Nitrous Oxide Emission Fluxes in Coffee Plantations during Fertilization: A Case Study in Costa Rica
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Fertilization
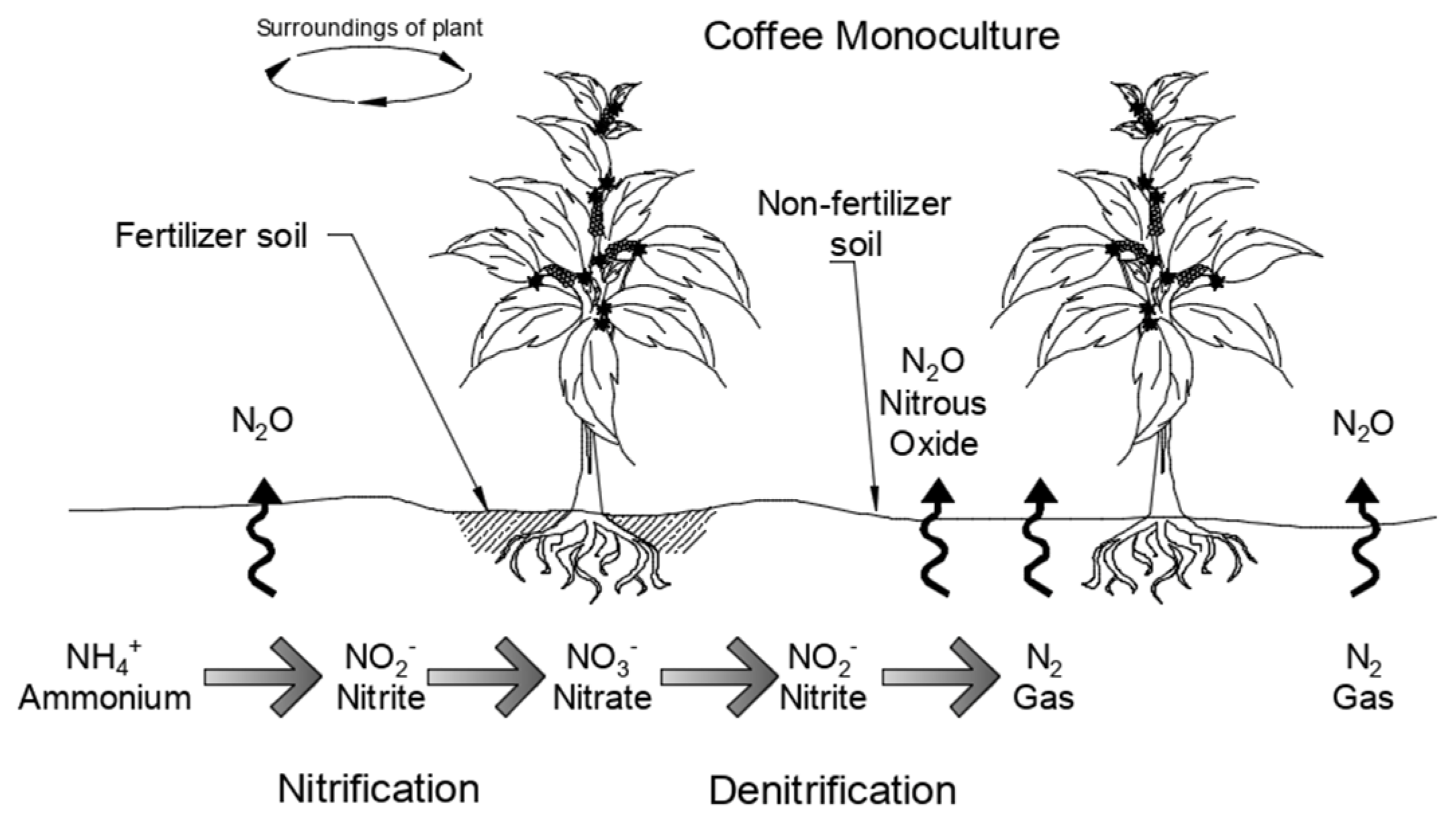
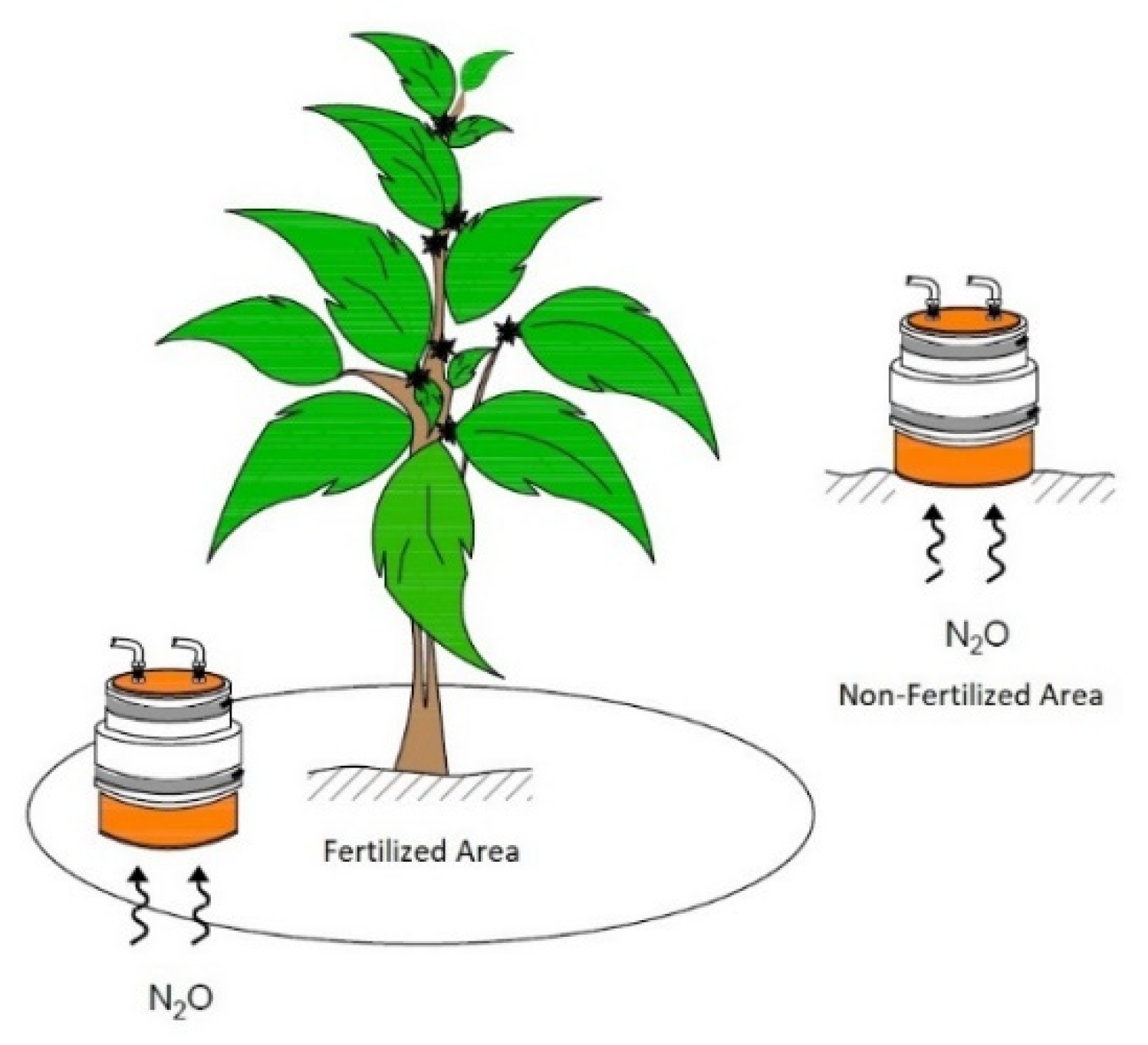
2.2. Experimental Design and Sampling
- ‑
- Direct emissions of CO2eq:where: GWP = 310 (100 to 110 years lifetime global warming potential).
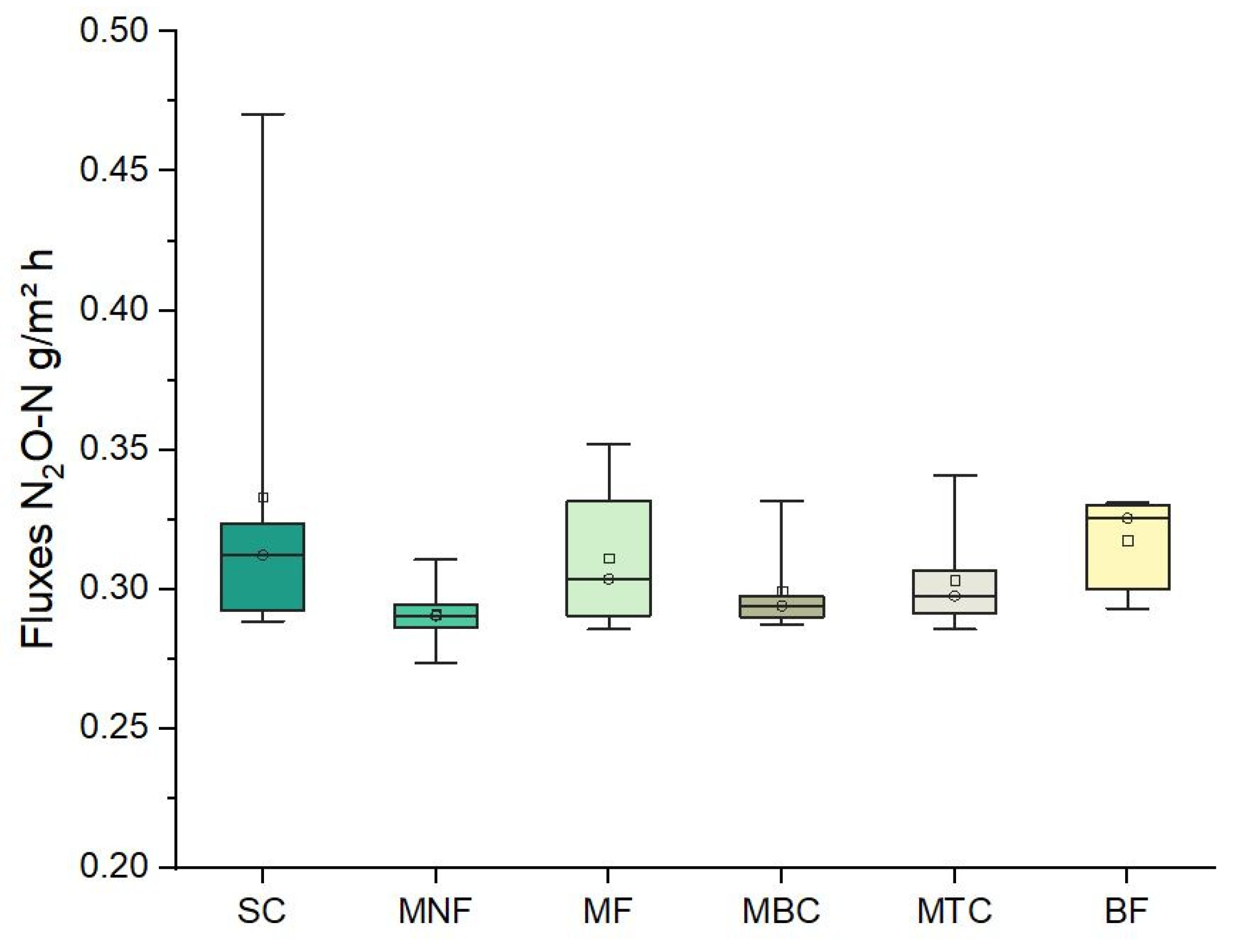
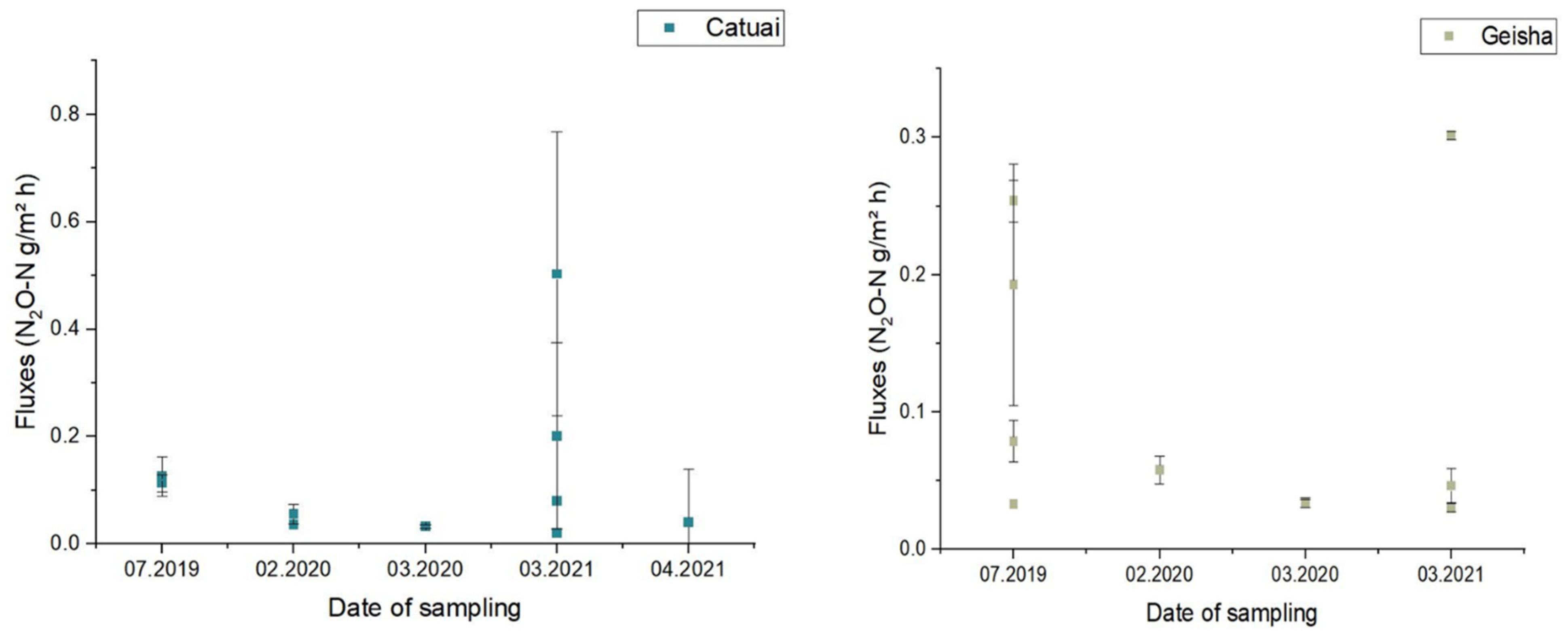
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kelliher, F.M.; Reisinger, A.R.; Martin, R.J.; Harvey, M.J.; Price, S.J.; Sherlock, R.R. Measuring nitrous oxide emission rate from grazed pasture using Fourier-transform infrared spectroscopy in the noctumal boundary layer. Agric. For. Meteorol. 2002, 111, 29–38. [Google Scholar] [CrossRef]
- EPA. Overview of Greenhouse Gases. 2012. Available online: https://www.epa.gov/ghgemissions/overview-greenhouse-gases (accessed on 3 August 2018).
- Intergovernmental Panel on Climate Change. Summary for Policymakers. In Climate Change 2013—The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014; pp. 1–30. [Google Scholar] [CrossRef]
- IPNI. Agronomic Fact Sheets on Crop Nutrients: Nitrogen. 2005. Available online: https://www.ipni.net/publication/nutrifacts-na.nsf/0/E6EA88FF25BC684985257F07006E1B5D/$FILE/NutriFacts-NA-18.pdf (accessed on 23 October 2021).
- Hue, N.V.; Silva, J.A. Organic Soil Amendments for Sustainable Agriculture: Organic Sources of Nitrogen, Phosphorus, and Potassium. In Plant Nutrition in Hawaiss’s Soil for Tropical and Subtropical Agriculture; University of Hawaii: Honolulu, HI, USA, 2000; pp. 133–144. [Google Scholar]
- Feng, J.; Li, F.; Deng, A.; Feng, X.; Fang, F.; Zhang, W. Integrated assessment of the impact of enhanced-efficiency nitrogen fertilizer on N2O emission and crop yield. Agric. Ecosyst. Environ. 2016, 231, 218–228. [Google Scholar] [CrossRef]
- Bouwman, A.F. Direct emission of nitrous oxide from agricultural soils. Nutr. Cycl. Agroecosyst. 1996, 46, 53–70. [Google Scholar] [CrossRef]
- Nevison, C. Indirect N2O Emissions from Agriculture. In Good Practice Guidance and Uncertainty Management in National Greenhouse Gas Inventories (IPCC Guidelines); IGES: Geneva, Switzerland, 1998; pp. 381–397. [Google Scholar]
- Gillenwater, M.; Saarinen, K.; Ajavon, A.N. Precursors and Indirect. In IPCC 2006, 2006 IPCC Guidelines for National Greenhouse Gas Inventories, Prepared by the National Greenhouse Gas Inventories Programme; Eggleston, H.S., Buendia, L., Miwa, K., Ngara, T., Tanabe, K., Eds.; Institute for Global Environmental Strategies (IGES): Hayama, Japan, 2006. [Google Scholar]
- Smith, K.; Bouwman, L.; Braatz, B. N2O: Direct Emissions From Agricultural Soils. In Good Practice Guidance and Uncertainty Management in National Greenhouse Gas Inventories; Intergovernmental Panel on Climate Change: Kanagawa, Japan, 1999; pp. 361–380. [Google Scholar]
- Ehhalt, D.; Prather, M. Atmospheric Chemistry and Greenhouse Gases. In Climate Change 2001: The Scientific Basis 3rd Assesment Report of the Intergovernmental Panel on Climate Change; Pacific Northwest National Lab. (PNNL): Richland, WA, USA, 2001; pp. 239–287. Available online: http://scholar.google.com/scholar?hl=en&btnG=Search&q=intitle:Agriculture.+In+Climate+Change+2007:+Mitigation.+Contribution+of+Working+Group+III+to+the+Fourth+Assessment+Report+of+the+Intergovernmental+Panel+on+Climate+Change+[B.#0 (accessed on 29 September 2021).
- Wang, F.; Chen, S.; Zhang, K.Q.; Shen, S.Z.; Zhu-Barker, X. Impact of nitrogen fertilizer source on nitrous oxide (N2O) emissions from three different agricultural soils during freezing conditions. Toxicol. Environ. Chem. 2016, 98, 551–560. [Google Scholar] [CrossRef]
- Hergoualc’h, K.; Skiba, U.; Harmand, J.M.; Oliver, R. Processes responsible for the nitrous oxide emission from a Costa Rican Andosol under a coffee agroforestry plantation. Biol. Fertil. Soils 2007, 43, 787–795. [Google Scholar] [CrossRef]
- Stevens, R.J.; Laughlin, R.J. Measurement of nitrous oxide and di-nitrogen emissions from agricultural soils. Nutr. Cycl. Agroecosyst. 1998, 52, 131–139. [Google Scholar] [CrossRef]
- Rochette, P.; Eriksen-Hamel, N.S. Chamber Measurements of Soil Nitrous Oxide Flux: Are Absolute Values Reliable? Soil Sci. Soc. Am. J. 2008, 72, 331–342. [Google Scholar] [CrossRef]
- Cai, Z.; Laughlin, R.J.; Stevens, R.J. Nitrous oxide and dinitrogen emissions from soil under different water regimes and straw amendment. Chemosphere 2001, 42, 113–121. [Google Scholar] [CrossRef]
- Chalk, P.M.; Smith, C.J. Chemodenitrification. In Gaseous Loss of Nitrogen from Plant-Soil Systems; Springer: Dordrecht, The Netherlands, 1983; pp. 65–89. [Google Scholar] [CrossRef]
- Wang, C.; Amon, B.; Schulz, K.; Mehdi, B. Factors That Influence Nitrous Oxide Emissions from Agricultural Soils as Well as Their Representation in Simulation Models: A Review. Agronomy 2021, 11, 770. [Google Scholar] [CrossRef]
- Noponen, M.R.A.; Edwards-Jones, G.; Haggar, J.P.; Soto, G.; Attarzadeh, N.; Healey, J.R. Greenhouse gas emissions in coffee grown with differing input levels under conventional and organic management. Agric. Ecosyst. Environ. 2012, 151, 6–15. [Google Scholar] [CrossRef] [Green Version]
- Food And Agriculture Organization of the United Nations (FAO). Land Use Statistics and Indicators Statistics. Global, Regional and Country Trends; FAOSTAT Analytical Brief Series No 28; FAO: Rome, Italy, 2019. [Google Scholar]
- Goulding, K.; Jarvis, S.; Whitmore, A. Optimizing nutrient management for farm systems. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 667–680. [Google Scholar] [CrossRef] [Green Version]
- Moldvaer, A. Coffee Obsession, 1st ed.; Dorling Kindersley Limited: London, UK, 2014. [Google Scholar]
- Nieters, A.; Grabs, J.; Jimenez, G.; Alpizar, W. NAMA Café Costa Rica: A Tool for Low Carbon Development. NAMA Facility Technical Support Unit on behalf of German Federal Ministry for the Environment, Nature Conservation, Building and Nuclear Safety (BMUB)/UK Department for Energy and Climate Change (DECC). Available online: http://www.namacafe.org/sites/default/files/files/NAMA_Facility_factsheet_Costa%20Rica.pdf (accessed on 7 December 2021).
- Insituto del Café de Costa Rica. Clima del Café–ICAFE. 2020. Available online: http://www.icafe.cr/sector-cafetalero/clima/?zona=LS (accessed on 3 October 2021).
- Vignola, R.; Watler, W.; Coto, K.P.; Céspedes, A.V. Ficha Técnica Cultivo de Café: Prácticas Efectivas Para la Reducción de Impactos por Eventos Climáticos en el Cultivo de Café en Costa Rica. Costa Rica. 2018. Available online: http://www.mag.go.cr/bibliotecavirtual/reduccion-impacto-por-eventos-climaticos/Informe-final-cafe.pdf (accessed on 16 September 2021).
- Chinchilla, M.; Mata, R.; Alvarado, A. Caracterización y Clasificación de Algunos Ultisoles de la Región de Los Santos, Talamanca, Costa Rica. Available online: https://revistas.ucr.ac.cr/index.php/agrocost/article/view/6687 (accessed on 7 December 2021).
- Bornemisza, E.; Segura, A.; Bertrand, B.; Rapidel, B. Café-América Centra! In Capitul03 Los Suelos Cafetaleros de América Central y su Fertilización; Desafíos de la Caficultura en Centroamérica; No. IICA-E11 12; IICA: San José, Costa Rica; PROMECAFE CIRAD: París, France, 1999; ISBN 92-9039-391-2. [Google Scholar]
- Viguera, B.; Alpízar, F.; Harvey, C.A.; Martínez-Rodríguez, M.R.; Saborío-Rodríguez, M. Climate change perceptions and adaptive responses of small-scale coffee farmers in Costa Rica|Percepciones de cambio climático y respuestas adaptativas de caficultores costarricenses de pequeña escala. Agron. Mesoam. 2019, 30, 333–351. [Google Scholar] [CrossRef] [Green Version]
- ICAFE. Informe Sobre La Actividad Cafetalera De Costa Rica, Heredia, Costa Rica. 2017. Available online: http://www.icafe.cr/wp-content/uploads/informacion_mercado/informes_actividad/anteriores/2017.pdf (accessed on 1 September 2021).
- VDI: Verein Deutscher Ingenieure. VDI 3475 Part 1: Emission Control Biological Waste Treatment Facilities Composting and Anaerobic Digestion Plant Capacities more than Approx. 6.000 Mg/a.; Beuth Verlag GmbH: Berlin, Germany, 2003. [Google Scholar]
- VDI: Verein Deutscher Ingenieure. VDI 3880: Olfactometry Static Sampling. Beuth Publishing Düsseldorf. 2011. Available online: www.vdi-richtlinien.de (accessed on 7 November 2021).
- Ruiz, M.S.M.; Reiser, M.; Kranert, M. Composting and Methane Emissions of Coffee By-Products. Atmosphere 2021, 12, 1153. [Google Scholar] [CrossRef]
- Pavelka, M.; Acosta, M.; Kiese, R.; Altimir, N.; Brümmer, C.; Crill, P.; Darenova, E.; Fuß, R.; Gielen, B.; Graf, A.; et al. Standardisation of chamber technique for CO2, N2O and CH4 fluxes measurements from terrestrial ecosystems. Int. Agrophys. 2018, 32, 569–587. [Google Scholar] [CrossRef]
- Blackshaw, R.E. Nitrogen Fertilizer, Manure, and Compost Effects on Weed Growth and Competition with Spring Wheat. Agron. J. 2005, 97, 1612–1621. [Google Scholar] [CrossRef] [Green Version]
- Cowan, N.J.; Famulari, D.; Levy, P.E.; Anderson, M.; Bell, M.J.; Rees, R.M.; Reay, D.S.; Skiba, U.M. An improved method for measuring soil N2O fluxes using a quantum cascade laser with a dynamic chamber. Eur. J. Soil Sci. 2014, 65, 643–652. [Google Scholar] [CrossRef] [Green Version]
- Gasmet. FTIR-Portable Multigas Analyzer. Available online: https://www.gasmet.com/products/category/portable-gas-analyzers/gt5000-terra/ (accessed on 30 September 2021).
- Pihlatie, M.; Christiansen, J.; Aaltonen, H.; Korhonen, J.; Nordbo, A.; Rasilo, T.; Benanti, G.; Giebels, M.; Helmy, M.; Sheehy, J.; et al. Comparison of static chambers to measure CH4 emissions from soils. Agric. For. Meteorol. 2013, 171–172, 124–136. [Google Scholar] [CrossRef] [Green Version]
- Food and Agriculture Organization of the United Nations. Estimating Greenhouse Gas Emissions in Agriculture. A Manual to Address Data Requirements for Developing Countries; Food and Agricultural Organization: Rome, Italy, 2015. [Google Scholar]
- Chinchilla, M.; Mata, R.; Alvarado, A. Caracterización Y Clasificación De Algunos Ultisoles. Agron. Costarric. 2011, 35, 59–81. [Google Scholar] [CrossRef]
- Mata, R.A.; Ramírez, J.E. Estudio de Caracterización de Suelos y su Relación con el Manejo del Cultivo de Café en la Provincia de Heredia; No. 633.7332 M425; Instituto del Café de Costa Rica: San José, Costa Rica, 1999. [Google Scholar]
- Cubero, D.; Vieira, M.J. Conferencia 70. In Abonos Orgánicos y Fertilizantes Químicos...¿Son Compatibles con la Agricultura? 1999, pp. 61–62. Available online: http://www.mag.go.cr/congreso_agronomico_xi/a50-6907-III_061.pdf (accessed on 24 September 2021).
- ŠImek, M.; Cooper, J.E. The influence of soil pH on denitrification: Progress towards the understanding of this interaction over the last 50 years. Eur. J. Soil Sci. 2002, 53, 345–354. [Google Scholar] [CrossRef]
- Shaaban, M.; Wu, Y.; Khalid, M.S.; Peng, Q.-A.; Xu, X.; Wu, L.; Younas, A.; Bashir, S.; Mo, Y.; Lin, S.; et al. Reduction in soil N2O emissions by pH manipulation and enhanced nosZ gene transcription under different water regimes. Environ. Pollut. 2018, 235, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Wrage, N.; Velthof, G.L.; van Beusichem, M.L.; Oenema, O. Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol. Biochem. 2001, 33, 1723–1732. [Google Scholar] [CrossRef]
- Oertel, C.; Matschullat, J.; Zurba, K.; Zimmermann, F.; Erasmi, S. Greenhouse gas emissions from soils—A review. Geochemistry 2016, 76, 327–352. [Google Scholar] [CrossRef] [Green Version]
- Buchkina, N.P.; Rizhiya, E.Y.; Pavlik, S.v.; Balashov, E.V. Soil Physical Properties and Nitrous Oxide Emission from Agricultural Soils. Adv. Agrophys. Res. 2013, 193–220. [Google Scholar] [CrossRef] [Green Version]
- Oktarita, S.; Hergoualc’H, K.; Anwar, S.; Verchot, L.v. Substantial N2O emissions from peat decomposition and N fertilization in an oil palm plantation exacerbated by hotspots. Environ. Res. Lett. 2017, 12, 104007. [Google Scholar] [CrossRef]
- Arias-Navarro, C.; Diaz-Pines, E.; Klatt, S.; Brandt, P.; Rufino, M.C.; Butterbach-Bahl, K.; Verchot, L.V. Spatial variability of soil N2O and CO2 fluxes in different topographic positions in a tropical montane forest in Kenya. J. Geophys. Res. Biogeosci. 2017, 122, 514–527. [Google Scholar] [CrossRef]
- Eichner, M.J. Nitrous Oxide Emissions from Fertilized Soils: Summary of Available Data. J. Environ. Qual. 1990, 19, 272–280. [Google Scholar] [CrossRef]
- Harrison, R.B. Composting and Formation of Humic Substances; Jørgensen, S.E., Fath, B.D., Eds.; Academic Press: Oxford, UK, 2008; pp. 713–719. ISBN 9780080454054. [Google Scholar] [CrossRef]
- Gilbert, J.; Ricci-Jurgensen, M.; Ramola, A. Benefits of Compost and Anaerobic Digestate When Applied to Soil. Report 2. 2020, pp. 68–70. Available online: https://www.iswa.org/knowledge-base/benefits-of-compost-and-anaerobic-digestate-when-applied-to-soil/?v=1ee0bf89c5d1 (accessed on 10 September 2021).
- Diacono, M.; Montemurro, F. Long-term effects of organic amendments on soil fertility. A review. Agron. Sustain. Dev. 2010, 30, 401–422. [Google Scholar] [CrossRef] [Green Version]
- Ros, M.; Klammer, S.; Knapp, B.; Aichberger, K.; Insam, H. Long-term effects of compost amendment of soil on functional and structural diversity and microbial activity. Soil Use Manag. 2006, 22, 209–218. [Google Scholar] [CrossRef]
- Larney, F.J.; Hao, X. A review of composting as a management alternative for beef cattle feedlot manure in southern Alberta, Canada. Bioresour. Technol. 2007, 98, 3221–3227. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Lang, M.; Li, C.; Hao, X. Nitrous oxide and carbon dioxide emissions from soils amended with compost and manure from cattle fed diets containing wheat dried distillers’ grains with solubles. Can. J. Soil Sci. 2016, 97, 522–531. [Google Scholar] [CrossRef] [Green Version]
- Hoben, J.P.; Gehl, R.J.; Millar, N.; Grace, P.R.; Robertson, G.P. Nonlinear nitrous oxide (N2O) response to nitrogen fertilizer in on-farm corn crops of the US Midwest. Glob. Chang. Biol. 2010, 17, 1140–1152. [Google Scholar] [CrossRef]
- Montenegro, J.; Herrera, J. Emisión del óxido nitroso (N2O) en el sistema de producción café sin sombra en Costa Rica. Tóp. Metereol. Oceanogr. IMN 2013, 12, 22–34. [Google Scholar] [CrossRef] [Green Version]
- Martinez Rayo, J.L. Manual Técnico: Para el Manejo de la Fertilizacion de Suelos Cafetaleros. 2012. Available online: https://jorgemartinezrayo.files.wordpress.com/2013/07/manual-de-fertilidad-de-suelos.pdf (accessed on 7 December 2021).
- Harsono, S.S.; Wibowo, R.K.K.; Supriyanto, E. Energy Balance and Green House Gas Emisson on Smallholder Java Coffee Production at Slopes Ijen Raung Plateau of Indonesia. J. Ecol. Eng. 2021, 22, 271–283. [Google Scholar] [CrossRef]
- Wu, L.; Jiang, Y.; Zhao, F.; He, X.; Liu, H.; Yu, K. Increased organic fertilizer application and reduced chemical fertilizer application affect the soil properties and bacterial communities of grape rhizosphere soil. Sci. Rep. 2020, 10, 9568. [Google Scholar] [CrossRef] [PubMed]


| Parameters | Units | A* | B** | C*** |
|---|---|---|---|---|
| pH | H2O | 5.5 | 4.9 | 5.0 |
| Acidity | cmol(+)/L | 0.14 | 1.38 | 0.50 |
| Ca | 8.13 | 4.96 | 6.79 | |
| Mg | 3.04 | 1.33 | 2.75 | |
| K | 2.27 | 0.56 | 1.85 | |
| CEC | 13.58 | 8.23 | 11.89 | |
| AS | % | 1 | 17 | 4 |
| P | mg/L | 12 | 1 | 4 |
| Zn | 7.4 | 3.2 | 6.3 | |
| Cu | 8 | 12 | 8 | |
| Fe | 312 | 312 | 242 | |
| Mn | 19 | 39 | 85 | |
| EC | mS/cm | 0.4 | 0.2 | 0.4 |
| C | % | 3.63 | 2.61 | 2.93 |
| N | 0.35 | 0.23 | 0.27 | |
| C/N | Ratio | 10.4 | 11.3 | 10.9 |
| Fertilization Period Costa Rica 2019–2020 | ||||
|---|---|---|---|---|
| 22 June 2019 | 28 August 2019 | 27 October 2019 | 31 May 2020 | 20 August 2020 |
| Formula/Amount per plant | ||||
| 17 (N)-6 (P2O5)-18 (K2O)-5 (MgO)-0.2(B)-0.1(Z)-1.6(S) | 17(N)-6(P2O5)-18(K2O)-5 (MgO)-0.2(B)-0,1(Z)-1.6(S) | 20(N)-8(MgO)-11(Ca) | 18 (N)-5-(P2O5)18 (K2O)-0.2(B)-7.3(S) | 17 (N)-6(P2O5)-18 (K2O)-5(Mg)-0.2(B)-0.1(Z)-1.6(S) |
| Amount added: 90 g/plant | ||||
| Percentage of Fertilizer Used in Nursery Greenhouse in the Botanical Garden | ||||
| Component | Week 5 July 2019vol in % | Week 9 July 2019vol in % | Volume Mixture for Small-Sized Plant (mL) | Volume Mixture for Medium-Sized Plant (mL) |
| Nitrogen (N) | 20 | 15 | 100 | 200 |
| Potassium (K2O) | 20 | 20 | ||
| Phosphorus (P2O5) | 20 | 25 | ||
| Fertilizer Assay—04.2021 in Costa Rica | ||||
| Chemical Fertilizer (F1) | Physical Mixture Fertilizer (F2) | Soil Amendment (SA)—Compost Coffee Byproducts | ||
| 17 (N)-6(P2O5)-18 (K2O)-5(Mg)-0.2(B)-0.1(Z)-1.6(S) | 18(N)-5(P2O5)-15(K2O)-7.3(S)-6(MgO)-0.2(B) | 1.37(N)-0.46(P2O5)-3.19(K2O)-0.35(MgO) | ||
| Amount added: 90 g/plant | Amount added: 2 kg/plant | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
San Martin Ruiz, M.; Reiser, M.; Kranert, M. Nitrous Oxide Emission Fluxes in Coffee Plantations during Fertilization: A Case Study in Costa Rica. Atmosphere 2021, 12, 1656. https://doi.org/10.3390/atmos12121656
San Martin Ruiz M, Reiser M, Kranert M. Nitrous Oxide Emission Fluxes in Coffee Plantations during Fertilization: A Case Study in Costa Rica. Atmosphere. 2021; 12(12):1656. https://doi.org/10.3390/atmos12121656
Chicago/Turabian StyleSan Martin Ruiz, Macarena, Martin Reiser, and Martin Kranert. 2021. "Nitrous Oxide Emission Fluxes in Coffee Plantations during Fertilization: A Case Study in Costa Rica" Atmosphere 12, no. 12: 1656. https://doi.org/10.3390/atmos12121656
APA StyleSan Martin Ruiz, M., Reiser, M., & Kranert, M. (2021). Nitrous Oxide Emission Fluxes in Coffee Plantations during Fertilization: A Case Study in Costa Rica. Atmosphere, 12(12), 1656. https://doi.org/10.3390/atmos12121656







