The Synthesis of FeCl3-Modified Char from Phoenix Tree Fruit and Its Application for Hg0 Adsorption in Flue Gas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Char Preparation from Phoenix Tree Fruit and FeCl3 Modification
2.2. Analytical Methods
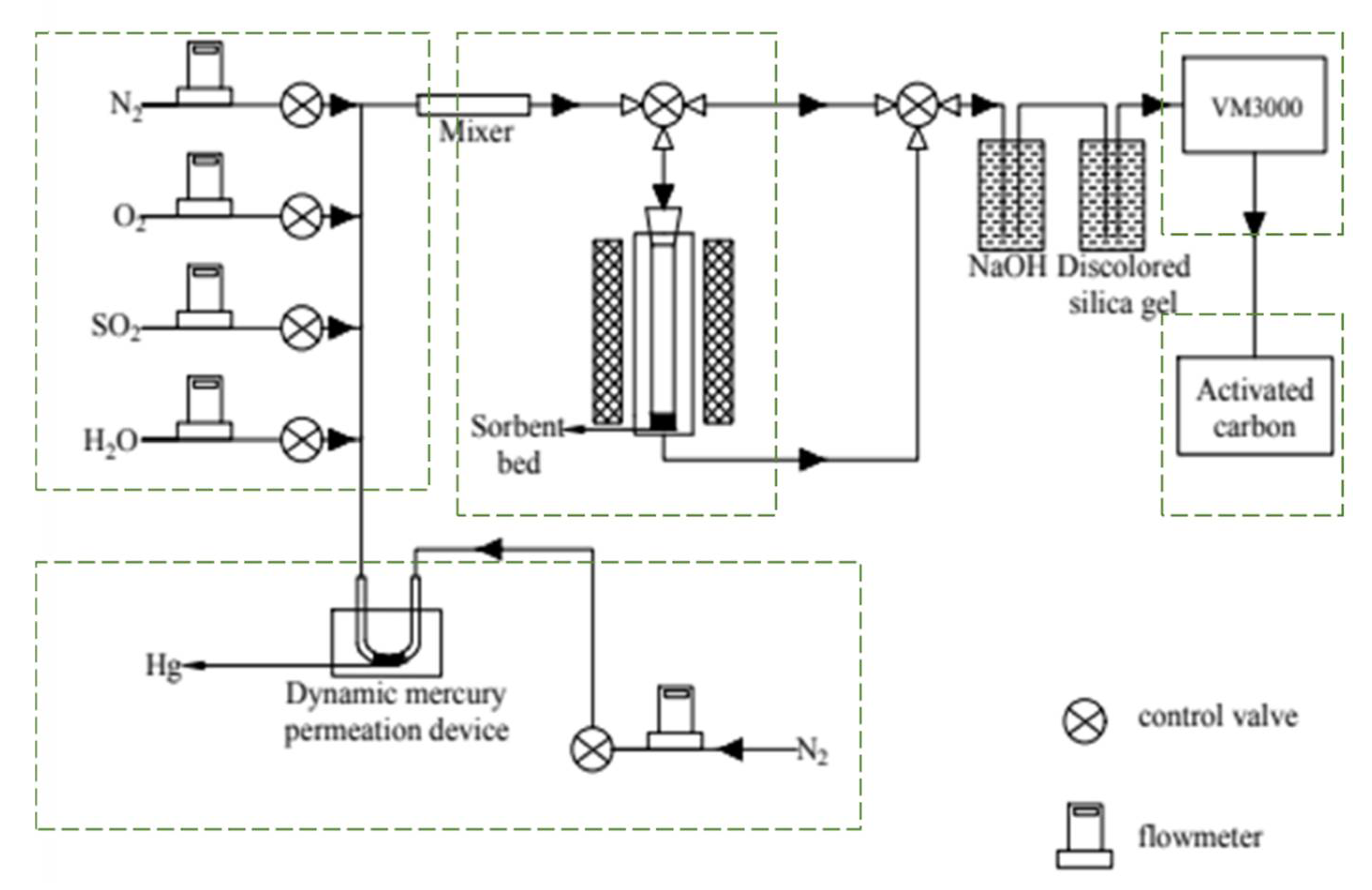
2.3. Hg Adsorption Experiments
3. Results and Discussions
3.1. Properties of the Phoenix Tree Fruit
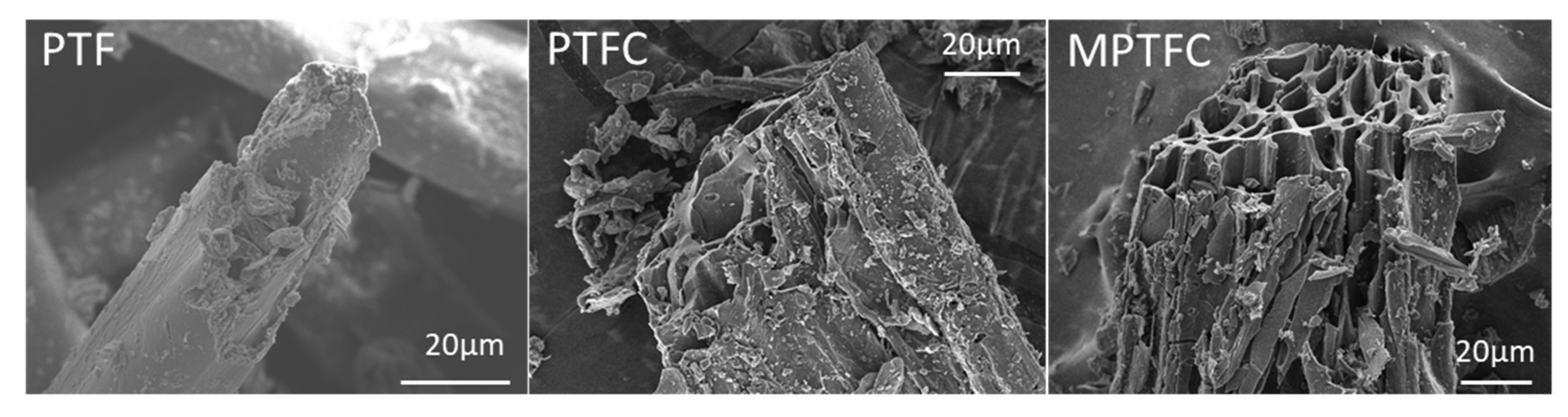
3.2. SEM Observations
3.3. BET Analysis of the Sorbents
3.4. Hg0 Adsorption Performance
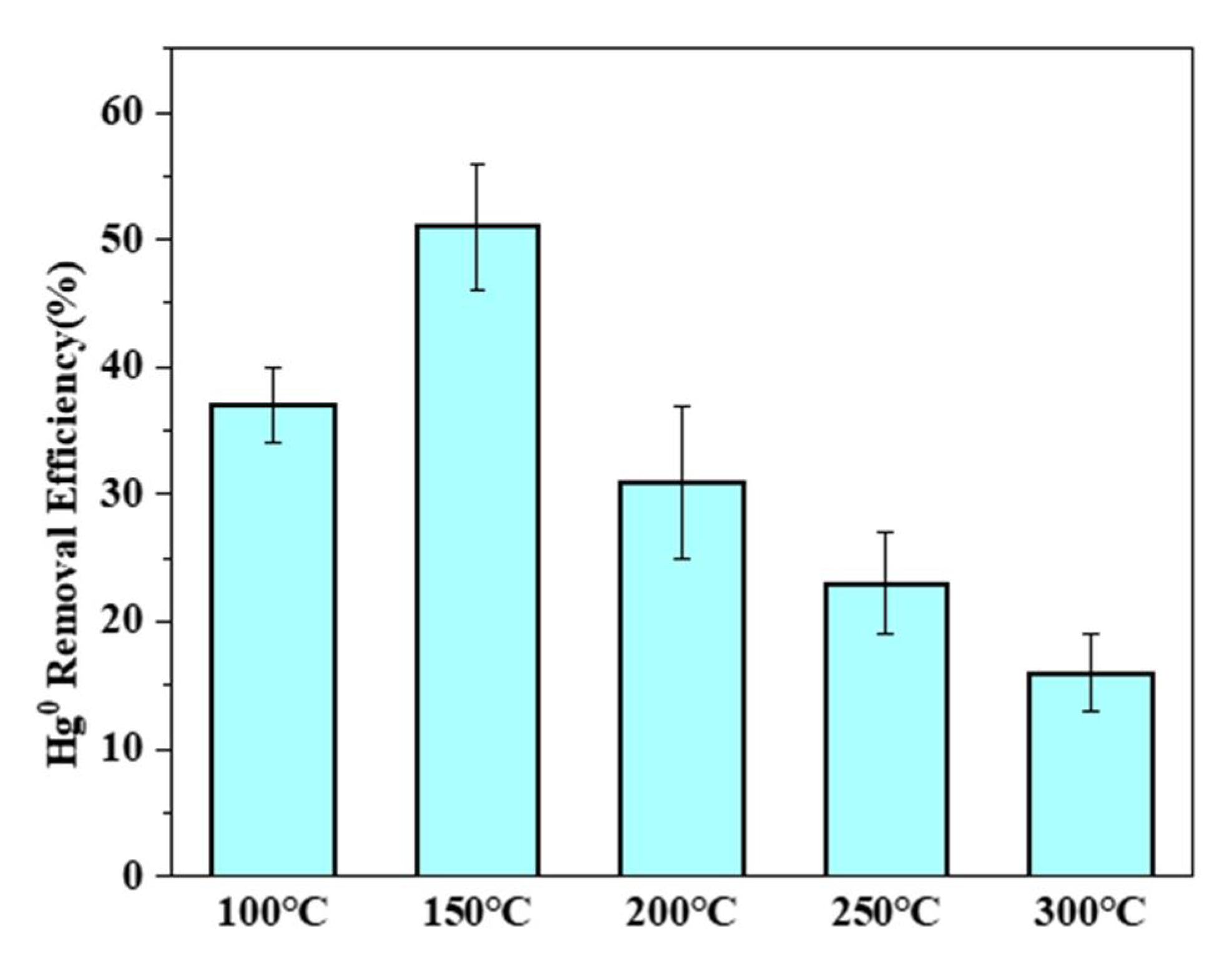
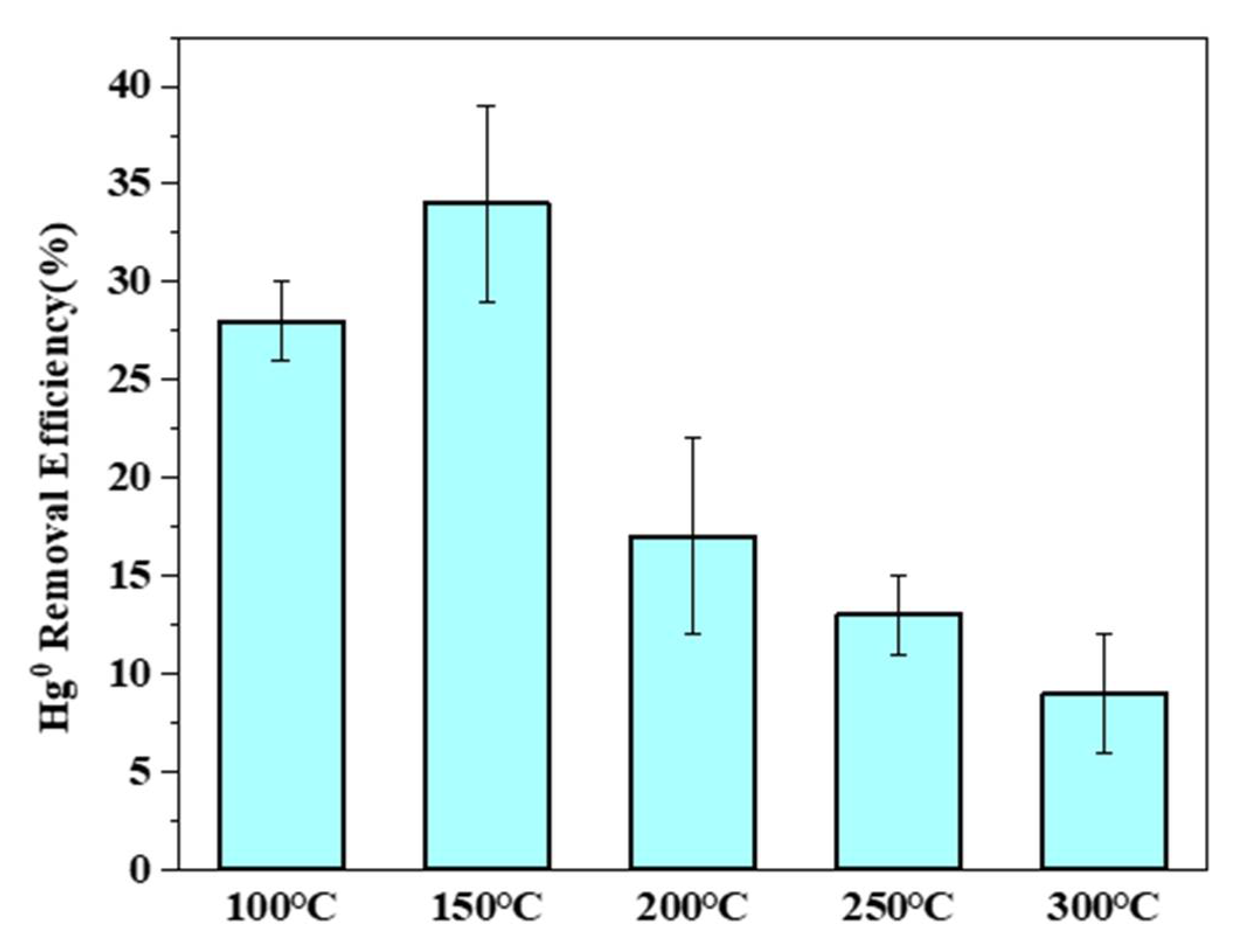
3.4.1. Effects of Temperature on Hg0 Removal by PTFC
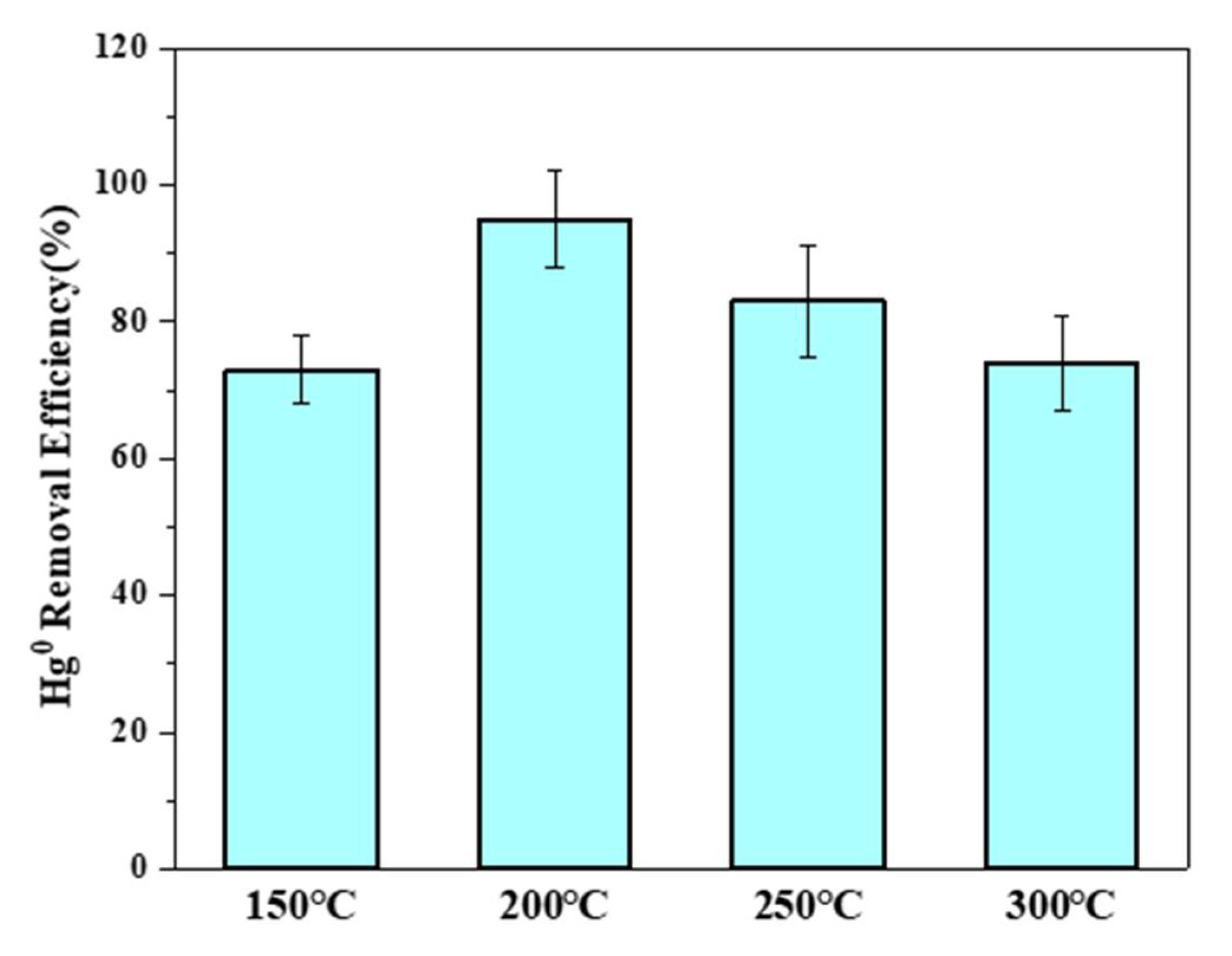
3.4.2. Effects of Temperature on Hg0 Removal by MPTFC
3.4.3. Effects of FeCl3 Concentrations on MPTFC for Hg0 Removal
3.4.4. Effects of O2 on Hg0 Removal by the Sorbents
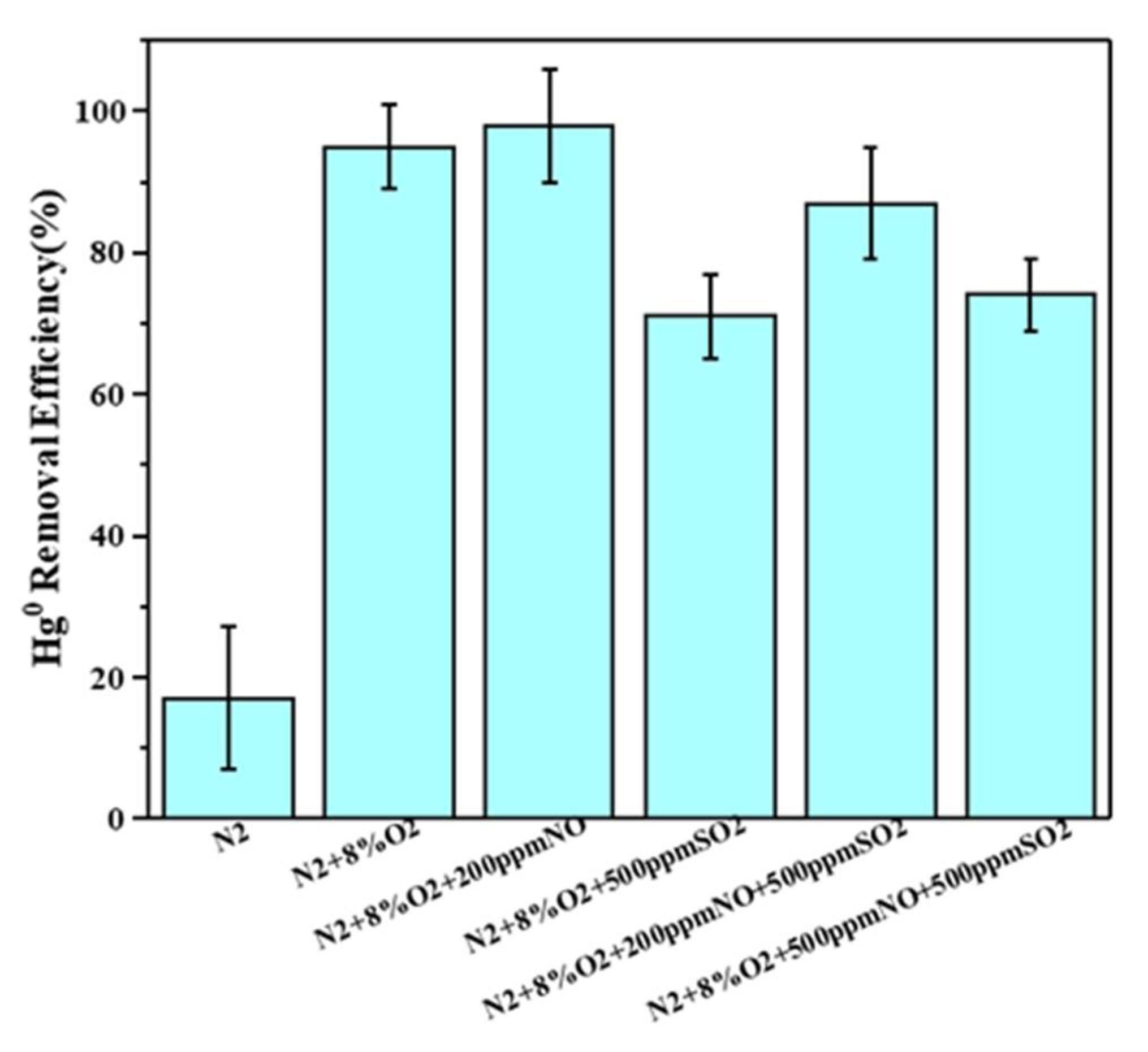
3.4.5. Effects of Flue Gas Composition on the Hg0 Removal Performance of MPTFC
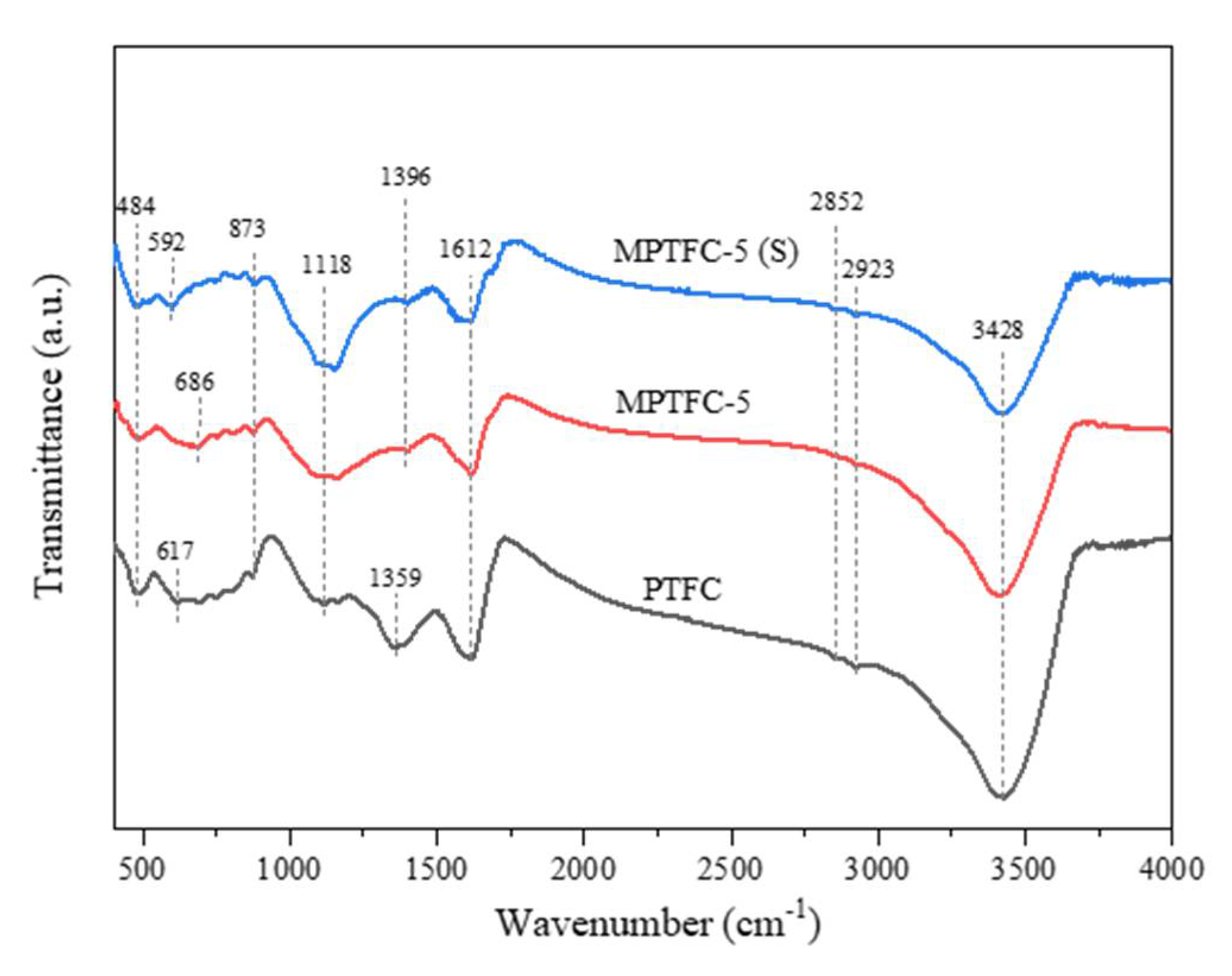
3.5. FTIR Measurements of MPTFC
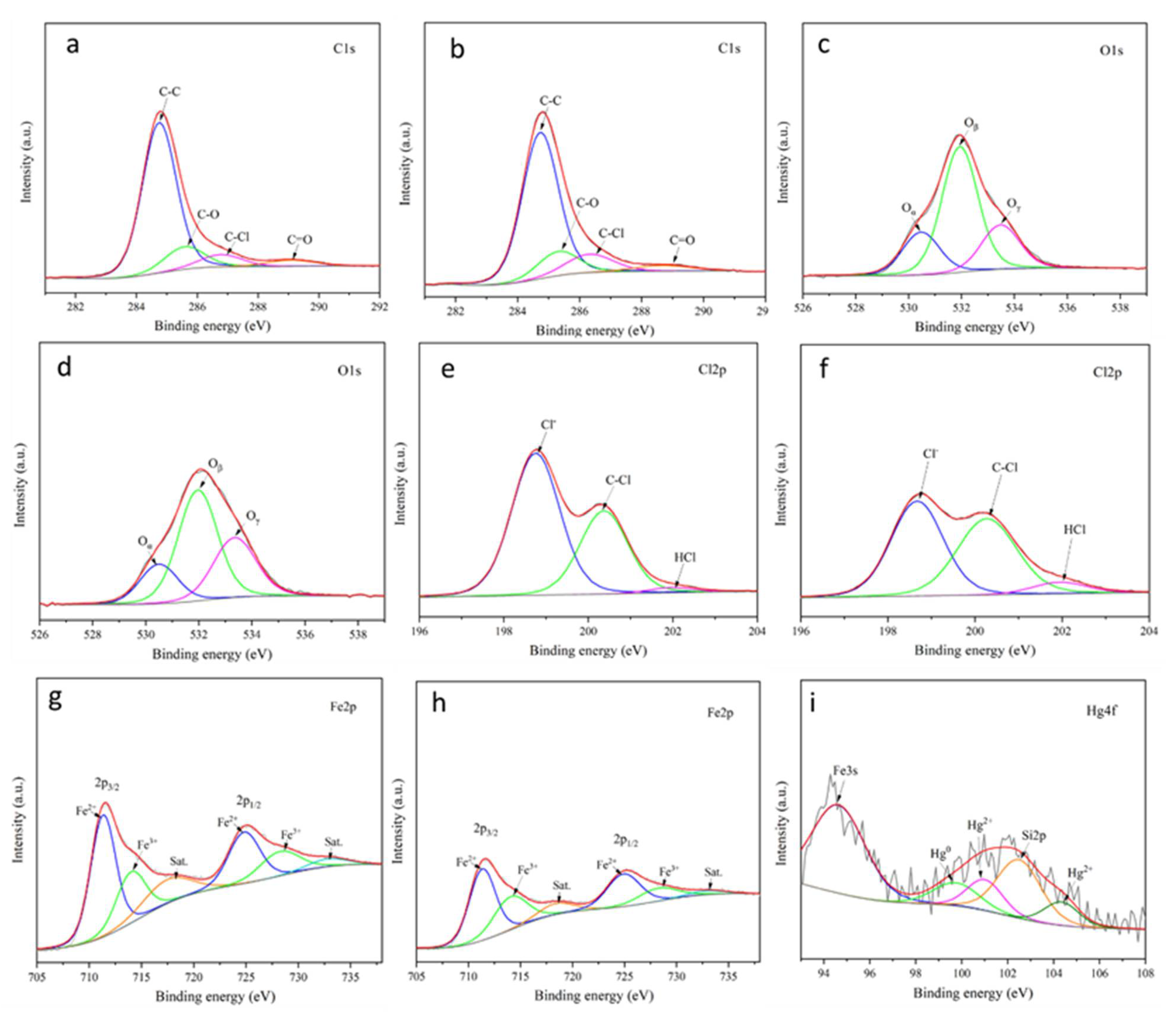
3.6. XPS Analysis of MPTFC before and after the Reactions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, H.; Zhu, L.; Wang, J.; Li, L.; Shih, K. Development of Nano-Sulfide Sorbent for Efficient Removal of Elemental Mercury from Coal Combustion Fuel Gas. Environ. Sci. Technol. 2016, 50, 9551–9557. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liang, S.; Liu, M.; Yi, Y.; Mi, Z.; Zhang, Y.; Li, Y.; Qi, J.; Meng, J.; Tang, X.; et al. Trans-provincial health impacts of atmospheric mercury emissions in China. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Luo, G.; Zhang, Q.; Li, Z.; Zhang, S.; Cui, W. Cost-effective sulfurized sorbents derived from one-step pyrolysis of wood and scrap tire for elemental mercury removal from flue gas. Fuel 2020, 285, 119221. [Google Scholar] [CrossRef]
- Liu, H.; Chang, L.; Liu, W.; Xiong, Z.; Zhao, Y.; Zhang, J. Advances in mercury removal from coal-fired flue gas by mineral adsorbents. Chem. Eng. J. 2020, 379, 122263. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, S.; Liu, K.; Li, G.; Hao, J. Emission-Limit-Oriented Strategy To Control Atmospheric Mercury Emissions in Coal-Fired Power Plants toward the Implementation of the Minamata Convention. Environ. Sci. Technol. 2018, 52, 11087–11093. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Ding, J.; Yu, Y.; Zhang, J. Mercury/oxygen reaction mechanism over CuFe2O4 catalyst. J. Hazard. Mater. 2022, 424, 127556. [Google Scholar] [CrossRef]
- Sasmaz, E.; Kirchofer, A.; Jew, A.D.; Saha, A.; Abram, D.; Jaramillo, T.F.; Wilcox, J. Mercury chemistry on brominated activated carbon. Fuel 2012, 99, 188–196. [Google Scholar] [CrossRef]
- Wang, S.; Hao, J. Air quality management in China: Issues, challenges, and options. J. Environ. Sci. 2012, 24, 2–13. [Google Scholar] [CrossRef]
- Yang, W.; Wang, Z.; Liu, Y. Review on Magnetic Adsorbents for Removal of Elemental Mercury from Flue Gas. Energy Fuels 2020, 34, 13473–13490. [Google Scholar] [CrossRef]
- Zhao, S.; Pudasainee, D.; Duan, Y.; Gupta, R.; Liu, M.; Lu, J. A review on mercury in coal combustion process: Content and occurrence forms in coal, transformation, sampling methods, emission and control technologies. Prog. Energy Combust. Sci. 2019, 73, 26–64. [Google Scholar] [CrossRef]
- Zhou, Q.; Tao, X.; Di, G.; Shang, Y.; Lu, P.; Xu, G.; Liu, M.; Zheng, Y.; Dong, L. SO2 Tolerance and Mechanism of Elemental Mercury Removal from Flue Gas by a Magnetic Recyclable Fe6Mn0.8Ce0.2Oy Sorbent. Energy Fuels 2021, 35, 5101–5109. [Google Scholar] [CrossRef]
- Li, H.; Zu, H.; Yang, Z.; Yang, J.; Xu, H.; Qu, W. The adsorption mechanisms of Hg0 on marcasite-type metal selenides: The influences of metal-terminated site. Chem. Eng. J. 2021, 406, 126723. [Google Scholar] [CrossRef]
- Yang, J.; Xu, H.; Zhao, Y.; Li, H.; Zhang, J. Mercury Removal from Flue Gas by Noncarbon Sorbents. Energy Fuels 2021, 35, 3581–3610. [Google Scholar] [CrossRef]
- Wu, S.; Li, H.; Li, L.; Wu, C.-Y.; Zhang, J.; Shih, K. Effects of flue-gas parameters on low temperature NO reduction over a Cu-promoted CeO2–TiO2 catalyst. Fuel 2015, 159, 876–882. [Google Scholar] [CrossRef]
- Zhang, J.; Duan, Y.; Zhou, Q.; Zhu, C.; She, M.; Ding, W. Adsorptive removal of gas-phase mercury by oxygen non-thermal plasma modified activated carbon. Chem. Eng. J. 2016, 294, 281–289. [Google Scholar] [CrossRef]
- Zheng, J.-M.; Shah, K.J.; Zhou, J.-S.; Pan, S.-Y.; Chiang, P.-C. Impact of HCl and O2 on removal of elemental mercury by heat-treated activated carbon: Integrated X-ray analysis. Fuel Process. Technol. 2017, 167, 11–17. [Google Scholar] [CrossRef]
- Tang, R.; Yang, W.; Wang, H.; Zhou, J.; Zhang, Z.; Wu, S. Preparation of Fly-Ash-Modified Bamboo-Shell Carbon Black and Its Mercury Removal Performance in Simulated Flue Gases. Energy Fuels 2016, 30, 4191–4196. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, Y.; Zhang, J.; Zheng, C. Removal of elemental mercury from flue gas by recyclable CuCl2 modified magnetospheres catalyst from fly ash. Part 1. Catalyst characterization and performance evaluation. Fuel 2016, 164, 419–428. [Google Scholar] [CrossRef]
- Yang, W.; Chen, H.; Han, X.; Ding, S.; Shan, Y.; Liu, Y. Preparation of magnetic Co-Fe modified porous carbon from agricultural wastes by microwave and steam activation for mercury removal. J. Hazard. Mater. 2020, 381, 120981. [Google Scholar] [CrossRef]
- Li, H.; Feng, S.; Yang, Z.; Yang, J.; Liu, S.; Hu, Y.; Zhong, L.; Qu, W. Density Functional Theory Study of Mercury Adsorption on CuS Surface: Effect of Typical Flue Gas Components. Energy Fuels 2019, 33, 1540–1546. [Google Scholar] [CrossRef]
- Zhang, X.; Duan, Y.; Wang, H.; Ren, S.; Wei, H. Effect of flue gas components on Hg0 oxidation and adsorption by modified walnut shell coke in O2/CO2 atmosphere. Asia-Pac. J. Chem. Eng. 2020, 15, e2423. [Google Scholar] [CrossRef]
- Gao, L.; Li, C.; Zhang, J.; Du, X.; Li, S.; Zeng, J.; Yi, Y.; Zeng, G. Simultaneous removal of NO and Hg0 from simulated flue gas over CoOx-CeO2 loaded biomass activated carbon derived from maize straw at low temperatures. Chem. Eng. J. 2018, 342, 339–349. [Google Scholar] [CrossRef]
- Shan, Y.; Yang, W.; Li, Y.; Liu, Y.; Pan, J. Preparation of microwave-activated magnetic bio-char adsorbent and study on removal of elemental mercury from flue gas. Sci. Total Environ. 2019, 697, 134049. [Google Scholar] [CrossRef]
- Yang, W.; Liu, Z.; Xu, W.; Liu, Y. Removal of elemental mercury from flue gas using sargassum chars modified by NH4Br reagent. Fuel 2018, 214, 196–206. [Google Scholar] [CrossRef]
- Shen, B.; Tian, L.; Li, F.; Zhang, X.; Xu, H.; Singh, S. Elemental mercury removal by the modified bio-char from waste tea. Fuel 2017, 187, 189–196. [Google Scholar] [CrossRef]
- Yang, W.; Liu, Y.; Pan, J. Experimental and kinetic study on Hg0 removal by microwave/hydrogen peroxide modified seaweed-based porous biochars. Environ. Technol. Innov. 2021, 22, 101411. [Google Scholar] [CrossRef]
- Altaf, A.R.; Teng, H.; Zheng, M.; Ashraf, I.; Arsalan, M.; Rehman, A.U.; Gang, L.; Pengjie, W.; Ren, Y.; Lu, X. One-step synthesis of renewable magnetic tea-biochar derived from waste tea leaves for the removal of Hg0 from coal-syngas. J. Environ. Chem. Eng. 2021, 9, 105313. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Yang, W.; Liu, L.; Pan, J. Adsorption of elemental mercury in flue gas using biomass porous carbons modified by microwave/hydrogen peroxide. Fuel 2021, 291, 120152. [Google Scholar] [CrossRef]
- Shen, B.; Liu, Z.; Xu, H.; Wang, F. Enhancing the absorption of elemental mercury using hydrogen peroxide modified bamboo carbons. Fuel 2019, 235, 878–885. [Google Scholar] [CrossRef]
- Lee, S.J.; Seo, Y.-C.; Jurng, J.; Lee, T.G. Removal of gas-phase elemental mercury by iodine- and chlorine-impregnated activated carbons. Atmos. Environ. 2004, 38, 4887–4893. [Google Scholar] [CrossRef]
- Cai, J.; Shen, B.; Li, Z.; Chen, J.; He, C. Removal of elemental mercury by clays impregnated with KI and KBr. Chem. Eng. J. 2014, 241, 19–27. [Google Scholar] [CrossRef]
- Tan, Z.; Niu, G.; Chen, X. Removal of elemental mercury by modified bamboo carbon. Chin. J. Chem. Eng. 2015, 23, 1875–1880. [Google Scholar] [CrossRef]
- Xu, W.; Adewuyi, Y.G.; Liu, Y.; Wang, Y. Removal of elemental mercury from flue gas using CuOx and CeO2 modified rice straw chars enhanced by ultrasound. Fuel Process. Technol. 2018, 170, 21–311. [Google Scholar] [CrossRef]
- Xie, Y.; Li, C.; Zhao, L.; Zhang, J.; Zeng, G.; Zhang, X.; Zhang, W.; Tao, S. Experimental study on Hg0 removal from flue gas over columnar MnOx-CeO2/activated coke. Appl. Surf. Sci. 2015, 333, 59–67. [Google Scholar] [CrossRef]
- Li, G.; Wu, Q.; Wang, S.; Li, Z.; Liang, H.; Tang, Y.; Zhao, M.; Chen, L.; Liu, K.; Wang, F. The influence of flue gas components and activated carbon injection on mercury capture of municipal solid waste incineration in China. Chem. Eng. J. 2017, 326, 561–569. [Google Scholar] [CrossRef]
- Han, L.; He, X.; Yue, C.; Hu, Y.; Li, L.; Chang, L.; Wang, H.; Wang, J. Fe doping Pd/AC sorbent efficiently improving the Hg0 removal from the coal-derived fuel gas. Fuel 2016, 182, 64–72. [Google Scholar] [CrossRef]
- Liu, D.; Xu, W.; Liu, Y. Seaweed bio-chars modified with metal chloride for elemental mercury capture from simulated flue gas. Atmos. Pollut. Res. 2020, 11, 122–130. [Google Scholar] [CrossRef]
- Zhao, L.; Li, C.; Zhang, J.; Zhang, X.; Zhan, F.; Ma, J.; Xie, Y.; Zeng, G. Promotional effect of CeO2 modified support on V2O5–WO3/TiO2 catalyst for elemental mercury oxidation in simulated coal-fired flue gas. Fuel 2015, 153, 361–369. [Google Scholar] [CrossRef]

| Conditions | Gas Components | Total Gas Flow Rate (L/mol) | Initial Hg0 Concentration (µg/m3) | Reaction Temperature (°C) | Sorbent Mass (mg) | |
|---|---|---|---|---|---|---|
| No. | ||||||
| 1 | 8% O2, N2 | 1 | 50 | 200 | 100 | |
| 2 | 8% O2, N2 | 1 | 50 | 150/200/250/300 | 100 | |
| 3 | N2 | 1 | 50 | 100/150/200/250/300 | 100 | |
| 4 | 8% O2, N2 500 ppm SO2 0~700 ppm NO | 1 | 50 | 200 | 100 | |
| 5 | 8% O2, N2 0~1500 ppm SO2 200 ppm NO | 1 | 50 | 200 | 100 | |
| 6 | 8% O2, N2 | 1 | 50 | 200 | 100 | |
| Sample | Proximate Analysis (wt.%, Dry Basis) | Ultimate Analysis (wt.%, Dry and Ash-Free Basis) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C daf | H daf | O daf | N daf | S t | M ad | V daf | A d | FC daf | |
| PTF | 65.98 | 3.42 | 34.74 | 1.86 | 0.52 | 5.41 | 72.08 | 6.58 | 15.93 |
| Sample | PTF | PTFC | MPTFC-3 | MPTFC-5 | MPTFC-7 |
|---|---|---|---|---|---|
| Surface area | 2.4 | 3.4 | 5.8 | 10.6 | 8.4 |
| Functional Groups | Before Reactions | After Reactions | ||
|---|---|---|---|---|
| Position (eV) | Content (%) | Position (eV) | Content (%) | |
| C–C/C–H | 284.75 | 70.70 | 284.73 | 74.71 |
| C–O/C–OH | 285.58 | 13.21 | 285.31 | 13.37 |
| C–Cl | 286.78 | 11.59 | 286.32 | 7.62 |
| C=O | 289.08 | 4.47 | 288.69 | 4.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Li, M.; Hu, Z.; Tian, C. The Synthesis of FeCl3-Modified Char from Phoenix Tree Fruit and Its Application for Hg0 Adsorption in Flue Gas. Atmosphere 2022, 13, 1093. https://doi.org/10.3390/atmos13071093
Chen W, Li M, Hu Z, Tian C. The Synthesis of FeCl3-Modified Char from Phoenix Tree Fruit and Its Application for Hg0 Adsorption in Flue Gas. Atmosphere. 2022; 13(7):1093. https://doi.org/10.3390/atmos13071093
Chicago/Turabian StyleChen, Wei, Ming Li, Zirui Hu, and Chong Tian. 2022. "The Synthesis of FeCl3-Modified Char from Phoenix Tree Fruit and Its Application for Hg0 Adsorption in Flue Gas" Atmosphere 13, no. 7: 1093. https://doi.org/10.3390/atmos13071093
APA StyleChen, W., Li, M., Hu, Z., & Tian, C. (2022). The Synthesis of FeCl3-Modified Char from Phoenix Tree Fruit and Its Application for Hg0 Adsorption in Flue Gas. Atmosphere, 13(7), 1093. https://doi.org/10.3390/atmos13071093






