1. Introduction
The annual loss of property caused by metal corrosion is about 4% of the gross national product, according to the American Society for Metals, and the annual scrap of metal due to corrosion is 30% of the production. The annual economic loss due to rusting in China is 300 billion yuan, and the loss of the steel is equivalent to the annual output of Baotou Iron and Steel Company. If indirect losses are considered, the total cost of corrosion is estimated to be up to 500 billion yuan, accounting for about 5% of the total output value of the national economy [
1].
Atmospheric corrosion is the electrochemical corrosion of metal surfaces under extremely thin liquid films. The corrosion process is a comprehensive and intersecting engineering problem involving a gas phase and liquid phase. The atmospheric environment in different regions is not identical, and the corrosion mechanism, influencing factors, and degrees of corrosion of local transmission and substation equipment are also different. If a single anti-corrosion measure is taken, in different atmospheric corrosion environments, it may lead to unsatisfactory anti-corrosion effects in areas with harsh environments [
2], or an increase in the cost of anti-corrosion in some mildly corrosive areas. In order to enhance the corrosion protection effect of Shanxi State Grid transmission and transformation equipment and improve the reliability of power supply of the power grid, it is necessary to carry out research on the corrosion mechanism of the atmospheric environment on power transmission and transformation equipment in accordance with the characteristics of Shanxi’s atmospheric environment.
2. Power Transmission and Transformation Metal Materials
A large number of metal materials are used in power transmission and transformation equipment and components. Their main functions can be divided into two categories: One is the bearing role, as structural parts have a support, transmission, tightening function, are generally steel-based, such as transmission towers, have a switchgear operation transmission mechanism, including various types of fastening bolts and steel cores in the wire. The other category plays a role in carrying current, as a conductive function plays the role of transmitting current, most of which are aluminum and copper and their alloys, such as various aluminum and aluminum alloy strands on transmission lines, conductive rods and tentacles in switchgear, windings and leads in transformers, etc. In this section, several metal materials are selected, such as carbon steel, stainless steel, aluminum and aluminum alloys, copper and copper alloys that are most used in transmission and substation equipment, as shown in
Table 1.
3. Investigation of Shanxi Atmospheric Environment
With its abundant coal resources, Shanxi has made significant contributions to the country’s industrial construction and was once known as “one of the eight major industrial cities in China”. As a large province of coal resources [
3], the industrial structure of Shanxi Province is heavy with energy and raw material industry as the main body. Among them, the air pollution in Linfen is more severe, and the sulfur dioxide in Linfen mainly originates from civil loose coal, industrial coal, and pollution generated in the industrial process, reflecting the characteristics of soot-type pollution. The main coal used by residents is medium- and high-sulfur coal, especially the scattered coal combustion of urban village residents, as well as the combustion of coal in small and medium-sized boilers, which is an important cause of the current sulfur dioxide pollution in Linfen. The corrosion effect of sulfur dioxide on the metal equipment of the transmission and substations is more severe, which makes the local atmospheric environmental pollution level in Linfen higher.
In addition, the provincial capital city Taiyuan, as a resource city, is developed by resources. However, with the deterioration of the environment and the transformation and upgrading of the industrial structure, the pollution of this city is becoming more and more severe, mainly with dust pollution, and the main impact of dust on the equipment of the transmission and substations is pollution flash failure, resulting in massive power outages.
4. Influence of Gas Phase Factors on Corrosion
Meteorological factors in the atmosphere directly affect the corrosive effect of the atmosphere, including relative humidity in the atmosphere, metal surface wetting time, air temperature, sunshine time, rainfall, and other factors [
4].
4.1. Relative Humidity
From the perspective of corrosion mechanisms, atmospheric corrosion is essentially a chemical or electrochemical reaction of underwater film. The water film condensed by the moisture in the air on the surface of the material is one of the basic conditions for atmospheric corrosion, and the formation of the water film is closely related to the humidity in the atmosphere.
Depending on the degree of moisture on the surface of the corroded metal, atmospheric corrosion can be divided into three categories: Dry atmospheric corrosion, tidal atmospheric corrosion, and wet atmospheric corrosion [
5]. The effect of air humidity on metal corrosion depends on the relative humidity (RH), which refers to the ratio of water vapor pressure in the air to saturated water vapor pressure at the same temperature, generally expressed as a percentage. The effect of relative humidity on the atmospheric corrosion of metal materials is not linear. There is a certain relative humidity value. When the relative humidity of the environment exceeds this value, the corrosion rate of the metal will increase rapidly, and this value is called critical relative humidity. The relative humidity of different metals is different, and commonly used metal materials such as iron it is 65%, in zinc it is 70%, in aluminum it is 76%, and in nickel it is 70%.
Li et al. [
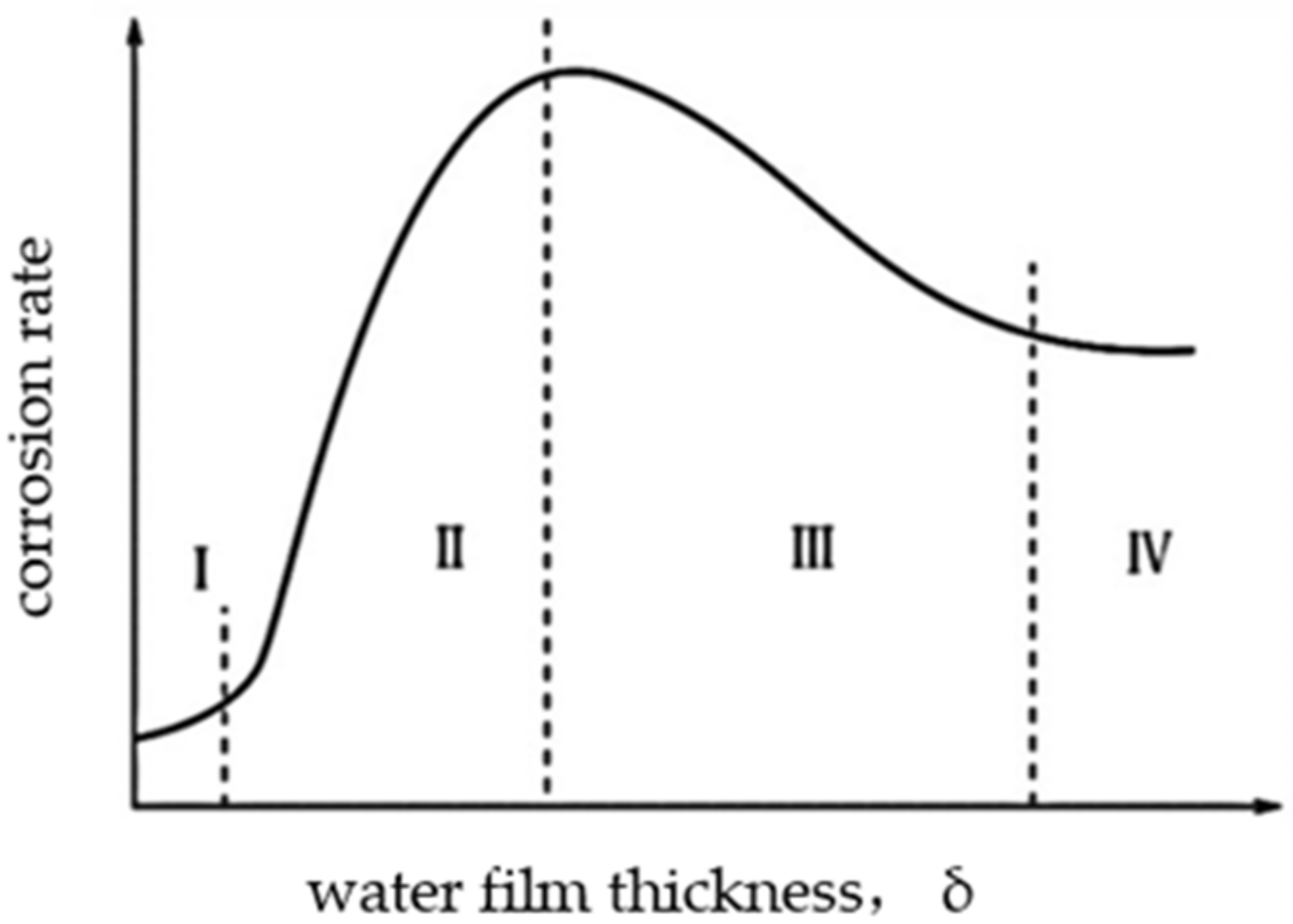
6] used the electrochemical impedance spectroscopy (EIS) method to study the atmospheric corrosion behavior of weathering steel in different relative humidities in an experimental simulation environment. The results show that when the RH is greater than 60%, the water molecules adsorbed on the smooth metal surface reach three monolayers, and when the humidity is greater than 70%, the corrosion process begins to occur. The relationship between the thickness of the water film and the corrosion rate is shown in
Figure 1 [
7].
It can be seen that at the beginning, with the increase in the thickness of the water film, a continuous electrolyte film layer is formed, and the oxygen is more easily diffused into the interface, and the metal corrosion rate increases rapidly. However, as the surface water film continues to thicken, the process of oxygen diffusion through the liquid film to the interface is blocked, and the reaction rate gradually decreases, and when the liquid film thickness is greater than 1 mm, it is equivalent to the corrosion of full immersion in the electrolyte, and the corrosion rate is basically unchanged. At the beginning, because the surface liquid film is very thin, oxygen can easily reach the metal surface, and the equilibrium potential of oxygen is more positive than the equilibrium potential of hydrogen, so the depolarization corrosion of oxygen occurs first in the aerobic solution. The reaction of the cathodic process is as follows:
In neutral or alkaline media:
In acidic media such as acid rain:
The anode process of corrosion is the process of dissolution of the metal as an anode, the loss of electrons, and, under the condition of atmospheric corrosion, the reaction of the anode process is:
Guo et al. [
8] studied the effects of relative humidity changes on stainless steel pitting under MgCl
2 droplets by in situ X-ray microtomography and optical microscopy. It was found that relative humidity fluctuations (between 12% and 33% or between 33% and 85%) can lead to many small pit nuclei, while continuous exposure below a constant 33% leads to the growth of individual pits. This conclusion shows that changes and fluctuations in relative humidity affect the formation process of metal corrosion morphology.
4.2. The Temperature
Temperature is also considered an important factor in corrosion in temperate climate zones [
9]. The ambient temperature and its changes affect the condensation of water vapor on the surface of the metal, the solubility of various corrosive gases and salts in the water film, the resistance of the water film, and the reaction rate of the cathode and anode processes in the corrosion battery. On the one hand, according to the Arrhenius formula:
where
k is the rate constant,
R is the molar gas constant,
T is the thermodynamic temperature,
Ea is the apparent activation energy, and
A is the frequency factor.
Increasing temperature speeds up the rate of anode reaction, which in turn accelerates corrosion. On the other hand, the increase in the greenhouse will also affect the thickness of the liquid film on the surface of the metal and reduce the relative humidity.
Considering the influence of temperature and atmospheric relative humidity, when the relative humidity is lower than the critical relative humidity of metals, the influence of temperature on atmospheric corrosion is very small, but when the relative humidity reaches the critical humidity of metals, the influence of temperature is obvious. Violent changes in temperature also affect atmospheric corrosion, when the temperature difference between day and night is too large and, coupled with suitable humidity, the water vapor in the atmosphere will condense on the metal surface. Xu et al. [
10] have shown that surface condensation is an important feature of the initial stage of atmospheric corrosion of metals, and changes in temperature will promote this process. It follows that the key factor in atmospheric corrosion of metals and alloys is the combined effect of temperature–humidity.
5. The Effect of Atmospheric Pollutants on Corrosion
Atmospheric pollutants are mainly composed of gases containing elements S, C, and N, as well as other solid particles. It is generally believed that the main components of atmospheric pollutants are shown in
Table 2 [
11].
Atmospheric pollutants are extremely important for the corrosion of metals, such as harmful gases in the atmosphere and solid particulate matter, etc. Sulfur oxide SOx is the most seriously affected in gases, especially SO2, which is very high in the atmosphere of cities or industrial areas. The composition of solid particles is more complex, among which those with a more severe impact on corrosion are chloride-containing particles, soluble and corrosive dust particles such as ammonium salts, and particles that can adsorb corrosive substances, such as carbon particles.
5.1. Cl−
Chloride ions in sea salt particles are highly penetrating and can easily penetrate the matrix through corrosion products on metal surfaces [
12]. In the early stages of corrosion, due to the presence of chloride (NaCl), moisture absorption is very strong, the surface of the steel quickly absorbs moisture from the humid atmosphere, and quickly forms countless corrosive microcells, resulting in enhanced conductivity in the thin liquid film. Salt is the core to absorb water and, with the formation of saturated solution, begins to corrode a small area as the reaction progresses. Na
+, Fe
2+ move to the cathode region and OH
−, Cl
− move to the anode dissolution region, generating Fe(OH)
2 near the active anode region. The weak point of the Fe(OH)
2 membrane ruptures, and the outflow of electrolyte forms a “head” outside the original corrosion point, which in turn causes corrosion, and a series of processes (rupture–corrosion–film formation) are repeated countless times to form a continuous filament. However, the Fe(OH)
2 corrosion product film is not stable, it decomposes into FeO or gradually oxidizes with O
2 dissolved in the thin liquid film to FeOOH (Equations (5) and (6)):
Meanwhile, some FeOOH gradually sheds water to form a more stable insoluble substance—Fe
2O
3:
In addition, Fe(OH)
2 is slowly oxidized to Fe
3O
4:
Due to the strong erosive nature of Cl
−, it not only acts as a conductive medium, but also destroys the protective film on the metal surface at the same time as the reactions occur. This process can be described by the following mechanism:
According to the above chemical reaction equation, during atmospheric corrosion, Cl
− causes local dissolution of Fe(OH)
2. This leads to the appearance of a fine crack in the dense oxide film through which the corrosive medium penetrates into the matrix, promoting the continuous extension and development of the filament, thereby accelerating the corrosion of the matrix [
13]. However, as corrosion progresses, the β formed in the inner layer FeOOH gradually decreases and is converted to Fe
2O
3 during the wet and dry cycle. The generation of insoluble Fe
2O
3 effectively protects the matrix and inhibits corrosion [
14].
5.2. SO2
At present, the mechanism of SO
2 accelerating the corrosion rate of metals has two main causes. First, due to the thickening of the water film condensation, SO
2 participates in the anode depolarization under high humidity conditions. This effect is significantly increased when the SO
2 content is greater than 0.5%. Although the content of SO
2 in the atmosphere is very low, its solubility in solution is about 1300 times higher than that of oxygen, which has a great impact on corrosion. The second is that a part of SO
2 is adsorbed on metal surfaces (such as iron) and reacts with iron to form soluble ferrous sulfate. Further oxidation of ferrous sulfate and formation of sulfate due to strong hydrolysis occur. Sulfuric acid reacts with iron, and the whole process has the characteristics of a self-catalytic reaction. The reaction is as follows:
Metals such as iron and zinc form soluble sulfate compounds in SO
2 atmospheres, and their corrosion rates are linear with SO
2 content [
15]. In addition, the corrosive effect of SO
2 on metals is also affected by temperature, and the temperature rise intensifies the corrosion process of SO
2 on metals.
5.3. Solid Deposits
The composition of solid particles is more complex as, in addition to sea salt particles, there are carbon and carbon compounds, nitrates, metal oxides, sand, etc. Ammonium salt particles are inherently soluble and corrosive, and when they dissolve in a liquid film to become a corrosive medium, they increase the corrosion rate. Under the same conditions, NH
4Cl has the most obvious corrosion acceleration effect on Q235 steel compared with NaCl, (NH
4)
2SO
4, Na
2SO
4. This is because the chloride ion radius is smaller than the sulfate ion radius, which more easily penetrates the matrix of Q235 steel. Moreover, ammonium chloride easily absorbs moisture and undergoes hydrolysis, producing strong acidity and promoting the dissolution of steel. Although the carbon particle itself is non-corrosive and does not dissolve, it can adsorb corrosive substances, and when the corrosive substances are dissolved in the water film, it can promote the corrosion process. There are also some dusts that are inherently non-corrosive and adsorbent, but can form crevices with the metal surface when they fall on the metal surface. These crevices can increase the condensation of water vapor, which in turn promotes the formation of an electrolyte film on the metal surface. At the same time, this will affect the size of the air contact opportunity at the interface, forming a local oxygen concentration difference, causing crevice corrosion, and accelerating the corrosion of the metal [
16]. Considered comprehensively, the ammonium salts with strong water absorption in solid sediments may bind chloride ions and accelerate the corrosion of metals.
6. Study on the Influence of Shanxi Atmospheric Environment on Corrosion of Power Transmission and Transformation Metal Equipment
In this study, the hanging experiment is carried out in 11 cities and nearly one hundred 220KV substations in Taiyuan, Yuncheng, Linfen, Jincheng, Changzhi, Lüliang, Jinzhong, Xinzhou, Datong, Shuozhou, and Yangquan in Shanxi. The metal materials of the hanging sheet include galvanized steel and carbon steel. After 380–420 days of hanging tablet experiments, the hanging sample was pickled to obtain the weight after pickling, and then the corrosion rate was calculated. The corrosion rates of each locality were compared and analyzed according to the data of environmental factors such as temperature, humidity, SO2 concentration, cl- deposition rate, etc., and the relationship was explored.
6.1. Corrosion of Hanging Sheets in Shanxi Substation
The hanging plate experiment was carried out near the selected substation, the hanging plate size was 150 × 100 × 4 mm, and each group of 3 pieces was parallel sampled. The original weight of the specimen w
0 was recorded. The experimental apparatus is shown in
Figure 2. After a test of about a year, the hanging piece was retrieved and the corrosion loss measurement was performed on the hanging piece. The descaling for measuring corrosion quality loss was carried out with reference to GB/T16545-1996 “Corrosion of metals and alloys—Removal of corrosion products from corrosion test specimens”.
Using 500 mL of concentrated hydrochloric acid +500 mL of distilled water +3.5 g of six times methenamine rust removal solution, the specimen was soaked at room temperature, ultrasonicated, and brushed for 10 min, until the rust was removed. At the same time, the corrosion of the descaling liquid on the matrix was corrected by uncorroded steel samples. The rust-removed specimen was washed, dehydrated with absolute ethanol, blown dry and placed in a dryer, and taken out and weighed after 24 h. In each group, 3 parallel specimens were used to determine the corrosion quality loss value. The corrosion quality loss rate is calculated as follows:
where
v is the corrosion quality loss rate, the unit is g/cm
2·a.
w0 represents the original weight of the specimen, the unit is g.
wt represents the weight of the specimen after rust removal, the unit is g.
S is the corrosion area of the specimen, the unit is cm
2.
t is the exposure time, the unit is a.
The corrosion thickness loss rate is calculated as follows:
where
R is the corrosion loss thickness rate, the unit is mm/a;
S is the corrosion area of the specimen, the unit is cm
2;
is the density of the metal, the unit is g/cm
3;
t is the exposure time, the unit is a.
Through the above method, the corrosion rate of each city is measured, and the specific data are shown in
Table 3.
According to GB/T19292.2-2018 “Corrosion of metal and alloys—Corrosivity of atmospheres—Part 2: Guiding values for the corrosivity categories”, the atmospheric corrosiveness grades (C1–CX) of each region can be determined according to the corrosion rate of standard metals (carbon steel, zinc, copper).
Table 4 shows the correspondence between the atmospheric corrosion level and the standard specimen corrosion rate.
According to the data of
Table 3 and
Table 4, 6 of the 11 Shanxi cities are in the C3 level atmospheric environment, 4 are in the C4 level atmospheric environment, and 1 is in the C5 level atmospheric environment. From the corrosion hanging film experiment, it can be seen that the overall air pollution level of Shanxi city is medium to high, of which Linfen has the most serious corrosion and is at the C5 level.
6.2. Monitoring Results of Atmospheric Corrosion Environment of Shanxi Power Grid
The monitoring of the atmospheric corrosion environment of the above 11 cities includes: The annual average temperature of the atmospheric environment, the annual rainfall, the annual average sulfur dioxide concentration, and the annual average chloride ion deposition rate. Among them, the annual average temperature, relative humidity, and annual rainfall data of the atmospheric environment are from the Shanxi Meteorological Bureau [
17], and the sulfur dioxide concentration is from the Department of Ecology and Environment of Shanxi Province [
18]. The chloride ion deposition rate is determined in accordance with the standard GB/T19292.3-2018 with a chloride ion monitoring device, the atmospheric environment data are accurate to the municipal level, and the monitoring data are shown in
Table 5.
According to the relative humidity and rainfall, the atmospheric environment can be divided into different types, such as dry type (RH < 60%), ordinary type (RH 60~75%), and wet type (RH > 75%). These cities are all dry type and the coupon test was a one-year short-term test. The climate factor of relative humidity has little effect on the corrosion of carbon steel. When the humidity in the atmosphere reaches 60%, the surface of carbon steel will be corroded slowly and, when the humidity reaches 80–100%, the main factor of corrosion is air pollution [
19].
These 11 cities all belong to a typical industrial atmospheric environment. At the initial stage of atmospheric corrosion, SO
2, Cl
−, and other pollutants have a great impact on the corrosion of carbon steel. According to the calculation of a carbon steel corrosion rate prediction equation [
20], the sum of SO
2 and Cl
− air pollutant concentrations in Linfen city is the highest, so the corrosion grade is the highest. When the temperature is less than or equal to 10 °C, the temperature does not promote or even hinders SO
2 corrosion. Therefore, although the SO
2 concentrations in Datong city and Shuozhou city are the highest, the corrosion grade is low.
Combined with the concentration of major pollutants in Shanxi cities this year and the year-on-year change rate, as shown in
Table 6, according to the Ambient Air Quality Standards [
21], the PM2.5 standard is an annual average concentration of 35 and the PM10 standard is an annual average concentration of 70. The PM2.5 in Shanxi Province exceeded the standard by 0.37 times, the PM10 exceeded the standard by 0.33 times, and the concentrations of the remaining two pollutants reached the standard. It can be found that Linfen, Jincheng, and Taiyuan exceed the standard in PM2.5 and PM10 concentrations. The main components of PM particulate pollutants in Shanxi are heavy metals (Zn, Pb, As, Cd, Cu), water-soluble inorganic ions (SO
42−, NO
3−, NH
4+), and carbon particles. However, Linfen’s chloride ion deposition rate and average annual temperature are higher than those of the other two cities, and such solid deposits can absorb water, making it easier for the metal surface to reach the relative critical humidity to form a water film. In turn, it promotes the formation of water film in an environment with low relative humidity. Coupled with the combined effect of chloride ions and temperature, the corrosion of the metal is accelerated, resulting in the highest atmospheric corrosion level. This conclusion explains the corrosion situation in Shanxi cities very well.
7. Summary
Therefore, through the above analysis, in the scope of Shanxi, the main pollutants are particulate pollutants. Although the overall relative humidity is not high, because the metal surface is attached to more solid particles, it is easier for the metal surface to reach critical relative humidity. The deposition rate of chloride ions has a greater impact. Chloride ions have a small ionic radius, which can easily diffuse and contact the matrix at the defect of the oxide film on the surface of the material, causing corrosion. After atmospheric corrosion occurs, the increase in temperature is particularly important for the impact of the corrosion process.
For the atmospheric environment of Shanxi, the corrosion situation can be effectively alleviated by using galvanized steel materials. According to the comparison of the experimental data of galvanized steel and carbon steel hanging sheets in
Table 3, the anti-corrosion effect of galvanized steel is obvious. In addition, for heavily polluted areas, the sediment on key metal parts of the power grid ought to be cleaned regularly to avoid the acceleration of metal corrosion caused by sediments.
Author Contributions
Conceptualization, X.C.; methodology, X.C. and Z.Z.; validation, Z.Z. and H.Z.; formal analysis, X.C. and H.Z.; investigation, F.L.; resources, H.Y.; data curation, S.T.; writing—original draft preparation, X.C.; writing—review and editing, Z.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Research on anti-corrosion technology of power transmission and transformation engineering equipment in high pollution areas and development and application of atmospheric monitoring system, grant number 52053020002R.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hongjun, H.; Hongjing, W.; Guangzhi, L. Material and Technology of Vapor Phase Rust Prevention; Metallurgical Industry Press: Beijing, China, 2011. [Google Scholar]
- Chao, H. Study on the distribution characteristics of coal resources and the distribution of exploration and development in Shanxi. Coal Geol. China 2020, 32, 159–162. [Google Scholar]
- Yun, C.; Limin, X.; Ningna, Y. Atmospheric corrosion and protection of power transmission and transformation steel components. North China Electr. Power Technol. 2014, 12, 10–14. [Google Scholar]
- Ye, W. Atmospheric Corrosion of Metals and Its Experimental Methods; Beijing Chemical Industry Press: Beijing, China, 2013. [Google Scholar]
- Chinese Society for Corrosion and Protection. Corrosion and Protection of Natural Environment; Chemical Industry Press: Beijing, China, 1996. [Google Scholar]
- Li, C.; Ma, Y.; Li, Y. EIS monitoring study of atmospheric corrosion under variable relative humidity. Corros. Sci. 2010, 52, 3677–3686. [Google Scholar] [CrossRef]
- Yuxia, P.; Mei, W.; Zhigao, W. Influence of atmospheric corrosive environment on the corrosion of transmission and transformation equipment in Sichuan Power Grid. Mater. Prot. 2018, 51, 110–113. [Google Scholar]
- Guo, L.; Street, S.R.; Mohammed-Ali, H.B. The effect of relative humidity change on atmospheric pitting corrosion of stainless steel 304L. Corros. Sci. 2019, 150, 110–120. [Google Scholar] [CrossRef] [Green Version]
- GB/T19292.1-2018; Corrosion of Metals and Alloys Atmospheric Corrosivity Part 1: Classification, Determination and Evaluation. Standardization Administration of China: Beijing, China, 2018.
- Naixin, X.; Lingyuan, Z.; Cuihong, D. A new technology to study the condensation behavior of atmospheric corrosion metal surface. Chin. J. Corros. Prot. 2001, 5, 46–50. [Google Scholar]
- Ye, W. Influence of Pollution Factors on Atmospheric Corrosion Behavior of Metals; Materials Science, Institute of Metals, Chinese Academy of Sciences: Beijing, China, 2005. [Google Scholar]
- Morcillo, M.; Rosales, B.; Almeida, E. Atmospheric corrosion of mild steel. Part II—Marine atmospheres. Mater. Corros. 2000, 51, 865–874. [Google Scholar]
- Xiaogang, L.; Chaofang, D.; Kui, X.; Du, C.; Zhou, H.; Lin, C. Initial Behavior and Mechanism of Metal Atmospheric Corrosion; Science Press: Beijing, China, 2009. [Google Scholar]
- Yuantai, M. Study on Corrosion Behavior of Low Carbon Steel in Typical Atmospheric Environment; Corrosion Science and Protection, Institute of Metals, Chinese Academy of Sciences: Beijing, China, 2009. [Google Scholar]
- Qiuxia, S. Material Corrosion and Protection; Metallurgical Press: Beijing, China, 2001. [Google Scholar]
- Xu, N.; Zhao, L. Laboratory observation of dew formation at an early stage of atmospheric corrosion of metals. Corros. Sci. 2002, 44, 163–170. [Google Scholar] [CrossRef]
- Shanxi Meteorological Bureau. Available online: http://sx.cma.gov.cn/ (accessed on 30 December 2021).
- Department of Ecology and Environment of Shanxi Province. Available online: https://sthjt.shanxi.gov.cn (accessed on 30 December 2021).
- Hui, S. Study on classification of steel corrosive environment. Sichuan Architecture. Build. Equip. Build. Mater. 2018, 41, 220–224. [Google Scholar]
- ISO 9223-2012; Corrosion of Metals and Alloys—Corrosivity of Atmospheres—Classification. ISO: Geneva, Switzerland, 2012.
- GB 3095-2012; Ambient Air Quality Standard. Standardization Administration of China: Beijing, China, 2012.
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).