings, Applications, and Solving Methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the Use and Interpretation of Adsorption Isotherm Models: A Review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef]
- Piccin, J.S.; Cadaval, T.R.S.A.; De Pinto, L.A.A.; Dotto, G.L. Adsorption Isotherms in Liquid Phase: Experimental, Modeling, and Interpretations. In Adsorption Processes for Water Treatment and Purification; Bonilla-Petriciolet, A., Mendoza-Castillo, D., Reynel-Ávila, H., Eds.; Springer: Cham, Switzerland, 2017; pp. 19–51. [Google Scholar] [CrossRef]
- Dobe, N.; Abia, D.; Tcheka, C.; Tejeogue, J.P.N.; Harouna, M. Removal of Amaranth Dye by Modified Ngassa Clay: Linear and Non-Linear Equilibrium, Kinetics and Statistical Study. Chem. Phys. Lett. 2022, 801, 139707. [Google Scholar] [CrossRef]
- Lima, E.C.; Gomes, A.A.; Tran, H.N. Comparison of the Nonlinear and Linear Forms of the van’t Hoff Equation for Calculation of Adsorption Thermodynamic Parameters (∆S° and ∆H°). J. Mol. Liq. 2020, 311, 113315. [Google Scholar] [CrossRef]
- Apel, P.Y.; Biesheuvel, P.M.; Bobreshova, O.V.; Borisov, I.L.; Vasil’eva, V.I.; Volkov, V.V.; Grushevenko, E.A.; Nikonenko, V.V.; Parshina, A.V.; Pismenskaya, N.D.; et al. Concentration Polarization in Membrane Systems. Membr. Membr. Technol. 2024, 6, 133–161. [Google Scholar] [CrossRef]
- Etienne, M.; Walcarius, A. Analytical Investigation of the Chemical Reactivity and Stability of Aminopropyl-Grafted Silica in Aqueous Medium. Talanta 2003, 59, 1173–1188. [Google Scholar] [CrossRef]
- Bondarenko, L.; Illés, E.; Tombácz, E.; Dzhardimalieva, G.; Golubeva, N.; Tushavina, O.; Adachi, Y.; Kydralieva, K. Fabrication, Microstructure and Colloidal Stability of Humic Acids Loaded Fe3O4/APTES Nanosorbents for Environmental Applications. Nanomaterials 2021, 11, 1418. [Google Scholar] [CrossRef] [PubMed]
- Ip, A.W.M.; Barford, J.P.; McKay, G. Reactive Black Dye Adsorption/Desorption onto Different Adsorbents: Effect of Salt, Surface Chemistry, Pore Size and Surface Area. J. Colloid Interface Sci. 2009, 337, 32–38. [Google Scholar] [CrossRef] [PubMed]






| Separation Method | Adsorbent | Analyte | Adsorption Capacity | Ref. |
|---|---|---|---|---|
| Adsorption | Bentonite functionalized with APTES * | Methylene blue | 217.4 mg g−1 | [34] |
| Adsorption | Bentonite functionalized with APTES | Basic violet 10 Direct blue 1 Acid red 27 | 5.6 mg g−1 10.1 mg g−1 9.7 mg g−1 | [35] |
| Adsorption | Bentonite grafted with poly(2-(dimethylamino)ethyl methacrylate) | Orange 1 | 700 mg g−1 | [36] |
| Adsorption | Bentonite functionalized with acids (HCl and H2SO4) | Methyl orange | 67.4 mg g−1 (HCl) 47.8 mg g−1 (H2SO4) | [37] |
| Adsorption | Montmorillonite functionalized with APTES | Acid red 1 Acid green 25 | 364.1 mg g−1 397.0 mg g−1 | [38] |
| Adsorption | Pozzolan functionalized with APTES | Brilliant green 1 Reactive black 5 | 350.6 mg g−1 300.9 mg g−1 | [39] |
| Adsorption—microfiltration | Polyethylene oxide/bentonite/polyaniline composite membrane | Methyl orange Methylene blue | 94% 96% | [40] |
| Adsorption—microfiltration | Synthesized mesoporous material | Methyl green | 97% | [41] |
| Adsorption—microfiltration | Synthesized nanoporous material | Methyl Orange | 151.5 mg g−1 | [42] |
| Coagulation/flocculation—microfiltration | Potato starch | Solophenyl blue | 100% | [43] |
| Sample | Particle Size | Zeta Potential | Elemental Composition (%) | |||||
|---|---|---|---|---|---|---|---|---|
| (nm) | (mV) | O | Si | Al | Fe | N | C | |
| BAPTES | 1625 ± 137 | 3.5 ± 1.0 | 41.67 | 24.02 | --- | 6.82 | 8.22 | 4.11 |
| Bentonite | 268 ± 16 | −16.6 ± 2.2 | 50.82 | 24.07 | 22.37 | 22.16 | --- | --- |
| Sample | Dye Retention (%) | Dye Retention (mg/g) |
|---|---|---|
| AR27 | ||
| BAPTES | 86.06 ± 0.12 | 35.52 ± 0.05 |
| Bentonite | 2.10 ± 0.12 | 0.81 ± 0.14 |
| Qe (exp) (mg g−1) | Pseudo-First Order Model | Pseudo-Second Order Model | ||||
|---|---|---|---|---|---|---|
| k1 (h−1) | Qe (mg g−1) | R2 | k2 (g mg−1 h−1) | Qe (mg g−1) | R2 | |
| 39.5 ± 1.1 | 0.178 ± 0.032 ** | 40.38 ± 2.21 *** | 0.975 | 3.95 × 10−3 ± 1.82 × 10−3 ’ | 47.80 ± 5.20 *** | 0.935 |
| Langmuir model | ||
|---|---|---|
| QL (mg g−1) | KL (L mg−1) | R2 |
| 64.81 ± 4.24 *** | 0.055 ± 0.021’ | 0.913 |
| Freundlich model | ||
| kF (Ln mg1-n g−1) | n | R2 |
| 17.58 ± 1.28 *** | 0.204 ± 0.012 *** | 0.989 |
| Temkin model | ||
| KT (L mg−1) | f (J mol−1) | R2 |
| 2.42 ± 0.54 ** | 280.04 ± 10.79 *** | 0.993 |
| Variable | Blank | E1 | E2 | E3 | E4 | E5 |
|---|---|---|---|---|---|---|
| Ci (mg L−1) | 109.8 | 99.1 | 97.3 | 100.4 | 105.4 | 98.8 |
| mBAPTES (g) | --- | 0.0501 | 0.0502 | 0.0501 | 0.0501 | 0.0503 |
| Rsys (mg m−2) | 82.06 | 82.06 | 82.06 | 82.06 | 82.06 | 82.06 |
| RBAPTES (mg g−1) | 0.0 | 26.70 | 18.14 | 33.63 | 14.25 | 15.07 |
| RBAPTES (%) | 1.88 | 29.03 | 20.77 | 35.56 | 15.48 | 17.33 |
| Cc (mg L−1) | 2.06 | 28.76 | 20.21 | 35.69 | 16.31 | 17.12 |
| texp (h) | 9.54 | 2.17 | 2.19 | 4.31 | 0.91 | 1.04 |
| Variable | Value | QD (mg g−1) | % |
|---|---|---|---|
| pH | 5 | 3.05 ± 0.38 | 15.3 ± 1.9 |
| 7 | 4.42 ± 1.07 | 22.2 ± 5.3 | |
| 9 | 6.28 ± 0.38 | 31.6 ± 1.8 | |
| Ionic strength (% NaCl) | 0.0 | 4.02 ± 0.76 | 20.2 ± 3.82 |
| 0.5 | 5.24 ± 0.38 | 26.3 ± 2.1 | |
| 1.0 | 5.34 ± 0.20 | 26.8 ± 1.0 | |
| Ethanol | --- | 0.10 ± 0.03 | 0.5 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lerma, T.A.; Chamorro, A.F.; Palencia, M.; Combatt, E.; Valle, H. Organoclay Microparticle-Enhanced Microfiltration for the Removal of Acid Red 27 in Aqueous Systems. Water 2025, 17, 2817. https://doi.org/10.3390/w17192817
Lerma TA, Chamorro AF, Palencia M, Combatt E, Valle H. Organoclay Microparticle-Enhanced Microfiltration for the Removal of Acid Red 27 in Aqueous Systems. Water. 2025; 17(19):2817. https://doi.org/10.3390/w17192817
Chicago/Turabian StyleLerma, Tulio A., Andrés Felipe Chamorro, Manuel Palencia, Enrique Combatt, and Hernán Valle. 2025. "Organoclay Microparticle-Enhanced Microfiltration for the Removal of Acid Red 27 in Aqueous Systems" Water 17, no. 19: 2817. https://doi.org/10.3390/w17192817
APA StyleLerma, T. A., Chamorro, A. F., Palencia, M., Combatt, E., & Valle, H. (2025). Organoclay Microparticle-Enhanced Microfiltration for the Removal of Acid Red 27 in Aqueous Systems. Water, 17(19), 2817. https://doi.org/10.3390/w17192817







